Key Points
The variants in FIX’s SP cause HB at the stage of posttranscription, translation, and posttranslational processing.
The missense variants in FIX’s SP mainly disturb the cotranslational translocation and/or cleavage of SP.
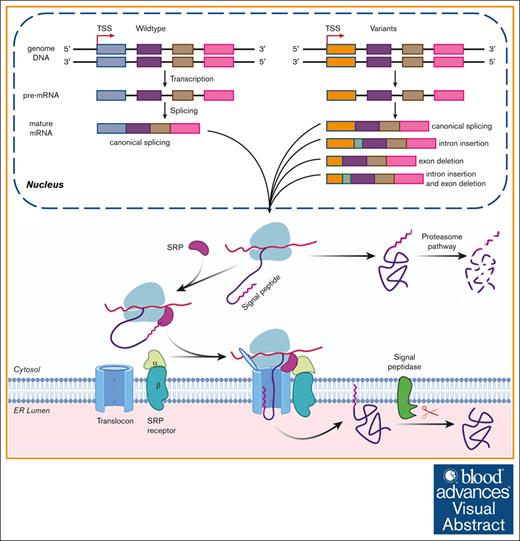
Visual Abstract
Signal peptide (SP) is essential for protein secretion, and pathogenic variants in the SP of factor IX (FIX) have been identified in hemophilia B (HB). However, the underlying mechanism for the genotype-phenotype correlation of these variants has not been well studied. Here, we systematically examined the effects of 13 pathogenic point variants in the SP of FIX using different approaches. Our results showed that these point variants lead to HB by missense variants and/or aberrant premessenger RNA (pre-mRNA) splicing. The missense variants in a hydrophobic core (h-region) mainly affected the cotranslational translocation function of the SP, and those in C-terminal containing cleavage site (c-region) caused FIX deficiency mainly by disturbing the cotranslational translocation and/or cleavage of the SP. Almost absolute aberrant pre-mRNA splicing was only observed in variants of c.82T>G, but a slight change of splicing patterns was found in variants of c.53G>T, c.77C>A, c.82T>C, and c.83G>A, indicating that these variants might have different degrees of impact on pre-mRNA splicing. Although two 6-nt deletion aberrant pre-mRNA splicing products caused FIX deficiency by disturbing the SP cleavage, they could produce some functional mature FIX, and vitamin K could increase the secretion of functional FIX. Taken together, our data indicated that pathogenic variants in the SP of FIX caused HB through diverse molecular mechanisms or even a mixture of several mechanisms, and vitamin K availability could be partially attributed to varying bleeding tendencies in patients carrying the same variant in the SP.
Introduction
Hemophilia B (HB) is caused by deficiency of coagulation factor IX (FIX), a vitamin K–dependent secretory glycoprotein. The prevalence of HB is ∼1 in 30 000 in male live births.1 Based on the FIX procoagulant activity (FIX:C) level, HB has been classified into 3 different severities: severe, moderate, and mild.2 Severe patients have <1% of normal FIX:C, and spontaneous bleeding occurs frequently. Moderate cases have 1% to 5% of normal FIX:C, and bleeding primarily happens after injury. Those with 5% to 40% of normal FIX:C are defined as mild cases, and they usually experience bleeding only with surgery or major trauma.3,4
Many human diseases, including HB, are connected with a number of variants in signal peptides (SPs) of secretory proteins.5-7 The SPs are recognized by signal recognition particle (SRP) and direct the translocation of secretory proteins into the endoplasmic reticulum (ER) lumen.8,9 Although the SPs do not have strong amino acid homology, majority of them have similar features and structural organization5: a positively charged N-terminal (n-region), a hydrophobic core (h-region), and a C-terminal containing cleavage site (c-region) recognized by a signal peptidase (Figure 1A). Some studies reported that variants in the h-region triggered messenger RNA (mRNA) degradation through the regulation of aberrant protein production (RAPP) pathway.10,11 Generally, variants in c-region did not inhibit SRP interaction but linked to defects in the SP cleavage.5 However, the underlying mechanism to explain the genotype-phenotype correlation of the variants in FIX’s SP has not been well studied.
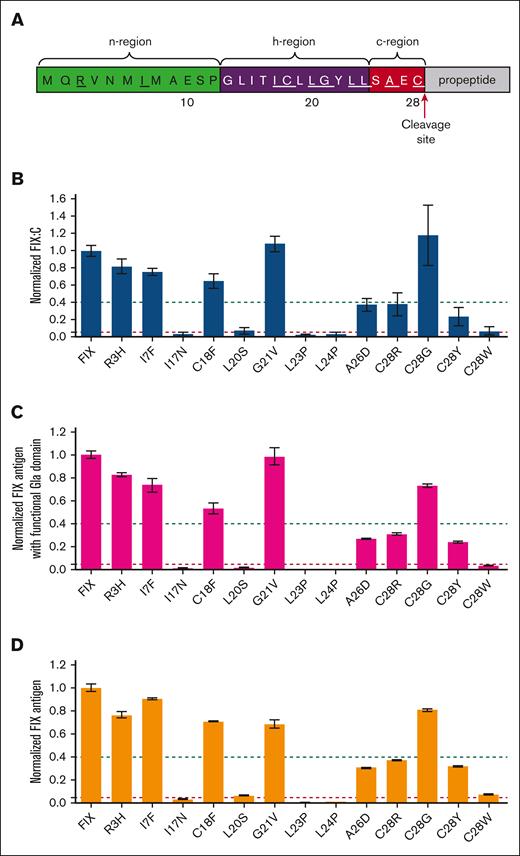
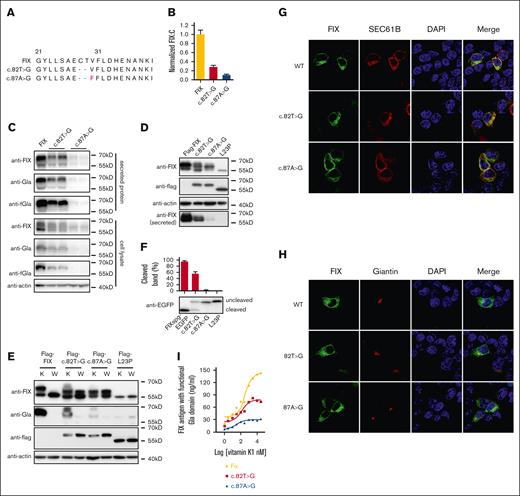
Characterizing FIX procoagulant activity and FIX antigen of missense variants in FIX’s SP by an in vitro cell system. (A) Sequence of FIX’s SP. The n-region (green), h-region (purple), and c-region (red) are indicated. The residues with reported missense variants were underlined. (B-D) The constructs of the variants were overexpressed in HEK293T cells in the medium with 10 μM vitamin K, and the FIX procoagulant activity (FIX:C), FIX antigen with functional Gla domain, and total FIX antigen were determined by using corresponding methods (see “Methods”). Relative values were normalized by wild-type (WT) FIX, which was defined as 1. The dashed green and red lines indicate the 40% and 5% level of WT FIX, respectively. Error bars are standard deviation calculated from 3 repeats. (B) Normalized FIX:C; (C) Normalized FIX antigen with functional Gla domain; and (D) Normalized total FIX antigen.
