Key Points
Administration of vitamin D to patients with CLL in a watch-and-wait is significantly associated with a longer TFS.
Administration of vitamin D to young patients with CLL <65 years old is significantly associated with a longer TTFT.
Visual Abstract
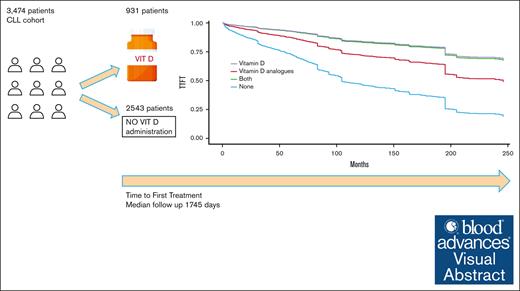
Low levels of vitamin D are associated with a shorter time to first treatment (TTFT) and inferior overall survival in patients with chronic lymphocytic leukemia (CLL). But whether vitamin D supplement affects the clinical course of patients with CLL, remains an open question. In this study, we aimed to retrospectively explore the clinical benefit of vitamin D supplement or one of its analogs, on TTFT and treatment-free survival (TFS) in a large cohort of patients with asymptomatic CLL, who were under watch-and-wait approach. Among the 3474 patients included in the study, 931 patients (26.8%) received either vitamin D supplement or its analog, for a minimum of 6 months. We found that vitamin D supplement was statistically significant for longer TTFT in the young cohort (age ≤65) and was associated with a longer TFS for all ages (P = .004). Among non–vitamin-D users, the median TFS was found to be 84 months, whereas among vitamin D supplement users the median TFS extended to 169 months. In conclusion, our long-term retrospective study demonstrates that the administration of vitamin D to patients with CLL in a watch-and-wait active surveillance is significantly associated with a longer TFS (in any age) and a longer TTFT among young patients (age ≤65). A prospective clinical trial is needed to validate results.
Introduction
Vitamin D deficiency or insufficiency is common globally and in Israel.1,2 There have been reports that patients with lymphoproliferative disorders, including both Hodgkin and non-Hodgkin lymphomas, are associated with increased incidence of low levels of 25-hydroxyvitamin D. Furthermore, these low levels have been associated with significant adverse prognostic implications.3,4
Retrospective and prospective studies exploring vitamin D insufficiency among patients with with chronic lymphocytic leukemia (CLL) have found an association between the condition and a shorter time to first treatment (TTFT) and inferior overall survival (OS).5,6
The mechanisms underlying the involvement of vitamin D and lymphomagenesis are yet to be fully comprehended, and several hypotheses have been proposed. For instance, an in vitro model of Hodgkin lymphoma demonstrated that adding vitamin D3 and its chemical analogs may favor lymphoma growth.7 Another study demonstrated an inverse correlation between blood levels of vitamin D and levels of inflammatory cytokines that are known to stimulate lymphoma growth.8
It has been reported that, in CLL, B cells exhibit a higher expression of the vitamin D receptor compared with normal B cells. This might suggest a role in regulating critical pathways of CLL proliferation.9 In addition, given in vitro pharmacologic doses of vitamin D derivatives are reported to be involved in apoptosis of B cells by inducing caspase 3- and 9-dependent pathways.10,11 Another explanation is the effect of low vitamin D levels that enhances the activity of myeloid–derived suppressor cells, which favor leukemia growth through high miR-155 expression.12
A case report described a 13-month remission of CLL in an older patient with vitamin D deficiency, after the administration of cholecalciferol.11 The only prospective study in the field was conducted by the Mayo Clinic group and encompassed both patients with non-Hodgkin lymphoma and those with CLL; vitamin D replacement was administered to patients with insufficient vitamin D levels, aiming to achieve 25(OH)D levels of ≥30 ng/mL. The study was reported as feasible, but its main limitation was that it included only 13 patients with CLL, and the effects on CLL course were not evaluated due to a short follow-up period.13
Whether vitamin D supplements affect the clinical course of patients with CLL or not remains an open question.
