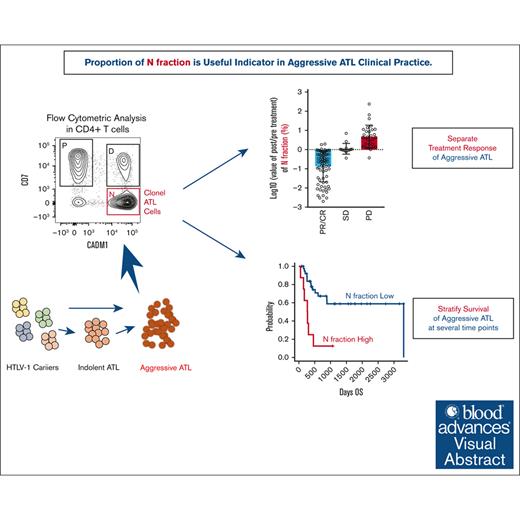
Key Points
Data from CD7 and CADM1 plots (HAS-Flow data) correlate with other disease status markers and reflects treatment response of aggressive ATL.
HAS-Flow data can be used as a prognostic marker for long time survival at various critical times in the treatment of aggressive ATL.
Visual Abstract
Adult T-cell leukemia/lymphoma (ATL) is a poor prognosis hematological malignancy originating from human T-cell leukemia virus 1 (HTLV-1)–infected CD4+ T cells. Flow cytometric plots of CADM1 and CD7 in CD4+ T cells are useful for separating HTLV-1–uninfected T cells and ATL cells. They are indicators of clonal evolution of HTLV-1–infected cells and disease progression of asymptomatic carriers or indolent ATL. However, the impacts of the plots on the clinical course or prognosis of ATL, especially in aggressive ATL, remain unclear. We focused on the N fraction (CD4+ CADM1+ CD7–) reflecting ATL cells and analyzed the flow cytometric profiles and clinical course of 497 samples from 92 HTLV-1–infected patients who were mainly aggressive ATL. The parameters based on N fractions showed significant correlations with known indicators of ATL disease status (soluble interleukin-2 receptor, lactate dehydrogenase, abnormal lymphocytes, etc.) and sensitively reflected the treatment response of aggressive ATL. The parameters based on N fractions significantly stratified the prognosis of aggressive ATL at 4 different time points: before treatment, after 1 course of chemotherapy, at the best response after chemotherapy, and before allogeneic hematopoietic cell transplantation. Even after mogamulizumab administration, which shows potent effects for peripheral blood lesions, the N fraction was still a useful indicator for prognostic estimation. In summary, this report shows that CADM1 vs CD7 plots in CD4+ T cells are useful indicators of the clinical course and prognosis of aggressive ATL. Therefore, this CADM1 and CD7 profile is suggested to be a useful prognostic indicator consistently from HTLV-1 carriers to aggressive ATL.
Introduction
Adult T-cell leukemia/lymphoma (ATL) is a disease that originates from tumorigenesis of human T-cell leukemia virus 1 (HTLV-1)–infected T cells.1,2 HTLV-1 is a retrovirus endemic to specific areas, including southwestern Japan, sub-Saharan Africa, South America, the Caribbean, and foci in the Middle East and central Australia.3 Approximately 5% of HTLV-1–infected carriers develop ATL during their lifetime.4 ATL is classified into 4 clinical forms: smoldering, chronic, lymphoma, and acute types.5 They can be broadly divided into (1) indolent ATL, which includes smoldering and chronic types without unfavorable factor (favorable chronic) and does not necessarily require immediate treatment; and (2) aggressive ATL, which includes chronic type with unfavorable factor (unfavorable chronic), lymphoma, and acute type and requires immediate treatment followed by allogeneic hematopoietic cell transplantation (allo-HCT) whenever possible and has a poor prognosis.6,7 Various prognostic models have been proposed specifically for ATL (prognostic index for acute- and lymphoma-type ATL: ATL-PI, prognostic index for indolent ATL: iATL-PI, Japan Clinical Oncology Group prognostic index: JCOG-PI, and modified ATL-PI),8-11 and recently, prognostic factors based on genetic mutations have also been proposed.12,13 Several factors such as patient age, pretransplant disease status, time from diagnosis to transplant, and pretransplant soluble interleukin-2 receptor (sIL-2R) levels have been reported to influence the prognosis of transplantation in ATL.14-18 sIL-2R and lactate dehydrogenase (LDH) are widely used as indicators of ATL disease status at any time.6,7 These prognostic factors at various time points in the clinical course influence subsequent treatment decisions and provide more accurate prognosis estimation. This may contribute to improved outcomes for patients with ATL. However, it is important to note that these prognostic factors are not universal. Most of these prognostic factors are targeted to the pretreatment period; genomic examination cannot be easily repeated during the treatment period. sIL-2R and LDH can be measured repeatedly and are useful for assessing disease status. However, they lack specificity because they can be affected by factors other than disease status (inflammation, tissue damage, other malignancies, etc.).19,20 Thus, the presence of an indicator that can be easily and repeatedly measured and directly reflects the tumor burden has an important role in treating ATL.