Characterizing FIX procoagulant activity and FIX antigen of missense variants in FIX’s SP by an in vitro cell system. (A) Sequence of FIX’s SP. The n-region (green), h-region (purple), and c-region (red) are indicated. The residues with reported missense variants were underlined. (B-D) The constructs of the variants were overexpressed in HEK293T cells in the medium with 10 μM vitamin K, and the FIX procoagulant activity (FIX:C), FIX antigen with functional Gla domain, and total FIX antigen were determined by using corresponding methods (see “Methods”). Relative values were normalized by wild-type (WT) FIX, which was defined as 1. The dashed green and red lines indicate the 40% and 5% level of WT FIX, respectively. Error bars are standard deviation calculated from 3 repeats. (B) Normalized FIX:C; (C) Normalized FIX antigen with functional Gla domain; and (D) Normalized total FIX antigen.
Thirteen missense variants in FIX’s SP had been associated to HB (supplemental Figure 1A).6,7 Our previous study indicated that these variants only partially explained FIX deficiency.7 Additionally, it remained unclear why patients with HB carrying the same variant in the SP usually exhibited varying bleeding tendency (supplemental Figure 1B).6,7 Unveiling their pathogenesis was not only essential for understanding the varying bleeding tendency but also for patients’ genetic counseling or even personalized therapy. Here, we systematically characterized the mechanisms of 13 pathogenic variants in FIX’s SP. Our results showed that diverse molecular mechanisms were involved in FIX deficiency.
Material and methods
Variants selection and nomenclature of FIX
The apparent missense variants in FIX’s SP (supplemental Table 1) were selected from a database.6 The variants were described following the nomenclature system of the Human Genome Variation Society. The NP_000124.1 was used as a reference for amino acid numbers of FIX precursor. The NM_000133.3 was used as a reference for F9 mRNA.
Plasmid constructions
The complementary DNAs of F9 and Metridia luciferase12 were subcloned into the elongation factor 1 alpha (EF1ɑ) and cytomegalovirus (CMV) multicloning site of pBudCE4.1 vector, respectively. Based on this construct, a flag tag was inserted to the N-terminal of FIX to build the Flag-FIX construct. The FIXspg-EGFP (FIX's signal peptide, propeptide and gla domain fused with enhanced green fluorescent protein) chimeric reporter (sequence in supplemental File 1) was constructed and subcloned into the pcDNA3.1(+). The Sec61B-mCherry and mScarlet-Giantin fusion proteins were used as ER and Golgi marker, respectively.13,14 For the minigene splicing assay, a modified F9 exon 1 (sequence in supplemental File 1) was subcloned into the exon traping vector pSPL3. All variants were constructed by a fast-cloning technique.15
FIX:C assay
Human embryonic kidney 293T (HEK293T) cells were used to express FIX transiently. The cells were cultured in Opti-minimal mssential medium (Opti-MEM) containing 10 μM vitamin K after transfection. The mediums were concentrated to 50-fold by ultrafiltration centrifugal tubes (Millipore, Darmstadt, Germany) for evaluating FIX:C. The FIX:C was evaluated by 1-stage coagulation method based on activated partial thromboplastin time (aPTT) using coagulation FIX deficient plasma and aPTT assay kit from Siemens Healthcare Diagnostics (Marburg, Germany).
ELISA
The sandwich-based enzyme-linked immunosorbent assay (ELISA) was performed to detect the secreted total FIX antigen and FIX antigen with functional Gla domain. To detect the total FIX antigen, the ELISA plate was coated by sheep anti-human FIX polyclonal antibody (Affinity Biologicals, Ancaster, ON) overnight at 4°C, and goat anti-human factor IX-peroxidase conjugated IgG (GAFIX-HRP, Affinity Biologicals) was used as detection antibody. Substrate 2,2′-azino-bis(3ethylbenzothiazoline-6-sulfonic acid) (ABTS) was used for color development, and the absorbance value was measured at 405 nm. To detect the FIX antigen with functional Gla domain, the ELISA plate was coated by the antibody of mouse anti-human FIX’s Gla domain (Clone GMA-001; Green Mountain Antibodies, Burlington, VT), referred as anti-fGla and detected by sheep anti-human factor IX-peroxidase conjugated IgG (SAFIX-HRP, Affinity Biologicals). ELISA was performed as previous described.16
Immunoblotting
The cell lysate was prepared as previous described.17 The medium was precipitated by trichloroacetic acid and resuspended to perform immunoblotting. The samples were subjected to a reduced sodium dodecyl sulfate/polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride (PVDF) membrane. The primary antibodies included SAFIX, anti-Gla (BioMedica Diagnostics, Windsor, NS), anti-fGla, β-actin (Proteintech, Wuhan, People’s Republic of China), anti-Flag M2 (Sigma, Darmstadt, Germany), and anti-EGFP (Proteintech). Corresponding HRP-conjugated secondary antibodies were used to detect the primary antibodies. Signals were detected by an enhanced chemiluminescence (ECL) kit (Millipore).
Immunoprecipitation
The cell lysates were divided equally into 2 tubes, and anti-flag antibody and protein A/G-agarose beads were added into the lysates. The mixture was incubated on a rotating device overnight at 4°C. The immuno-precipitates were collected by centrifugation at 8000g and washed by lysis buffer 2 times. Then Peptide-N-Glycosidase F (PNGase F) was added in 1 tube, and both tubes were incubated at 37°C for 2 hours. Then protein loading buffer were added into each tube and boiled for 5 minutes. After centrifugation, the supernatants were applied to immunoblotting.
Subcellular localization of reporter protein
The coverslips were placed in a 6-well plate before seeding the HEK293T cells in which the reporter was transiently expressed. Transfected cells were cultured in medium with 10 μM vitamin K, then fixed by 4% paraformaldehyde. The coverslip was mounted to glass slide by 4′,6-diamidino-2-phenylindole (DAPI)–Fluoromount G medium. The fluorescent image was collected by the Nikon C2 plus confocal microscope.
Minigene splicing assay
The constructs were transfected into HEK293T cells. Total RNAs were extracted and were reverse transcribed to complementary DNAs for polymerase chain reaction (PCR) amplification. Electrophoresis of PCR products was performed by agarose gel, and the target DNA bands were ligated into the pMD18-T vector (Clontech, Changping, People’s Republic of China). The transformation was performed in DH5α competent Escherichia coli cells. The positive colonies were identified by PCR and confirmed by sequencing.
Statistical analysis
Statistical analysis was performed by GraphPad Prism 7. Data of ELISA and FIX activity were presented as mean ± standard deviation. The sequencing results of minigene splicing were determined by χ2 test, with significance at P value < .05. ImageJ was used to quantify the fluorescent signal and immunoblotting bands; t test was used to test the differences between groups.