Methods
In this study, we aimed to retrospectively explore the clinical benefits of vitamin D supplement or one of its analogs on TTFT and treatment-free survival (TFS) in a large cohort of patients with asymptomatic CLL, for whom the watch-and-wait approach was being adopted. TFS was defined as a composite end point of TTFT or death, whereas in TTFT, death was used for censoring. The cohort is based on anonymized data obtained from electronic medical records of Maccabi Healthcare Services (MHS) members, after receiving approval from the institution’s ethical committee. MHS is the second-largest health care organization in Israel, with 2.5 million insured patients. The study included data from 1 January 2000 to 31 December 2022. By using the ICD-9 coding system, we identified 4098 patients who received a diagnosis of CLL during this period. Following the approach of previous retrospective studies of CLL conducted on the MHS database, we excluded 430 patients who did not meet these specific criteria: (a) the diagnosis was confirmed by an expert hematologist, (b) the diagnosis was recorded in the MHS registry for hematologic neoplasm diseases, (c) meeting either of the following conditions: (1) Receiving anti-CLL therapy at least once after diagnosis, or (2) if treatment-the, have at least 1 complete blood count result indicative of an absolute lymphocyte count above 5 × 109/L at any time during the study. Furthermore, 194 patients were excluded from the cohort as they required immediate therapy after diagnosis. Consequently, the final study cohort included 3474 patients, and their characteristics are presented in Table 1.
The characteristics of the cohort and the differences among the vitamin D and non-vitamin D groups
| Variable . | Non-vitamin D (n = 2543) . | Vitamin D or analog (n = 931) . | P value . |
|---|---|---|---|
| Age during diagnosis | 66.381 (57.4-75) | 69.937 (62.903-77.101) | <.001 |
| Sex: male | 1671 (65.7%) | 329 (35.3%) | <.001 |
| Binet stage = C during diag. | 203 (8%) | 30 (3.2%) | <.001 |
| Osteoporosis (binary) | 259 (10.2%) | 278 (29.9%) | <.001 |
| Albumin | 4.241 (4.052-4.433) NA: 46% | 4.252 (4.062-4.401) NA: 41% | .631 |
| Calcium | 9.251 (9.001-9.551) NA: 46% | 9.442 (9.102-9.602) NA:36% | <.001 |
| Elevated LDH∗ | Elevated: 85.7% NA: 46% | Elevated: 88.8% NA: 43% | .016 |
| Phosphor | 3.502 (3.203-3.837) NA: 78% | 3.602 (3.213-3.924) NA:75% | .032 |
| Magnesium | 2.02 (1.924-2.186) NA: 89% | 2.048 (1.9-2.2) NA: 90% | .646 |
| PLT | 195.007 (155.9-242.201) | 215 (175-260) | <.001 |
| HB | 13.543 (12.4-14.6) | 13.247 (12.350-14.200) | <.001 |
| Vitamin D 25 (OH) | 25.217 (19.016-31.004) NA: 90% | 24.88 (18.751-29.853) NA: 84% | .294 |
| WBC | 16.41 (12.402-24.632) | 14.709 (12.421-19.95) | <.001 |
| % Lymp | 59.225 (47.002-69.831) | 57.800 (49.400-66.688) | .239 |
| Variable . | Non-vitamin D (n = 2543) . | Vitamin D or analog (n = 931) . | P value . |
|---|---|---|---|
| Age during diagnosis | 66.381 (57.4-75) | 69.937 (62.903-77.101) | <.001 |
| Sex: male | 1671 (65.7%) | 329 (35.3%) | <.001 |
| Binet stage = C during diag. | 203 (8%) | 30 (3.2%) | <.001 |
| Osteoporosis (binary) | 259 (10.2%) | 278 (29.9%) | <.001 |
| Albumin | 4.241 (4.052-4.433) NA: 46% | 4.252 (4.062-4.401) NA: 41% | .631 |
| Calcium | 9.251 (9.001-9.551) NA: 46% | 9.442 (9.102-9.602) NA:36% | <.001 |
| Elevated LDH∗ | Elevated: 85.7% NA: 46% | Elevated: 88.8% NA: 43% | .016 |
| Phosphor | 3.502 (3.203-3.837) NA: 78% | 3.602 (3.213-3.924) NA:75% | .032 |
| Magnesium | 2.02 (1.924-2.186) NA: 89% | 2.048 (1.9-2.2) NA: 90% | .646 |
| PLT | 195.007 (155.9-242.201) | 215 (175-260) | <.001 |
| HB | 13.543 (12.4-14.6) | 13.247 (12.350-14.200) | <.001 |
| Vitamin D 25 (OH) | 25.217 (19.016-31.004) NA: 90% | 24.88 (18.751-29.853) NA: 84% | .294 |
| WBC | 16.41 (12.402-24.632) | 14.709 (12.421-19.95) | <.001 |
| % Lymp | 59.225 (47.002-69.831) | 57.800 (49.400-66.688) | .239 |
The laboratory test variables refer to their values as reported during the first 6 months after diagnosis.