Quantifying ATL tumor cells in peripheral blood is essential for determining disease status, clinical course, and therapeutic efficacy.5,21 ATL cells typically represent morphologically abnormal lymphocytes with characteristic polylobate nuclei,1 but there are many cases in which it is difficult to accurately distinguish ATL cells from normal lymphocytes. Flow cytometry can be used to objectively and accurately detect ATL cells, and detection of ATL cells with various gating strategies has been reported.22-26 HTLV-1–infected cells express cell adhesion molecule 1 (CADM1), and CD7 expression decreases with the progression of ATL disease subtypes. We previously reported that flow cytometry using CADM1 and CD7 plots in CD3+ CD4+ T cells can clearly separate HTLV-1–noninfected CD4+ T cells from clonal ATL cells. This system is named the HTLV-1 analysis system by flow cytometry (HAS-Flow).23-25 This CADM1 and CD7 plot is an indicator reflecting the clonal evolution of HTLV-1–infected cells from HTLV-1 asymptomatic carriers to acute-type ATL and can predict disease progression of asymptomatic carriers or indolent ATL.24,27,28 However, the relationship between HAS-Flow data and ATL's clinical course or prognosis remains unclear, especially in aggressive ATL. Given the quantitative nature of this parameter for transformed ATL cells and the fact that HAS-Flow data are not affected by inflammation or bone marrow recovery, it is likely that these data could be used as a prognostic marker at diagnosis and during treatment.
In this study, we examined peripheral blood samples, mainly from patients with aggressive ATL, and revealed that there is a correlation between the data obtained from CADM1 CD7 plots of CD4+ T cells by flow cytometry and the other clinical data, clinical course of ATL, or prognosis of aggressive ATL at various treatment time points.
Methods
Patients and clinical data
The study included data from 497 peripheral blood samples from 92 patients with HTLV-1 asymptomatic carriers or ATL who received treatment at Research Hospital, the Institute of Medical Science, the University of Tokyo, between June 2012 and January 2023. Clinical subtypes of ATL were classified according to the Shimoyama classification5 and evaluated at the time of each sample collection.29 Data used in this study, including flow cytometric data, were extracted from medical records. Peripheral blood samples from patients with hematological malignancies other than HTLV-1 infection and healthy individuals were collected with written informed consent according to the procedures approved by the institutional review board and analyzed as other than clinical analysis. Complete blood counts and serum levels of sIL-2R and LDH were evaluated using peripheral blood samples taken the same day as the flow cytometric analysis. The proportions of lymphocytes and abnormal lymphocytes were quantified using manual differential leucocyte counts. The treatment response in the clinical course was classified into complete remission (CR), partial response (PR), stable disease (SD), and progressive disease (PD), according to the previous report.21 The institutional review board of the Institute of Medical Science, University of Tokyo, approved this study (2019-72-0217; 2022-37-1114).
Flow cytometric analysis
Details of the analysis are reported previously.24 Briefly, peripheral blood samples were treated with red blood cell lysis buffer for flow cytometric analysis. Cells were then stained with fluorescence-conjugated antibodies. The following antibodies were used in this study: fluorescein isothiocyanate–conjugated anti-human CD3 (clone SK7; BD Biosciences), peridinin chlorophyll protein cyanine 5.5–conjugated anti-human CD4 (clone SK3; BioLegend), biotin-conjugated anti-human/rat CADM1 (clone 3E1; MBL Life Science), phycoerythrin-conjugated streptavidin (BD Biosciences), and allophycocyanin-conjugated anti-human CD7 (clone CD7-6B7; BioLegend). Flow cytometric analyses were performed using a BD FACSCanto II (BD Bioscience) on the same day as the sample collection, and the data were analyzed with FlowJo software (Tree Star). The gating strategy in this study is determined according to a previous report24 and is shown in Figure 1A. The gate was adjusted according to the intensity of CD3 expression as shown in supplemental Figure 1 but was not adjusted by CD7 expression. N/P ratio was calculated as the proportion of N fraction (CD7– CADM1+) to P fraction (CD7+ CADM1–). The number of N fraction was calculated using data from the same day analysis of white blood cell (WBC) count, the proportion of lymphocytes and abnormal lymphocytes, and flow cytometry.