Results
Missense variants in FIX’s SP only partially explain the HB
Thirteen missense variants in HB had been reported at 10 residues of FIX’s SP, distributing in its n-region, h-region, and c-region (Figure 1A). Firstly, we characterized their effects on FIX:C by an in vitro HEK293T expression system. Our results showed that only 8 missense variants in h-region (I17N, L20S, L23P, and L24P) and c-region (A26D and C28R/Y/W) dramatically decreased FIX:C (Figure 1B). Because the SP regulated protein secretion, we further evaluated the antigen levels of secreted FIX with functional Gla domain and total FIX by ELISA. These variants affected the antigen levels similarly to that of FIX:C (Figure 1C-D; supplemental Figure 2). These results together suggested that these 8 variants in the h- and c-regions dramatically affected FIX secretion, which was consistent with the HB phenotype.6 However, the R3H, I7F, C18F, G21V, and C28G variants had significant amount of secreted functional FIX, which was inconsistent with the HB phenotype in patients.
Missense variants in h-region destroy the cotranslational translocation function of the SP
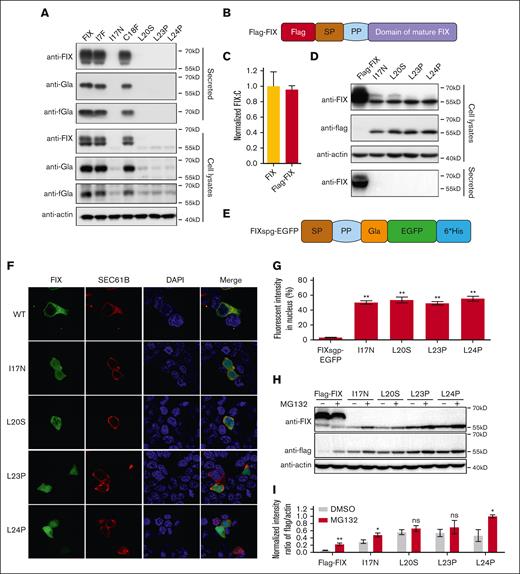
To explore the mechanism of variants in the h-region dramatically affecting FIX secretion, we examined the variant effect on the intracellular and extracellular FIX levels by immunoblotting (Figure 2A; supplemental Figure 3A). Results showed that the secreted FIX of I17N, L20S, L23P, and L24P variants were undetectable, and the intracellular FIX antigen of these variants was significantly decreased (Figure 2A). These results were consistent with that from FIX:C and ELISA, suggesting that the decrease of FIX secretion was caused by decreased intracellular expression of these variants. However, these variants also expressed a faint intracellular protein band with ∼55kD molecular weight detected by anti-FIX antibody (Figure 2A).
Missense variants in h-region destroy cotranslational translocation function of the SP. (A) The intracellular expressed and secreted FIX antigens were detected by immunoblotting using antibodies of anti-FIX, anti-Gla, and anti-FIX with functional Gla domain (anti-fGla), respectively. The variants were based on FIX construct. The β-actin was used as a loading control for cell lysate samples. (B) A scheme of Flag-FIX. A flag tag was inserted at the N-terminal of FIX. (C) Comparison of FIX:C between FIX and Flag-FIX. (D) Missense variants in h-region lead to the SP-retained FIX. The variants were constructed on the basis of Flag-FIX construct. The anti-flag antibody was used to detect the SP-retained FIX. (E) A scheme of chimeric fluorescent reporter FIXspg-EGFP. The construct contains the SP, propeptide (PP) and Gla domain of FIX, and was followed by EGFP. (F) The effect of c-region missense variants on subcellular localization of FIXspg-EGFP reporter. Variants in the SP h-region lead to mislocalization of the reporter FIXspg-EGFP in cytosol and nucleus. Sec61B-mcherry was used as an ER marker. Nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole). (G) Quantification of fluorescent intensity in nucleus from panel F. Green fluorescent intensity of FIXspg-EGFP in the whole cell and nucleus was quantified by ImageJ. The percentage of fluorescent intensity in nucleus was calculated by dividing the fluorescent intensity in nucleus to that in the whole cell. Six to 10 cells were analyzed for each construct, and t test was performed between WT and variants by GraphPad Prism 7. ∗∗P < .01. (H) Proteasome inhibitor (MG132) prevented the degradation of the SP-retained FIX. The variants were constructed on the basis of Flag-FIX. The cells were cultured in a medium with 10 μM vitamin K. (I) Quantification of protein bands from panel H. The protein band intensity of anti-flag and anti-actin was quantified by ImageJ. The intensity ratio of anti-flag/anti-actin was calculated and normalized by MG132 treatment of L24P, which was defined as 1. Three independent experiments were analyzed, and t test was performed between MG132-treated and -untreated sample of each construct by GraphPad Prism 7. ns indicates no significant difference (P > .05); ∗∗P < .01; ∗P < .05.
Missense variants in h-region destroy cotranslational translocation function of the SP. (A) The intracellular expressed and secreted FIX antigens were detected by immunoblotting using antibodies of anti-FIX, anti-Gla, and anti-FIX with functional Gla domain (anti-fGla), respectively. The variants were based on FIX construct. The β-actin was used as a loading control for cell lysate samples. (B) A scheme of Flag-FIX. A flag tag was inserted at the N-terminal of FIX. (C) Comparison of FIX:C between FIX and Flag-FIX. (D) Missense variants in h-region lead to the SP-retained FIX. The variants were constructed on the basis of Flag-FIX construct. The anti-flag antibody was used to detect the SP-retained FIX. (E) A scheme of chimeric fluorescent reporter FIXspg-EGFP. The construct contains the SP, propeptide (PP) and Gla domain of FIX, and was followed by EGFP. (F) The effect of c-region missense variants on subcellular localization of FIXspg-EGFP reporter. Variants in the SP h-region lead to mislocalization of the reporter FIXspg-EGFP in cytosol and nucleus. Sec61B-mcherry was used as an ER marker. Nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole). (G) Quantification of fluorescent intensity in nucleus from panel F. Green fluorescent intensity of FIXspg-EGFP in the whole cell and nucleus was quantified by ImageJ. The percentage of fluorescent intensity in nucleus was calculated by dividing the fluorescent intensity in nucleus to that in the whole cell. Six to 10 cells were analyzed for each construct, and t test was performed between WT and variants by GraphPad Prism 7. ∗∗P < .01. (H) Proteasome inhibitor (MG132) prevented the degradation of the SP-retained FIX. The variants were constructed on the basis of Flag-FIX. The cells were cultured in a medium with 10 μM vitamin K. (I) Quantification of protein bands from panel H. The protein band intensity of anti-flag and anti-actin was quantified by ImageJ. The intensity ratio of anti-flag/anti-actin was calculated and normalized by MG132 treatment of L24P, which was defined as 1. Three independent experiments were analyzed, and t test was performed between MG132-treated and -untreated sample of each construct by GraphPad Prism 7. ns indicates no significant difference (P > .05); ∗∗P < .01; ∗P < .05.