Diag., diagnosis; HB, hemoglobin; LDH, lactate dehydrogenase; Lymp, lymphocytes; NA, not available; PLT, platelet; WBC, white blood cells.
Because LDH normal range values varies among laboratories, we defined it as a binary variable (elevated or not elevated).
We employed a multivariate time–dependent Cox proportional-hazards model adjusted for age and sex to accommodate variations in vitamin D intake during the follow-up period. We use the inverse probability of treatment weighting (IPTW) with average treatment effect on the treated estimates to create a pseudopopulation that equalizes the distribution of confounding variables between the exposed and unexposed groups.14 Although both propensity matching and IPTW are known to mitigate bias when estimating marginal hazard ratios, we opted for the IPTW approach due to studies highlighting its superior precision.15 The IPTW procedure involves 2 primary steps. Firstly, we computed the propensity score, which quantifies the likelihood of being exposed to the vitamin D intervention based on individual characteristics (age, sex, white blood cells [WBCs], platelet [PLT], and hemoglobin [HB]) as recorded at the start of the follow-up period. Subsequently, we calculated weights as the inverse of the propensity score and fitted a weighted Cox model.
This Cox model considers the usage of vitamin D as a time-varying covariate measured monthly. We examined 5 different methods to define vitamin D exposure: (a) current month use (0/1), (b) cumulative months of use, (c) current month dose, (d) cumulative dose,thed (e) the logarithmic transformation of the current dose. Each exposure variable was tested separately, resulting in 5 variables for vitamin D supplementation and 5 variables for vitamin D analogs. All these variables are time-dependent, allowing for variations in a patient’s exposure status throughout the follow-up period. This approach enhances statistical power for detecting moderate effects and reduces the likelihood of biases, such as immortal time bias.
Similarly, we included various blood test results as time-varying covariates. Because blood tests are irregularly sampled, we imputed their values using linear interpolation. It should be noted that other time-varying variables (eg, vitamin D supplements are not imputed). Multicollinearity between different covariates was examined using the variance inflation factor (VIF). Schoenfeld’s global test was applied to test the proportional-hazards assumption for those variables. The Mantel-Byar test was used to ensure statistical significance after adjustment for immortal time bias.
To thoroughly investigate whether the observed effects of vitamin D supplementation on TTFT and TFS are solely attributable to lifestyle and health behavior, we incorporated vitamin C supplementation as a quasi-control variable in our analysis. By introducing vitamin C supplementation as a comparative measure, we sought to ascertain if it elicits a comparable influence on both TTFT and TFS, thus helping to delineate any potential confounding factors and providing deeper insights into the specific impacts of vitamin D supplementation.
All statistical analyses were performed using the R statistical software 4.2.2 (2022-10-31) (Foundation for Statistical Computing, Vienna, Austria).
MHS gave an approval from the institution’s ethical committee.
Results
Among the 3474 patients included in the study, 931 patients (26.8%) received either vitamin D supplement or its analog, for a minimum of 6 months during the watch-and-wait period. Within the 931 patients, 27% already began receiving vitamin D supplements within the first 6 months of the watch-and-wait period. Out of these 931 patients, 845 patients received only vitamin D supplement, 52 received only vitamin D analog, and 34 received both.
The median follow-up duration for the entire cohort, from the beginning of the study until the time of first treatment or death, was 1745 days (602-3700).
The most common forms of vitamin D supplements were vitamin D3 alone combined with calcium and as part of a multivitamin. The median dose of vitamin D3 was 400 IU (200 to 600) with median exposure of 28 months (13-56). Supplemental Table 1 provides a detailed distribution of the vitamin D dose in terms of months of exposure. We also separately assessed the usage of alfacalcidol, a nonendogenous analog of vitamin D, with a median dose of 0.5 MCG (0.25-1) and median exposure of 14 months (6-30).
Table 2 presents the results as hazard ratios (HRs) with 95% confidence intervals for fixed and time-dependent covariates fully adjusted multivariable analyses. The assessment period for measuring exposure started from the time diagnosis and continued until the end of the follow-up period.