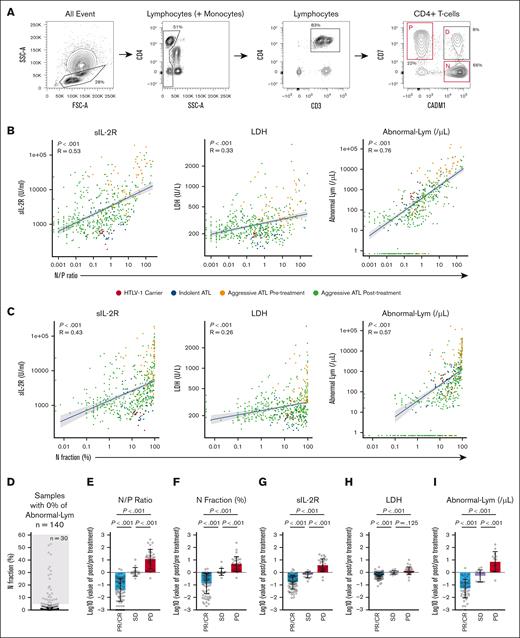
Effects of N fraction–based parameters on other data reflecting ATL disease status and treatment response of aggressive ATL. (A) Representative flow cytometric plots in this study. (B) Correlation between N/P ratio and sIL-2R, LDH, and abnormal lymphocytes (/μL), colored by the status of the patients (n = 497). (C) Correlation between N fraction (%) and sIL-2R, LDH, and abnormal lymphocytes (/μL), colored by the status of the patients (n = 497). (D) Proportion of N fraction in the sample with 0% of abnormal lymphocytes. (E-I) Before and after the ratio of N/P ratio (E), N fraction (%) (F), sIL-2R (G), LDH (H), and abnormal lymphocytes (/μL) (I) at 2 different time points of the same patients (n = 121), comparison between each treatment response (PR/CR, SD, and PD). All data are shown as the mean ± standard deviation; P values were calculated by Spearman rank correlation for panels B-C or 2-tailed unpaired Student t test for panels E-I.
Effects of N fraction–based parameters on other data reflecting ATL disease status and treatment response of aggressive ATL. (A) Representative flow cytometric plots in this study. (B) Correlation between N/P ratio and sIL-2R, LDH, and abnormal lymphocytes (/μL), colored by the status of the patients (n = 497). (C) Correlation between N fraction (%) and sIL-2R, LDH, and abnormal lymphocytes (/μL), colored by the status of the patients (n = 497). (D) Proportion of N fraction in the sample with 0% of abnormal lymphocytes. (E-I) Before and after the ratio of N/P ratio (E), N fraction (%) (F), sIL-2R (G), LDH (H), and abnormal lymphocytes (/μL) (I) at 2 different time points of the same patients (n = 121), comparison between each treatment response (PR/CR, SD, and PD). All data are shown as the mean ± standard deviation; P values were calculated by Spearman rank correlation for panels B-C or 2-tailed unpaired Student t test for panels E-I.
Statistical analysis
The Spearman rank correlation was calculated to assess the correlation between the data from flow cytometry and other data. Student t test was used to compare continuous variables between 2 groups. In the analysis of overall survival (OS) and progression-free survival (PFS), the log-rank test was used for univariate analysis, and the Cox proportional hazard model was used for univariate and multivariate analysis to show hazard ratios (HRs) and 95% confidence intervals (CIs). In the multivariate analysis, patient age, sex, LDH level, and sIL-2R level were used as factors, along with data obtained by flow cytometry. We applied the grid-search approach to determine the cutoff values. In other words, we tested all possible cutoff values and selected the one that showed the most significant stratification of the target population in terms of P values of log-rank test for OS on the condition that the smaller group included at least 15% of the target population. The same value was applied for PFS analysis for the same target cohort. Missing data were excluded from analyses when they were required. All statistical analyses were performed using GraphPad Prism software (version 7.0d; GraphPad Software), EZR (version 1.55; Saitama Medical Center, Jichi Medical University),30 or R (version 4.2.1; R Foundation for Statistical Computing). All P values were 2-sided, and P values <.05 were considered statistically significant.