It had been reported that decreased intracellular expression of secretory protein with h-region variants could result from mRNA degradation through the RAPP pathway.5 To test this hypothesis, we examined the mRNA levels of the h-region variants by transiently expressing their constructs in HEK293T cells. Our data showed that the mRNA levels slightly decreased in the L20S, L23P, and L24P variants (65% of wild type; supplemental Figure 3B). Nevertheless, the decreased mRNA level did not match the dramatically decreased protein level, suggesting that there might be another mechanism for the decreased FIX expression, besides the RAPP pathway.
The h-region was recognized by SRP, which was essential for cotranslational translocation of secretory protein from cytosol into ER.8 If the SP could not be recognized by SRP due to the h-region variants, the ribosome would complete the translation in cytosol, and the final product could not be translocated into ER for posttranslational modification, SP cleavage, and secretion. Therefore, we reasoned that the ∼55kD protein band observed in h-region variants (Figure 2A) might be a cytosol–located FIX polypeptide with the SP retained. To test this hypothesis, we designed a Flag-FIX construct with a flag tag fused at the N-terminus of FIX (Figure 2B), which had a similar FIX:C as the FIX construct (Figure 2C). The intracellular and secreted FIX antigen patterns of Flag-FIX–based h-region variants were also similar to the corresponding FIX-based variants (Figure 2D; supplemental Figure 4). As expected, the ∼55 kD protein band could be detected by anti-flag antibody in cell lysates (Figure 2D), suggesting that the SP was retained in FIX of these variants.
To demonstrate whether the h-region variants disrupted the cotranslational translocation function of FIX’s SP, we introduced these variants to a chimeric fluorescent reporter, FIXspg-EGFP (Figure 2E), to visualize their subcellular localization. Firstly, our results showed that these variants had a similar effect on protein secretion whether they were in FIXspg-EGFP reporter–based constructs (supplemental Figure 5) or in FIX-based constructs, suggesting that both constructs shared the same mechanism on protein secretion. Our results of fluorescent images showed that the FIXspg-EGFP reporters of h-region variants spread throughout the cell, whereas the reporter with wild-type SP was colocalized with an ER marker (Sec61B; Figure 2F). Quantitative analysis showed that the fluorescent intensity of these variants in the nucleus was significantly higher than that of the wild type (Figure 2G). Collectively, our data suggested that the variants in h-region disrupted the cotranslational translocation function of the SP and caused the cytosol-located FIX.
Protein mislocation could trigger protein quality control, and the proteasome system was the major pathway for protein quality control in cytosol, so we examined whether the proteasome pathway could affect the expression of the cytosol-located FIX. Our results showed that the degradation of the cytosol-located FIX was inhibited by a proteasome inhibitor MG132, indicating that proteasome pathway was involved its degradation (Figure 2H-I; supplemental Figure 6). Additionally, posttranslational modification in the ER was important for the stabilization of coagulation factors.18 Therefore, the lack of posttranslational modification might be the reason to trigger the degradation of the cytosol-located FIX.
Missense variants in c-region differentially disrupted the SP function
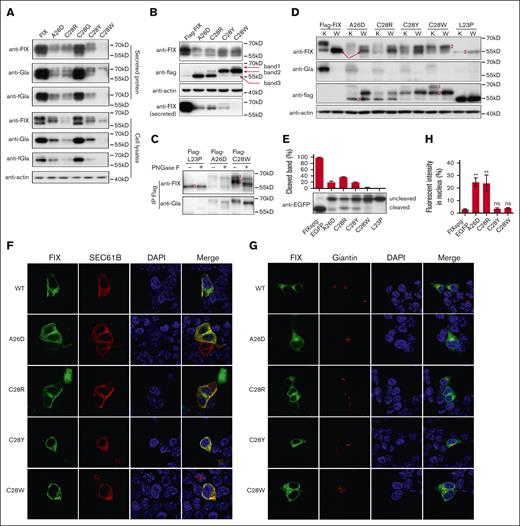
Next, we explored the mechanism of the c-region variants causing FIX deficiency. Our data showed that both intracellular and secreted FIX were significantly decreased in variants of A26D and C28R/Y/W (Figure 3A), consistent with the data of FIX:C (Figure 1B). The mRNA levels were not affected in c-region variants (supplemental Figure 3B), which was consistent with previous conclusion that the RAPP pathway was not the main reason for the decreased protein expression for c-region variants.5 Because c-region was essential for SP cleavage, the decreased FIX expression presumably resulted from the disruption of the SP cleavage. To test this hypothesis, we used the Flag-FIX construct to evaluate the effect of c-region variants on the SP cleavage. Results showed that their expression patterns of intracellular and secreted FIX in Flag-FIX–based constructs (Figure 3B; supplemental Figure 4) were similar to the corresponding FIX-based constructs. However, at least 3 protein bands with different sizes were observed when intracellular FIX was detected by anti-flag antibody (Figure 3B), suggesting the existence of 3 different forms of the SP-retained FIX.
Missense variants in c-region differently disturb the SP function. (A) The intracellular and secreted FIX antigens with c-region variants were detected by antibodies of anti-FIX, anti-Gla, and anti-FIX with functional Gla domain (anti-fGla), respectively. The constructs were based on FIX. The β-actin was used as a loading control for cell lysate samples. (B) The c-region variants had several forms of the SP-retained FIX. The constructs were based on Flag-FIX construct. The anti-flag antibody was used to detect the different forms of the SP-retained FIX, which were indicated by the band 1, 2, and 3. (C) Posttranslational modifications of glycosylation and γ-carboxylation lead to different forms of the SP-retained FIX. Band 1 was glycosylated and γ-carboxylated, band 2 was glycosylated but not γ-carboxylated, and band 3 had neither modification. (D) Comparison of protein expression patterns between vitamin K and warfarin treatment. The different forms of the SP-retained FIX were indicated by 1, 2, and 3. K and W indicated that the cells were treated by vitamin K (10 μM) or warfarin (10 μM). Warfarin was used to inhibit γ-carboxylation. The red arrows indicate the SP-cleaved and uncarboxylated FIX. (E) The c-region variants differently affected the SP cleavage. The HEK293T cells were transfected with FIXsgp-EGFP constructs and cultured in medium with 10 μM warfarin. The immunoblotting was performed by anti-EGFP antibody. The L23P was used as the SP-retained (uncleaved) control. The intensity of the SP cleaved and uncleaved bands was quantified by ImageJ, and the percentage of cleaved band was calculated from 3 independent experiments by dividing the cleaved band intensity to the total intensity of the cleaved and uncleaved bands. (F-G) The effect of c-region missense variants on subcellular localization of FIXspg-EGFP reporter. Sec61B-mcherry and mScarlet-Giantin were used as an ER marker and Golgi marker, respectively. Nuclei were stained with DAPI. Cells were cultured in a medium with 10 μM vitamin K. (H) Quantification of fluorescent intensity in nucleus from panels F-G. Green fluorescent intensity of FIXspg-EGFP in the whole cell and nucleus was quantified by ImageJ. The percentage of fluorescent intensity in nucleus was calculated by dividing the fluorescent intensity in nucleus to that in the whole cell. Six to 10 cells were analyzed for each construct, and t test was performed between WT and variants by GraphPad Prism 7. ns indicates no significant difference (P > .05); ∗∗P < .01.