Multivariable Cox regression with time-dependent covariates
| Variable . | TFS . | TTFT . | ||
|---|---|---|---|---|
| HR . | P value . | HR . | P value . | |
| Age during diag. | 1.013 (1.009-1.018) | <.0001 | 0.974 (0.969-0.98) | <.0001 |
| Sex: male | 1.697 (1.52-1.896) | <.0001 | 1.236 (0.784-1.95) | .36 |
| Osteoporosis (binary) | 0.944 (0.825-1.08) | <.0001 | 0.452 (0.251-0.816) | .0083 |
| Binet stage = C | 1.95 (1.704-2.231) | <.0001 | 2.419 (1.186-4.935) | .015 |
| Albumin | 1.011 (0.996-1.025) | .165 | 1.041 (0.992-1.092) | .0954 |
| Calcium | 0.94 (0.897-0.985) | <.001 | 1.288 (0.983-1.687) | .069 |
| Elevated LDH∗ | 0.981 (0.854-1.127) | .786 | 0.958 (0.795-1.153) | .227 |
| Phosphore | 1.157 (1.051-1.274) | .003 | 1.271 (0.601-2.688) | .53 |
| Magnesium | 0.931 (0.754-1.151) | <.0001 | 0.902 (0.574-1.42) | .65 |
| PLT | 0.999 (0.998-1) | <.001 | 0.996 (0.991-1.001) | .062 |
| HB | 0.739 (0.723-0.754) | <.0001 | 0.733 (0.713-0.753) | <.0001 |
| Vitamin D 25 (OH) | 1.004 (0.998-1.009) | .177 | 1.0273 (1.0165-1.0382) | <.0001 |
| WBC | 1.002 (1.001-1.002) | <.0001 | 1.0023 (1.0020-1.0027) | <.0001 |
| % Lymp† | 1.001 (1.0004-91.0016) | .0001 | 1.004 (1.003-1.005) | <.0001 |
| Log (current vitamin D dose) | 0.912 (0.856-0.971) | .004 | 0.879 (0.762-1.014) | .077 |
| Log (current vitamin C dose) | 0.541 (0.052-5.603) | .615 | 0.6535 (0.129-3.302) | .607 |
| Variable . | TFS . | TTFT . | ||
|---|---|---|---|---|
| HR . | P value . | HR . | P value . | |
| Age during diag. | 1.013 (1.009-1.018) | <.0001 | 0.974 (0.969-0.98) | <.0001 |
| Sex: male | 1.697 (1.52-1.896) | <.0001 | 1.236 (0.784-1.95) | .36 |
| Osteoporosis (binary) | 0.944 (0.825-1.08) | <.0001 | 0.452 (0.251-0.816) | .0083 |
| Binet stage = C | 1.95 (1.704-2.231) | <.0001 | 2.419 (1.186-4.935) | .015 |
| Albumin | 1.011 (0.996-1.025) | .165 | 1.041 (0.992-1.092) | .0954 |
| Calcium | 0.94 (0.897-0.985) | <.001 | 1.288 (0.983-1.687) | .069 |
| Elevated LDH∗ | 0.981 (0.854-1.127) | .786 | 0.958 (0.795-1.153) | .227 |
| Phosphore | 1.157 (1.051-1.274) | .003 | 1.271 (0.601-2.688) | .53 |
| Magnesium | 0.931 (0.754-1.151) | <.0001 | 0.902 (0.574-1.42) | .65 |
| PLT | 0.999 (0.998-1) | <.001 | 0.996 (0.991-1.001) | .062 |
| HB | 0.739 (0.723-0.754) | <.0001 | 0.733 (0.713-0.753) | <.0001 |
| Vitamin D 25 (OH) | 1.004 (0.998-1.009) | .177 | 1.0273 (1.0165-1.0382) | <.0001 |
| WBC | 1.002 (1.001-1.002) | <.0001 | 1.0023 (1.0020-1.0027) | <.0001 |
| % Lymp† | 1.001 (1.0004-91.0016) | .0001 | 1.004 (1.003-1.005) | <.0001 |
| Log (current vitamin D dose) | 0.912 (0.856-0.971) | .004 | 0.879 (0.762-1.014) | .077 |
| Log (current vitamin C dose) | 0.541 (0.052-5.603) | .615 | 0.6535 (0.129-3.302) | .607 |
Diag., diagnosis; HB, hemoglobin; LDH, lactate dehydrogenase; Lymp, lymphocytes; PLT, platelet; WBC, white blood cells.