Results
Patient and sample characteristics
We analyzed the data of 497 samples from 92 patients. The characteristics of the patients and the samples in this study are shown in Tables 1 and 2. At the time of the first sample collection, the median age was 65 years, and 61 cases (66%) were acute-type ATL. Eighty-one cases (88%) were diagnosed with aggressive ATL at least once during the clinical courses, and 76% of the samples were collected when patients had acute-type ATL. VCAP (vincristine, cyclophosphamide, doxorubicin, and predonisolone)-AMP (doxorubicin, ranimustine, and predonisolone)-VECP (vindesine, etoposide, carboplatin, and predonisolone) was the most frequently selected treatment as first-line therapy (44/74 [59%]), except for patients who did not receive systemic therapy for ATL (n = 14) and those with missing information about first-line therapy (n = 4). Thirty patients (33%) were treated with at least 1 course of mogamulizumab, and 178 samples (36%) were collected after mogamulizumab administration. In the flow cytometric analysis of CD4+ T cells, the P fraction (CD7+ CADM1–) reflects HTLV-1–noninfected CD4+ T cells, and the N fraction (CD7– CADM1+) reflects clonal ATL cells.24,25 The representative analysis of CD4+ T-cell fractions is shown in Figure 1A. Among CD4+ T cells, the median value of the P fraction was 49.1% (range, 0.4%-97.8%), and N fraction was 17.3% (range, 0%-89.3%); and the median value of the N/P ratio, which represents the ratio of ATL tumor cells to normal lymphocytes, was 0.37 (range, 0-246). The median values of other laboratory parameters associated with ATL disease status were WBC 5.43/× 103μL (range, 0.04-175.6), normal lymphocytes 19% (range, 0.5%-85.5%), abnormal lymphocytes 1.5% (range, 0%-95.3%), sIL-2R 1785 U/mL (range, 189-192 231), and LDH 246 U/L (range, 121-4174). The median value of the absolute number of N fractions calculated from the WBC count and flow cytometric data was 82/μL (range, 0-113 839).
Characteristics of patients
| Patient . | n = 92 . |
|---|---|
| Median age at first sample collection (range) | 65 (45-82) |
| Sex, male (%) | 42 (46%) |
| Disease type at first sample collection | |
| Asymptomatic carrier | 3 (3%) |
| Smoldering | 11 (12%) |
| Chronic (favorable) | 5 (5%) |
| Chronic (unfavorable) | 8 (9%) |
| Lymphoma | 4 (4%) |
| Acute | 61 (66%) |
| Aggressive ATL at any point in time (%) | 81 (88%) |
| First systemic therapy for ATL | |
| VCAP-AMP-VECP | 44 (48%) |
| CHOP-like regimen | 15 (16%) |
| Mogamulizumab ± chemotherapy | 12 (13%) |
| Low-dose VP-16 | 2 (2%) |
| High-dose MTX + AraC | 1 (1%) |
| No systemic therapy for ATL | 14 (15%) |
| N/A∗ | 4 (4%) |
| Mogamulizumab administration | |
| Yes | 30 (33%) |
| No | 62 (67%) |
| Patient . | n = 92 . |
|---|---|
| Median age at first sample collection (range) | 65 (45-82) |
| Sex, male (%) | 42 (46%) |
| Disease type at first sample collection | |
| Asymptomatic carrier | 3 (3%) |
| Smoldering | 11 (12%) |
| Chronic (favorable) | 5 (5%) |
| Chronic (unfavorable) | 8 (9%) |
| Lymphoma | 4 (4%) |
| Acute | 61 (66%) |
| Aggressive ATL at any point in time (%) | 81 (88%) |
| First systemic therapy for ATL | |
| VCAP-AMP-VECP | 44 (48%) |
| CHOP-like regimen | 15 (16%) |
| Mogamulizumab ± chemotherapy | 12 (13%) |
| Low-dose VP-16 | 2 (2%) |
| High-dose MTX + AraC | 1 (1%) |
| No systemic therapy for ATL | 14 (15%) |
| N/A∗ | 4 (4%) |
| Mogamulizumab administration | |
| Yes | 30 (33%) |
| No | 62 (67%) |
AMP, doxorubicin, ranimustine, and prednisolone; AraC, cytarabine; CHOP, cyclophosphamide, vincristine, doxorubicin, and prednisolone; MTX, methotrexate; VCAP, vincristine, cyclophosphamide, doxorubicin, and prednisolone; VECP, vindesine, etoposide, carboplatin, and prednisolone; VP-16, etoposide.