Missense variants in c-region differently disturb the SP function. (A) The intracellular and secreted FIX antigens with c-region variants were detected by antibodies of anti-FIX, anti-Gla, and anti-FIX with functional Gla domain (anti-fGla), respectively. The constructs were based on FIX. The β-actin was used as a loading control for cell lysate samples. (B) The c-region variants had several forms of the SP-retained FIX. The constructs were based on Flag-FIX construct. The anti-flag antibody was used to detect the different forms of the SP-retained FIX, which were indicated by the band 1, 2, and 3. (C) Posttranslational modifications of glycosylation and γ-carboxylation lead to different forms of the SP-retained FIX. Band 1 was glycosylated and γ-carboxylated, band 2 was glycosylated but not γ-carboxylated, and band 3 had neither modification. (D) Comparison of protein expression patterns between vitamin K and warfarin treatment. The different forms of the SP-retained FIX were indicated by 1, 2, and 3. K and W indicated that the cells were treated by vitamin K (10 μM) or warfarin (10 μM). Warfarin was used to inhibit γ-carboxylation. The red arrows indicate the SP-cleaved and uncarboxylated FIX. (E) The c-region variants differently affected the SP cleavage. The HEK293T cells were transfected with FIXsgp-EGFP constructs and cultured in medium with 10 μM warfarin. The immunoblotting was performed by anti-EGFP antibody. The L23P was used as the SP-retained (uncleaved) control. The intensity of the SP cleaved and uncleaved bands was quantified by ImageJ, and the percentage of cleaved band was calculated from 3 independent experiments by dividing the cleaved band intensity to the total intensity of the cleaved and uncleaved bands. (F-G) The effect of c-region missense variants on subcellular localization of FIXspg-EGFP reporter. Sec61B-mcherry and mScarlet-Giantin were used as an ER marker and Golgi marker, respectively. Nuclei were stained with DAPI. Cells were cultured in a medium with 10 μM vitamin K. (H) Quantification of fluorescent intensity in nucleus from panels F-G. Green fluorescent intensity of FIXspg-EGFP in the whole cell and nucleus was quantified by ImageJ. The percentage of fluorescent intensity in nucleus was calculated by dividing the fluorescent intensity in nucleus to that in the whole cell. Six to 10 cells were analyzed for each construct, and t test was performed between WT and variants by GraphPad Prism 7. ns indicates no significant difference (P > .05); ∗∗P < .01.
FIX is a vitamin K–dependent glycoprotein, and both glycosylation and γ-carboxylation are necessary for its maturation. We reasoned that these different FIX forms resulted from different states of posttranslational modifications. To determine which form could be glycosylated and/or γ-carboxylated, the intracellular SP-retained FIX was immunoprecipitated by anti-flag antibody and digested by PNGase F to remove the N-linked oligosaccharide, and immunoblotting was performed by anti-FIX or anti-Gla antibodies. Our results indicated that protein band 1 in Figure 3B had both glycosylation and γ-carboxylation, because it could be recognized by anti-Gla antibody and was sensitive to PNGase F treatment (Figure 3C). Similarly, band 2 was only glycosylated but not γ-carboxylated, and band 3 had neither modification. To further confirm that only protein band 1 was γ-carboxylated, we treated transfected cells with either vitamin K or warfarin. Results in Figure 3D of warfarin-treated samples showed that protein band 1 disappeared; in contrast, band 2 increased, which confirmed that the band 1 was γ-carboxylated. Additionally, band 3 was neither glycosylated (Figure 3C, L23P) nor γ-carboxylated (Figure 3D), so it should be the cytosol-located FIX, which was consistent with the conclusion from Figure 2.
By a SP prediction server, the probability of cleavage site was also declined for these c-region variants (supplemental Figure 7). Comparing the band patterns probed by anti-flag and anti-FIX in Figure 3B,D, the anti-FIX antibody could detect more bands, indicating that there was SP-cleaved FIX in variants. Comparing the bands among warfarin-treated samples, we observed a band in variants (at least in A26D) with a similar molecular weight (red arrow in Figure 3D) as that in wild-type Flag-FIX, which could correspond to the SP-cleaved FIX. To further validate the variant effect on the SP cleavage, we used FIXspg-EGFP to evaluate the cleavage under warfarin treatment. The data clearly showed that these c-region variants differently affected the SP cleavage, and C28W affected the SP cleavage more severely than others (Figure 3E). The L23P was a SP-retained control.
Because both glycosylation and γ-carboxylation were only processed in ER (or Golgi), bands 1 and 2 were located in the ER (or Golgi). The band 3 in A26D and C28R, similar to L23P, should be located in cytosol. So, both A26D and C28R should affect the cotranslational translocation slightly. The data of fluorescent images showed that most reporters of A26D and C28R were located in the ER or Golgi, and there was a faint signal in nucleus for both variants (∼20%); whereas the reporters of C28Y/W were similar to wild-type reporter (Figure 3F-H), which were consistent with the data from immunoblotting.
Several variants in the SP lead to aberrant pre-mRNA splicing
Based on in vitro cell-based FIX:C, the pathogenic mechanism of HB with the SP variants could only partially be explained, suggesting the existence of other possibilities. Several studies had reported that nucleotide variant could lead to aberrant pre-mRNA splicing19-21; thus, we used a minigene splicing assay to examine their effect on F9 pre-mRNA splicing.20
Because F9 exon 1, encoding the SP, lacked a native intron at its 5′ junction site, we used a modified exon 1 as shown in Figure 4A for the minigene splicing analysis. The modified construct was named E231. The modified intron-exon insert could be spliced by the endogenous machinery of HKE293T cells, resulting in a PCR fragment of ∼500bp (supplemental Figure 8A). To evaluate whether the modified exon 1 could be used to explore the variant effect on pre-mRNA splicing, the c.87A>G, a previously reported splicing variant in exon 1,22,23 was introduced into E231. Although the PCR product of c.87A>G was similar to that of E231 (supplemental Figure 8A), DNA sequencing result showed that the PCR product of c.87A>G mainly contained an aberrant splicing product with a 6-nucleotide (6-nt) deletion of exon 1 (Figure 4B), which was consistent with previous observation.22,23 This result suggested that the modified intron-exon arrangement could be used for the evaluation of pre-mRNA splicing in exon 1.