This variable refers to the logarithmic transformation of the current dose.
The variable %Lymp had a high VIF score and was suspected of multicollinearity, thus its hazard ratio and P value were calculated after removing ad hoc WBC (the corresponding variable with the highest correlation).
Comparing 5 different methods to define vitamin D exposure, each was analyzed separately within a multivariate Cox model for TTFT and TFS presented inTable 2
| Vitamin D or analog representation . | TTFT . | TFS . | ||
|---|---|---|---|---|
| Hazard ratio . | P value . | Hazard ratio . | P value . | |
| Current vitamin D use (binary) | 0.992 (0.61-1.615) | .975 | 0.961 (0.855-1.065) | .066 |
| Cumulative vitamin D use | 0.954 (0.861-1.047) | .651 | 0.922 (0.972-1.013) | .223 |
| Current vitamin D dose | 1 (0.999-1) | .264 | 0.933 (0.882-1.012) | .179 |
| Log (current vitamin D dose) | 0.879 (0.762-1.014) | .077 | 0.912 (0.856-0.971) | .004 |
| Cumulative vitamin D dose | 1 (1-1) | .659 | 0.984 (0.982-1.02) | .421 |
| Current analog use | 0.965 (0.937-0.993) | .266 | 0.71 (0.461-1.122) | .091 |
| Cumulative analog use | 0.976 (0.965-0.988) | .767 | 0.927 (0.923-1.042) | .182 |
| Current analog dose | 0.968 (0.899-1.036) | .092 | 0.936 (0.995-1.001) | .056 |
| Log (analog dose) | 0.97 (0.913-1.027) | .181 | 0.951 (0.882-1.015) | .082 |
| Cumulative analog dose | 1 (1-1) | .372 | 1 (1-1) | .418 |
| Vitamin D or analog representation . | TTFT . | TFS . | ||
|---|---|---|---|---|
| Hazard ratio . | P value . | Hazard ratio . | P value . | |
| Current vitamin D use (binary) | 0.992 (0.61-1.615) | .975 | 0.961 (0.855-1.065) | .066 |
| Cumulative vitamin D use | 0.954 (0.861-1.047) | .651 | 0.922 (0.972-1.013) | .223 |
| Current vitamin D dose | 1 (0.999-1) | .264 | 0.933 (0.882-1.012) | .179 |
| Log (current vitamin D dose) | 0.879 (0.762-1.014) | .077 | 0.912 (0.856-0.971) | .004 |
| Cumulative vitamin D dose | 1 (1-1) | .659 | 0.984 (0.982-1.02) | .421 |
| Current analog use | 0.965 (0.937-0.993) | .266 | 0.71 (0.461-1.122) | .091 |
| Cumulative analog use | 0.976 (0.965-0.988) | .767 | 0.927 (0.923-1.042) | .182 |
| Current analog dose | 0.968 (0.899-1.036) | .092 | 0.936 (0.995-1.001) | .056 |
| Log (analog dose) | 0.97 (0.913-1.027) | .181 | 0.951 (0.882-1.015) | .082 |
| Cumulative analog dose | 1 (1-1) | .372 | 1 (1-1) | .418 |
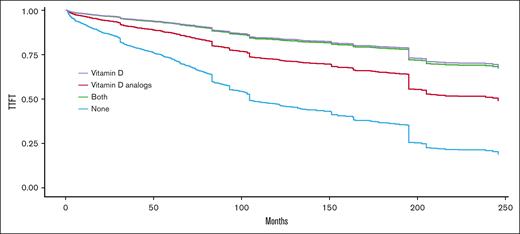
Figure 1 presents the expected TTFT curves adjusted for age and sex using the method discussed by B. L. Thomsen.16 Although vitamin D supplementation was found to have a positive effect on TTFT (as reflected by a HR <1 in Table 2), it was not statistically significant (P = .077). We hypothesized that a relatively high ratio of older patients was censored due to death unrelated to CLL without reaching the first treatment. Thus, we divided the cohort into 2 subcohorts: young (age ≤65) and old (age >65). We found that vitamin D supplement was statistically significant for longer TTFT in the young cohort (P = .038) but not for the older subcohort (P = .169). Notably, the young cohort comprised 42.4% of patients but accounted for 54.1% of patients reaching the first treatment. Moreover, 32.9% patients (659/1999) of the old cohort died without receiving any treatment for CLL. In contrast, only 8% (118/1475) of the young CLL cohort passed away before receiving their first treatment.