N/A, not available: cases with unknown initial treatment details.
Characteristics of samples
| Sample . | n = 497 . |
|---|---|
| Median age (range) | 66 (45-82) |
| Sex, male (%) | 191 (38%) |
| Type (%) | |
| Asymptomatic carrier | 10 (2%) |
| Smoldering | 36 (7%) |
| Chronic (favorable) | 13 (3%) |
| Chronic (unfavorable) | 48 (10%) |
| Lymphoma | 11 (2%) |
| Acute | 379 (76%) |
| Post-mogamulizumab administration (%) | |
| Yes | 178 (36%) |
| No | 319 (64%) |
| Median value of each data (range) | |
| P fraction/CD4+ T cells, % | 49.1 (0.4-97.8) |
| D fraction/CD4+ T cells, % | 3.1 (0-89.3) |
| N fraction/CD4+ T cells, % | 17.3 (0-98.9) |
| N fraction (/μL) | 82 (0-113 839) |
| N/P ratio | 0.37 (0-246) |
| WBC (/× 103μL) | 5.43 (0.04-175.6) |
| Lymphocytes, % | 19 (0.5-85.5) |
| Lymphocytes (/μL) | 944 (34-15 030) |
| Abnormal lymphocytes, % | 1.5 (0-95.3) |
| Abnormal lymphocytes (/μL) | 86 (0-165 942) |
| sIL-2R, U/mL | 1 785 (189-192 231) |
| LDH, U/L | 246 (121-4 174) |
| Sample . | n = 497 . |
|---|---|
| Median age (range) | 66 (45-82) |
| Sex, male (%) | 191 (38%) |
| Type (%) | |
| Asymptomatic carrier | 10 (2%) |
| Smoldering | 36 (7%) |
| Chronic (favorable) | 13 (3%) |
| Chronic (unfavorable) | 48 (10%) |
| Lymphoma | 11 (2%) |
| Acute | 379 (76%) |
| Post-mogamulizumab administration (%) | |
| Yes | 178 (36%) |
| No | 319 (64%) |
| Median value of each data (range) | |
| P fraction/CD4+ T cells, % | 49.1 (0.4-97.8) |
| D fraction/CD4+ T cells, % | 3.1 (0-89.3) |
| N fraction/CD4+ T cells, % | 17.3 (0-98.9) |
| N fraction (/μL) | 82 (0-113 839) |
| N/P ratio | 0.37 (0-246) |
| WBC (/× 103μL) | 5.43 (0.04-175.6) |
| Lymphocytes, % | 19 (0.5-85.5) |
| Lymphocytes (/μL) | 944 (34-15 030) |
| Abnormal lymphocytes, % | 1.5 (0-95.3) |
| Abnormal lymphocytes (/μL) | 86 (0-165 942) |
| sIL-2R, U/mL | 1 785 (189-192 231) |
| LDH, U/L | 246 (121-4 174) |
It has been shown that the D and N fractions are specific to HTLV-1–infected individuals and are barely detected in healthy individuals.24 To further validate this, we examined 10 patients with other hematological malignancies (4 with acute myeloid leukemia, 2 with myelodysplastic syndrome, 2 with acute lymphoblastic leukemia, and 2 with malignant lymphoma other than ATL) who were not infected with HTLV-1 and had received chemotherapy or allo-HCT. The D and N fractions were almost undetectable in HTLV-1–uninfected patients, whereas the D and N fractions were clearly detectable in HTLV-1–infected patients in this study cohort (supplemental Figure 2A), indicating the specificity of the D and N fractions in HTLV-1–infected individuals. Additionally, in dilution experiments using samples from patients with ATL and healthy individuals, N fraction detection sensitivity up to ∼0.2% was shown (supplemental Figure 2B).