Some point variants lead to aberrant pre-mRNA splicing. (A) A scheme of modified exon 1 construct for minigene splicing assay. The exon 1 of F9 was fused with the exon 3 of F9 as shown (sequence in supplemental File 1), and the modified sequence was subcloned into the multicloning site (MCS) of pSPL3 vector, which was named as E231. The c.88 indicated the position of the last nucleotide of 3′ site of exon 1. (B-C) Comparison of sequencing chromatograms between canonical and 2 major aberrant splicing products in c.87A>G (B) and c.82T>G (C). Sequence changes were indicated by red arrow lines. WT indicated the canonical splicing product in E231. (D) Statistics of splicing products. Approximately 100 sequencing results of each construct were analyzed. The splicing products were classified into 2 groups: canonical splicing product (N) and aberrant splicing product (Ab). The percentage of each group was calculated. The χ2 analysis was performed using GraphPad Prism 7. ns indicates no significant difference (P > .05); ∗P < .05; ∗∗P < .01. AmpR, Ampicillin resistance gene; MCS, multicloning site; SA, exon with splice acceptor site; SD, exon with splice donor site.
Some point variants lead to aberrant pre-mRNA splicing. (A) A scheme of modified exon 1 construct for minigene splicing assay. The exon 1 of F9 was fused with the exon 3 of F9 as shown (sequence in supplemental File 1), and the modified sequence was subcloned into the multicloning site (MCS) of pSPL3 vector, which was named as E231. The c.88 indicated the position of the last nucleotide of 3′ site of exon 1. (B-C) Comparison of sequencing chromatograms between canonical and 2 major aberrant splicing products in c.87A>G (B) and c.82T>G (C). Sequence changes were indicated by red arrow lines. WT indicated the canonical splicing product in E231. (D) Statistics of splicing products. Approximately 100 sequencing results of each construct were analyzed. The splicing products were classified into 2 groups: canonical splicing product (N) and aberrant splicing product (Ab). The percentage of each group was calculated. The χ2 analysis was performed using GraphPad Prism 7. ns indicates no significant difference (P > .05); ∗P < .05; ∗∗P < .01. AmpR, Ampicillin resistance gene; MCS, multicloning site; SA, exon with splice acceptor site; SD, exon with splice donor site.
Next, we introduced naturally occurring variations to the modified exon 1. The spliced PCR products from all variants had a similar size in agarose gel (supplemental Figure 8A). Therefore, we subcloned the PCR products into a T vector and picked up ∼100 positive colonies from each variant for sequencing. We identified 13 aberrant splicing types from all variants (supplemental Table 2). To evaluate whether the variants disturbed the pre-mRNA splicing patterns, the splicing products were divided into 2 groups: canonical product (N) and aberrant splicing products (Ab). Compared with E231, the aberrant splicing products were significantly increased in c.82T>G and c.87A>G, both mainly leading to a 6-nt deletion of exon 1 (Figure 4B-D; supplemental Table 2). Additionally, different degrees of aberrant splicing products were observed in c.53G>T, c.77C>A, c.82T>C, and c.83G>A (Figure 4D; supplemental Table 2). Together, these data indicated that the pathogenic variants in the SP of FIX may cause HB by aberrant pre-mRNA splicing.
Two major splicing variants with 6-nt deletion disturb SP cleavage and response to vitamin K
Among the aberrant splicing products, only 36-nt intron 1 insertion and/or 6-nt intron 1 deletion caused in-frame variants, which encoded FIX variants with normal mature domains (supplemental Figure 8B-C; Figure 5A). The splicing product of 36-nt intron 1 insertion (wild-type-splicing form 2 [WT-SF2] in supplemental Figure 8C) in the E231 construct with the same SP as wild-type FIX had normal FIX:C activity (supplemental Figure 8D), and this splicing product–based variants had an effect on the secretion of FIX antigen with functional Gla domain similar to FIX-based constructs (supplemental Figure 8E); therefore, the 36-nt intron 1 insertion-based variants should have similar behavior as FIX-based variants. Therefore, we only focused on the functional study of 2 major aberrant splicing products with 6-nt deletion in c.82T>G and c.87A>G, which encoded 2 FIX variants: p.C28-T29del (c.82T>G) and p.C28-V30delinsF (c.87A>G; Figure 5A). Because both variants changed the sequence of the SP cleavage site, we proposed that the SP function should be disturbed. The online SP prediction server24 predicted that the SP probability was significantly decreased in both variants, and cleavage site could shift to residue A26 (supplemental Figure 7).
Characterizations of FIX variants encoded by two 6-nt deletion splicing products. (A) Comparing the encoded protein sequences of 2 major FIX splicing variants caused by c.82T>G and c.87A>G. (B) Relative FIX:C of both splicing variants of c.82T>G and c.87A>G. Error bars were standard deviation calculated from 3 repeats. (C) The intracellular expressed and secreted FIX antigens of two 6-nt deletion splicing variants were detected by immunoblotting. (D) Two 6-nt deletion splicing variants disturbed the SP cleavage. (E) Comparison of protein expression patterns between vitamin K and warfarin treatment. K and W indicated that the cells were treated by vitamin K (10 μM) or warfarin (10 μM). Warfarin was used to inhibit γ-carboxylation. (F) The effect of splicing variants on the SP cleavage efficiency. The HEK293T cells were transfected with FIXsgp-EGFP constructs and cultured in medium containing 10 μM warfarin. The L23P was used as the SP-retained control (uncleaved). The intensity of the SP cleaved and uncleaved bands was quantified by ImageJ. The percentage of cleaved band was calculated from 3 independent experiments by dividing the cleaved band intensity to the total intensity of the cleaved and uncleaved bands. (G-H) The effect of the two 6-nt deletion splicing variants on subcellular localization of FIXspg-EGFP reporter. Sec61B-mcherry and mScarlet-Giantin were used as an ER marker and Golgi marker, respectively. Nuclei were stained with DAPI. Cells were cultured in a medium with 10 μM vitamin K. (I) Vitamin K level increased the FIX antigen with functional Gla domain in both splicing variants. The anti-FIX with functional Gla domain (anti-fGla) was used as capture antibody for ELISA, and the HRP-conjugated sheep anti-human FIX (SAFIX-HRP) was used as the detection antibody.
Characterizations of FIX variants encoded by two 6-nt deletion splicing products. (A) Comparing the encoded protein sequences of 2 major FIX splicing variants caused by c.82T>G and c.87A>G. (B) Relative FIX:C of both splicing variants of c.82T>G and c.87A>G. Error bars were standard deviation calculated from 3 repeats. (C) The intracellular expressed and secreted FIX antigens of two 6-nt deletion splicing variants were detected by immunoblotting. (D) Two 6-nt deletion splicing variants disturbed the SP cleavage. (E) Comparison of protein expression patterns between vitamin K and warfarin treatment. K and W indicated that the cells were treated by vitamin K (10 μM) or warfarin (10 μM). Warfarin was used to inhibit γ-carboxylation. (F) The effect of splicing variants on the SP cleavage efficiency. The HEK293T cells were transfected with FIXsgp-EGFP constructs and cultured in medium containing 10 μM warfarin. The L23P was used as the SP-retained control (uncleaved). The intensity of the SP cleaved and uncleaved bands was quantified by ImageJ. The percentage of cleaved band was calculated from 3 independent experiments by dividing the cleaved band intensity to the total intensity of the cleaved and uncleaved bands. (G-H) The effect of the two 6-nt deletion splicing variants on subcellular localization of FIXspg-EGFP reporter. Sec61B-mcherry and mScarlet-Giantin were used as an ER marker and Golgi marker, respectively. Nuclei were stained with DAPI. Cells were cultured in a medium with 10 μM vitamin K. (I) Vitamin K level increased the FIX antigen with functional Gla domain in both splicing variants. The anti-FIX with functional Gla domain (anti-fGla) was used as capture antibody for ELISA, and the HRP-conjugated sheep anti-human FIX (SAFIX-HRP) was used as the detection antibody.