Expected TTFT after adustment to age, sex, and other cofounders based on the method presented by Thomsen16for comparing 4 groups: nonvitamin D users, vitamin D supplement users, alfacalcidol users, and both alfacalcidol and vitamin D supplement users. Patients with at least 6 months of exposure are considered users.
Expected TTFT after adustment to age, sex, and other cofounders based on the method presented by Thomsen16for comparing 4 groups: nonvitamin D users, vitamin D supplement users, alfacalcidol users, and both alfacalcidol and vitamin D supplement users. Patients with at least 6 months of exposure are considered users.
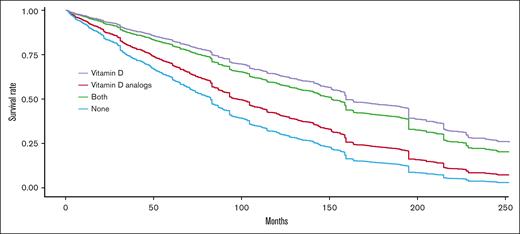
In addition, the intake of vitamin D was found to be associated with a longer TFS for all ages, after adjustment for immortal time bias (P = .004). Figure 2 presents the expected survival curves adjusted for age, sex, and using the method discussed in Chesnaye et al.16 Among non-vitamin D users, the median TFS was found to be 84 months, whereas among vitamin D-supplement users the median TFS extended to 169 months.
Expected treatment-free proportion after adjustment to age, sex, and other cofounders based on the method presented in Chesnaye et al16for comparing 4 groups: non-vitamin D users; vitamin D supplement users, alfacalcidol users, and both alfacalcidol and vitamin D supplement users. Patients with at least 6 months of exposure are considered as users.
Expected treatment-free proportion after adjustment to age, sex, and other cofounders based on the method presented in Chesnaye et al16for comparing 4 groups: non-vitamin D users; vitamin D supplement users, alfacalcidol users, and both alfacalcidol and vitamin D supplement users. Patients with at least 6 months of exposure are considered as users.
It is important to note that previous studies demonstrated that vitamin D supplements are more efficient than vitamin D analogs in increasing serum 25-hydroxyvitamin D.17 Indeed, alfacalcidol was also associated with prolonged TTFT and TFS, but it was not found to be statistically significant in a multivariable analysis (Table 3, P = .124 and .058, respectively). However, because alfacalcidol is commonly prescribed for osteoporosis, we performed an additional multivariable analysis on a subcohort that consists of 537 patients without comorbidity of osteoporosis. In this subanalysis, we found a statistically significant association between alfacalcidol usage and TFS (P = .02 and HR, 0.516 [0.293-0.908]), but it was not found to be statistically significant for TTFT (P = .069).
Interestingly, the vitamin D 25 (OH) serum levels are similar at diagnosis time for both individuals who were taking vitamin D and those who were not. However, our analysis shows that vitamin D takers tended to have more favorable prognostic markers at diagnosis (Table 1). This included a higher proportion of females, earlier Binet stage and lower WBC, and higher PLT and HB measured during the first 6 months of the follow-up. Vitamin D takers are also older (69.9 vs 66.4). Although these confounders were included in the multivariate Cox model, bias is still possible due to unmeasured confounding factors. Thus, it is prudent to interpret these results with caution.
Recall that to examine if vitamin D supplement is merely proxy to lifestyle and health behavior, we used vitamin C supplement as a control variable and checked if it has similar impact on TFS and TTFT. Vitamin C was determined to have no statistically significant impact on either TTFT or TFS (with P values of .607 and .615, respectively, reported in Table 2). Moreover, fitting a Cox model using a subcohort that consists of all patients without comorbidity of osteoporosis, reapproves that vitamin D supplement is associated with a prolonged TFS (P = .031) and TTFT for young patients (age <65, P = .036).
Although 25-hydroxyvitamin D serum levels were mostly not reported during the first 6 months (Table 1), these values were measured later for 60.3% of patients. The median time to the first 25-hydroxyvitamin D test was 20 months (9-53). Supplemental Table 2 provides the patient classification based on their first 25-hydroxyvitamin D serum level tests according to the reference values used by the MHS laboratory. We subsequently perform multivariate Cox regression separately for each vitamin D serum level subcohort and examine if the supplement of vitamin D is associated with longer TTFT and TFS. The results show that in all subcohorts, vitamin D supplementation dosage is linked to extended TTFT and TFS, as evidenced by mean HRs consistently <1. It is worth noting that the hypovitaminosis subcohort comprises a substantially larger patient population (1602 patients) than that in the deficiency and the sufficiency subcohorts (190 and 301 patients, respectively). In the hypovitaminosis subcohort, the vitamin D supplementation dosage had statistically significant impact on TTFT and TFS (P = .031 and .004, respectively).
Our database revealed 600 patients who underwent testing of serum 25-hydroxyvitamin D concentration levels before and after receiving vitamin D supplementation treatment. Analysis employing a linear regression model indicates a positive correlation (P < .001) between the dosage of vitamin D supplementation administered in the preceding 3 months and the increase in the serum 25-hydroxyvitamin D concentration levels. On average, serum concentrations of 25-hydroxyvitamin D increased by 0.6 ng/mL for every 100 units of daily vitamin D3 supplement intake.
Discussion
Vitamin D insufficiency was reported to be associated with inferior TTFT and OS in patients with CLL.5 The question of whether normalizing vitamin D levels in deficient patients with CLL would affect clinical course or not has been answered to some extent.
The Mayo Clinic performed a validation study on vitamin D replacement strategy in patients with lymphoma or CLL who had insufficient vitamin D levels.13 The advantage of their study was its prospective nature. The study provided evidence that vitamin D can be safely and efficiently replaced with achievement of target 25(OH)D levels of 30 ng/mL at 12 weeks without toxicity.13 Yet, follow-up was relatively short, and therefore, the study was not able to demonstrate whether vitamin D supplement has any effect on the natural history of the disease. In addition, they included both patients with lymphoma and those with CLL, who were treatment naïve and under chemotherapy.13
In this study, we aimed to retrospectively explore the clinical benefit of vitamin D supplement or one of its analogs on TTFT and TFS in a large cohort of patients with asymptomatic CLL, who were under watch-and-wait approach.
Major advantage of this study is its large cohort of 3474 patients with CLL, a uniform cohort of treatment-naïve patients only and a long follow-up of almost 5 years.
We were able to demonstrate that vitamin D supplement was statistically significant for longer TTFT in the young cohort (age ≤65) and was associated with a longer TFS for all ages (P = .004).
In addition, we used vitamin C supplement as a control variable and checked if it has similar impact on TFS and TTFT. Vitamin C was determined to have no statistically significant impact on either TTFT or TFS and therefore we may conclude that these results indicate a direct vitamin D effect and not a proxy to lifestyle, health behavior, or general vitamin supplements.
Our study has several limitations. The first was the retrospective nature of the study. Second was the lack of genetic background of CLL (IGHV [immunoglobulin heavy chain variable region] status and FISH [fluorescence in situ hybridization] results). Furthermore, a patient can purchase vitamin D supplements over the counter, and we do not have access to such information. Moreover, apart from complete blood count, all other laboratory tests (including 25-hydroxyvitamin D) were not performed regularly, thus limiting their role as time-dependent confounding factors. Although we employed the IPTW approach to balance the distribution of confounding variables between the exposed and unexposed groups, it is noteworthy that a statistically significant difference persists in the distribution of key characteristics, notably sex, age, and Binet stage at diagnosis between the 2 groups. This suggests a need for caution when interpreting the results.
In conclusion, our long-term retrospective study demonstrates that the administration of vitamin D to patients with CLL in a watch-and-wait active surveillance is significantly associated with a longer TFS (in any age) and a longer TTFT among young patients (age ≤65). A prospective clinical trial is needed to validate the results.
Acknowledgment
The research was supported by a grant from Kahn Sagol Maccabi Research & Innovation Center, Maccabi Healthcare Services.
Authorship
Contribution: T.T. designed, organized, and wrote the manuscript; H.A. and G.M. prepared and preprocessed the data; L.R. performed the statistical analysis and wrote the manuscript; and S.G. and T.P. critically reviewed the manuscript and supervised the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tamar Tadmor, Hematology Unit, Bnai Zion Medical Center, The Ruth and Bruce Rappaport Faculty of Medicine, Technion, Golomb 47 St., Haifa 31048, Israel; email: tamar.tadmor@b-zion.org.il.
References
Author notes
Data are available on request from the corresponding author, Tamar Tadmor (tamar.tadmor@b-zion.org.il).
The online version of this article contains a data supplement.