Our results showed that both variants significantly decreased the FIX:C (Figure 5B). Consistent with the FIX:C, the immunoblotting data of FIX-based constructs also showed that both variants decreased the intracellular and secreted FIX antigen (Figure 5C). By examining their mRNA stability, the data showed that both variants did not decrease mRNA stability (supplemental Figure 3B), indicating that the RAPP pathway was not the mechanism for their decreased FIX expression. Results from Flag-FIX–based and FIXspg-EGFP–based constructs indicated that both variants disturbed the SP cleavage (Figure 5D-F). The SP cleavage efficiency was affected more in c.87A>G than c.82A>G (Figure 5F), which was consistent with their FIX:C (Figure 5B). Results from fluorescent images of FIXspg-EGFP reporters showed that both variants were localized in the ER and Golgi, similar to the wild type (Figure 5G-H; supplemental Figure 8F).
We had shown previously that high concentration of vitamin K might improve the production of FIX with functional Gla domain.7 Here, we introduced both splicing variants to full-length FIX and our chimeric reporter protein FIX-Gla-PC16; our data showed that the production of FIX (Figure 5I) and FIX-Gla-PC (supplemental Figure 8G) with functional Gla domain were also dependent on vitamin K concentration, suggesting that the production of functional FIX responded to vitamin K availability for both splicing variants.
Discussion
HB is a rare bleeding disorder caused by FIX deficiency. Genetic variants in FIX’s SP have been reported in patients with HB.6,7 The SP, cleaved in the mature protein, is necessary for FIX secretion, which directs the translation complex to ER membrane for protein synthesis and posttranslational modification. A recent study suggested that the variants in the SP h-region triggered mRNA degradation through RAPP pathway.5 Our data showed that only a slight mRNA decrease was observed in some HB-associated h-region variants, indicating that RAPP was not the only mechanism leading to FIX deficiency. Our study suggested that HB-associated h-region variants also disrupted the cotranslational translocation function of SP and generated the dysfunctional cytosol-located FIX (Table 1). Based on the structure of signal recognition particle 54 kDa protein (SRP54)-SP complex,25 we proposed that the HB-associated variants in h-region directly perturbed the recognition of the SP by SRP through introducing helix-breaking proline (L23P and L24P) or interfering with h-region’s hydrophobicity (I17N and L20S). Additionally, our results showed that the cytosol-located FIX was unstable and degraded through ubiquitin-proteasome pathway (Figure 6).
Mechanisms of variants in the SP to cause FIX deficiency
| Variants . | Apparent variant effect . | Mechanisms to cause FIX deficiency . | |||
|---|---|---|---|---|---|
| Aberrant pre-mRNA splicing . | Missense variants . | ||||
| RAPP . | Disturbing cotranslational translocation of SP . | Disturbing cleavage of SP . | |||
| c.8G>A | Missense p.R3H | No | NA | No | No |
| c.19A>T | Missense p.I7F | No | NA | No | No |
| c.50T>A | Missense p.I17N | NA | No | Yes | No |
| c.53G>T | Missense p.C18F | Partially | NA | No | No |
| c.59T>C | Missense p.L20S | NA | Yes | Yes | No |
| c.62G>T | Missense p.G21V | No | NA | No | No |
| c.68T>C | Missense p.L23P | NA | Yes | Yes | No |
| c.71T>C | Missense p.L24P | NA | Yes | Yes | No |
| c.77C>A | Missense p.A26D | Partially | No | Partially | Yes |
| c.82T>C | Missense p.C28R | Partially | No | Partially | Yes |
| c.82T>G | Missense p.C28G | Yes | No | No | Yes∗ |
| c.83G>A | Missense p.C28Y | Partially | No | No | Yes |
| c.84T>G | Missense p.C28W | No | No | No | Yes |
| Variants . | Apparent variant effect . | Mechanisms to cause FIX deficiency . | |||
|---|---|---|---|---|---|
| Aberrant pre-mRNA splicing . | Missense variants . | ||||
| RAPP . | Disturbing cotranslational translocation of SP . | Disturbing cleavage of SP . | |||
| c.8G>A | Missense p.R3H | No | NA | No | No |
| c.19A>T | Missense p.I7F | No | NA | No | No |
| c.50T>A | Missense p.I17N | NA | No | Yes | No |
| c.53G>T | Missense p.C18F | Partially | NA | No | No |
| c.59T>C | Missense p.L20S | NA | Yes | Yes | No |
| c.62G>T | Missense p.G21V | No | NA | No | No |
| c.68T>C | Missense p.L23P | NA | Yes | Yes | No |
| c.71T>C | Missense p.L24P | NA | Yes | Yes | No |
| c.77C>A | Missense p.A26D | Partially | No | Partially | Yes |
| c.82T>C | Missense p.C28R | Partially | No | Partially | Yes |
| c.82T>G | Missense p.C28G | Yes | No | No | Yes∗ |
| c.83G>A | Missense p.C28Y | Partially | No | No | Yes |
| c.84T>G | Missense p.C28W | No | No | No | Yes |
NA, not available.
The aberrant pre-mRNA splicing product disturbs the cleavage of the SP.
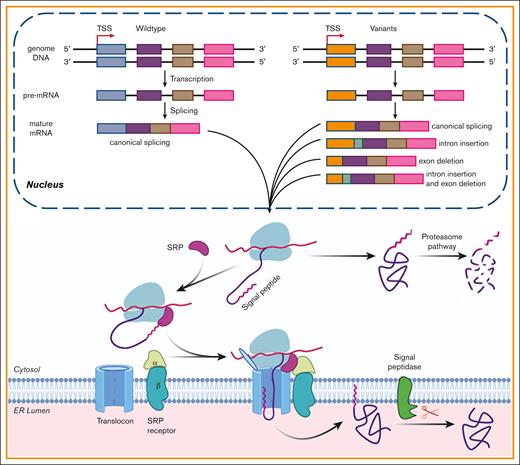
The effect of the SP variants on the biological processing of FIX. The pathogenic variants in FIX’s SP caused HB by missense variants and/or by aberrant pre-mRNA splicing. In nucleus, the gene was transcribed into pre-mRNA, and then the pre-mRNA was processed into mature mRNA. Some variants may disturb the processing of pre-RNA and lead to aberrant mature mRNA, which may generate aberrant protein products. The variants that did not disturb the process of splicing would cause missense variants. Missense variants in the SP h-region affected the cotranslational translocation function of the SP and produced the cytosol-located FIX, which was degraded by proteasome pathway. Those in c-region may disturb the cotranslational translocation and/or the SP cleavage. Additionally, a variation may cause HB by a mixture of above mechanisms. TSS, transcriptional start site; SRP, signal recognition particle.
The effect of the SP variants on the biological processing of FIX. The pathogenic variants in FIX’s SP caused HB by missense variants and/or by aberrant pre-mRNA splicing. In nucleus, the gene was transcribed into pre-mRNA, and then the pre-mRNA was processed into mature mRNA. Some variants may disturb the processing of pre-RNA and lead to aberrant mature mRNA, which may generate aberrant protein products. The variants that did not disturb the process of splicing would cause missense variants. Missense variants in the SP h-region affected the cotranslational translocation function of the SP and produced the cytosol-located FIX, which was degraded by proteasome pathway. Those in c-region may disturb the cotranslational translocation and/or the SP cleavage. Additionally, a variation may cause HB by a mixture of above mechanisms. TSS, transcriptional start site; SRP, signal recognition particle.
Based on the current dogma, the c-region was essential for the SP cleavage, so the c-region variants would disturb the cleavage of SP.5,8,9 However, our data showed that the c-region variants caused FIX deficiency through mixed mechanisms, including the disruption of cotranslational translocation function and/or cleavage of the SP (Table 1). The variants of A26D and C28R disturbed both cotranslational translocation function and cleavage of SP; whereas the C28Y/W mainly decreased the SP cleavage efficiency. Additionally, FIX with the uncleaved SP in C28Y/W appeared to attach to ER membrane through the SP22 (Figure 6), and this abnormal subcellular localization inhibited the posttranslational modifications and stability of FIX protein.
Besides missense variants, our data also showed that several variations resulted in FIX deficiency by aberrant pre-mRNA splicing by using minigene splicing assay (Figure 6). However, the minigene splicing assay could have several limitations. First, because exon 1 lacked a native intron at its 5′ junction site, the partial aberrant splicing products from the modified exon 1 construct (E231; supplemental Table 2) did not exist in the naturally occurring F9 transcripts. Second, because variants could affect pre-mRNA splicing far away from the variant site,26,27 the truncated intron 1 in construct E231 might not represent the splicing effect of variants on the intron 1 or even on the whole F9 gene. Nevertheless, our minigene analysis of the c.87A>G in the modified exon 1 still got the same splicing pattern as previously reported,22,23 suggesting the reliability of the results.
For c.77C>A (A26D), c.82T>C (C28R), and c.83G>A (C28Y), their FIX:C of missense variants (Figure 1B) indicated that their bleeding tendency should be mild. However, patients with these variants mostly showed severe or moderate bleeding tendency in clinical. The minigene splicing assay showed that these variants might partially result from aberrant pre-mRNA splicing. Therefore, FIX deficiency in these variants could result from both missense variants and aberrant splicing (Table 1). Additionally, the c.53G>T might cause FIX deficiency by partial aberrant pre-mRNA splicing (Table 1). In short, the current assay could partially reflect that several variants interfered with the process of pre-mRNA splicing (Figure 6). Further studies should be done to illuminate whether they disturbed the splicing process in vivo.
To elucidate the effect of aberrant pre-mRNA splicing on FIX:C, 2 major in-frame splicing products with 6-nt deletion were selected to explore their pathogenic mechanism. Our data showed that both FIX splicing variants disturbed the SP cleavage. However, they still produced partial functional FIX. Interestingly, our data indicated that vitamin K availability could be a factor affecting the production of functional FIX for both variants. We also observed that patients with the c.87A>G exhibited varying bleeding tendencies (supplemental Figure 1B).6 These varying bleeding tendencies could be explained by variable vitamin K levels in patients. Because FIX:C of splicing product in c.87A>G could reach ∼10% of wild-type FIX, patients should benefit from taking higher doses of vitamin K. When the vitamin K level decreased, patients could display moderate or severe bleeding tendencies. Similarly, vitamin K level could also explain that HB patients with pathogenic variants in the SP had varying bleeding tendencies (supplemental Figure 1B), which was consistent with our previous conclusion.7 Thus, we inferred that vitamin K administration might ameliorate the bleeding phenotype in patients with these variants.
Due to the limitation of methods, this study could still not explain some variants (c.8G>A, c.9A>T, and c.62G>T) causing HB (Table 1). Nevertheless, our data indicated that the variants in the SP cause FIX deficiency through diverse molecular mechanisms (Table 1), and most of the pathogenic variants could generate partial functional FIX. Additionally, it was worth noticing that vitamin K availability might be a factor causing different bleeding tendency in patients with some SP variants.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (82170133 and 81770140 [G.S.], 31900412 [M.G.], and 82370136 [Z.H.]), the Henan Department of Science & Technology (232102310110 [M.G.] and 212102310629 [Y.S.]), the National Heart Lung and Blood Institute (HL121718 [W.L.] and HL131690 [J.-K.T.]), the National Eye Institute (EY028705 [W.L.]), the Jiangsu Natural Science Foundation (BK20231333 [Z.H.]), and a Jiangsu Specially Appointed Professor Start-up Funds grant (Z.H.).
Authorship
Contribution: M.G. and J.Y. performed the experiments of FIX-based and Flag-FIX–based constructs with help from Y.S. and H.L.; L.C. performed the minigene splicing assay with help from J.Y. and H.Y.; S.D. performed the experiments of FIXspg-EGFP–based constructs with help from M.G., Q.C., Z.C., and Y.D.; G.S., J.-K.T., and W.L. designed the study, analyzed the data, and wrote the manuscript with help from L.Z., Z.H., and M.G.; and all authors reviewed and contributed to the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Guomin Shen, Department of Cell Biology, Harbin Medical University, Baojian Rd, Harbin 150081, People’s Republic of China; email: shenguomin@hrbmu.edu.cn/ shenba433@163.com; Weikai Li, Department of Biochemistry and Molecular Biophysics, Washington University in St. Louis School of Medicine, 660 S Euclid Ave, St. Louis, MO 63110; email: weikai@wustl.edu; and Jian-Ke Tie, Department of Biology, The University of North Carolina at Chapel Hill, 120 South Rd, Chapel Hill, NC 27599; email: jktie@email.unc.edu.
References
Author notes
M.G., L.C., J.Y., and S.D. contributed equally to this study.
Publication-related data are available on request from the corresponding authors, Guomin Shen (shenba433@163.com or shenguomin@hrbmu.edu.cn), Weikai Li (weikai@wustl.edu), and Jian-Ke Tie (jktie@email.unc.edu).
The full-text version of this article contains a data supplement.