Effects of HAS-Flow on other data reflecting ATL disease status and treatment response of aggressive ATL
To evaluate whether HAS-Flow could reflect ATL disease status, we examined the correlation between the flow cytometric data and other laboratory data that reflect ATL disease status. The proportion (%) and absolute number (per μL) of N fractions and N/P ratio showed a significantly positive correlation with the value of WBC (per μL), abnormal lymphocyte counts (% and per μL), LDH (U/L), and sIL-2R (U/mL) and negative correlation with the proportion of normal lymphocytes (%) in the whole cohort that includes HTLV-1 carriers, indolent ATL, and aggressive ATL before and after initial treatment (Figure 1B-C; supplemental Figure 3A-D). It is of note that 30 of the 140 samples (21%) with 0% of abnormal lymphocytes had an N fraction of >5% (Figure 1D). To confirm this discrepancy, we picked 5 samples with N fraction ≥25% despite 0% abnormal lymphocytes and had them reevaluated by an expert technician, but none of the 5 samples had >1% abnormal lymphocytes (supplemental Figure 4A-B), suggesting the presence of cases in which ATL cells defined by flow cytometry escape morphological identification. We then investigated the association between HAS-Flow data and the treatment response of aggressive ATL. We estimated the relative change of various parameters compared with before treatment according to the clinical response (PR/CR, SD, and PD) based on the standard criteria21 from 121 evaluable sample pairs in the same patients. The proportion of N fraction and N/P ratio significantly separated PR/CR, SD, and PD, along with sIL-2R and the absolute number of abnormal lymphocytes, whereas LDH did not significantly separate SD from PD (Figure 1E-I). These results indicate that the data obtained from HAS-Flow sensitively reflect the treatment response of aggressive ATL and significantly correlate with established indicators of ATL disease status.
Flow cytometric data and prognosis of aggressive ATL
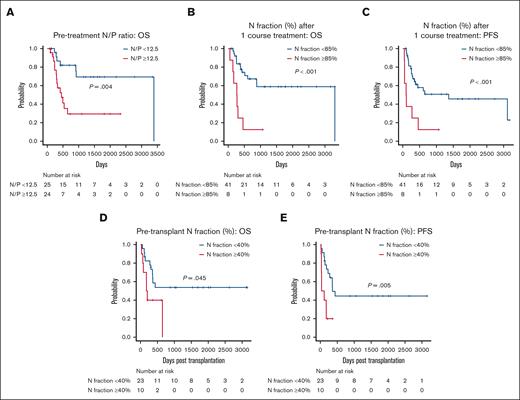
We then evaluated whether HAS-Flow data could predict the prognosis of aggressive ATL in which allo-HCT after intensive chemotherapy constitutes standard treatment. We analyzed survival at 3 critical time points during the clinical course: before initial treatment, after 1 course of chemotherapy, and before allo-HCT. First, we examined 49 cases of aggressive ATL whose samples with both before treatment and after 1 course of chemotherapy were available for analysis (supplemental Table 1). Patients with pretreatment N/P ratio ≥12.5 and N fraction ≥90% predicted significantly shorter OS than those with N/P ratio <12.5 (P = .004) and N fraction <90% (P = .042) (Figure 2A; supplemental Figure 5A). In a multivariate analysis, N/P ratio ≥12.5 was extracted as the significant factor predicting poor prognosis (P = .023; HR, 4.35; 95% CI, 1.23-15.44), but N fraction ≥90% was not (Table 3; supplemental Table 2). As possible causes, 6 cases with N fraction <90% were classified as N/P ≥12.5 due to low P fraction, and in all cases, infection was a problem early in treatment (supplemental Table 3). Characteristics of patients with pretreatment high and low N/P ratio are shown in supplemental Table 4.
Given that changes in the N fraction after initial chemotherapy vary widely from case to case (supplemental Figure 5B), we then analyzed 49 cases same to pretreatment analysis (supplemental Table 1) to examine the association between early treatment response and prognosis. Patients with N fraction ≥85% after 1 course of chemotherapy had significantly shorter OS and PFS than those with N fraction <85% (OS, P < .001; PFS, P < .001; Figure 2B-C). A multivariate analysis using data after 1 course of chemotherapy identified N fraction ≥85% as a significant poor prognostic factor for OS and PFS (OS, P = .004; HR, 5.37 [95% CI, 1.71-16.86]; PFS, P = .002; HR, 5.90 [95% CI, 1.92-18.10]), independent of male sex (Table 3). The same cutoff value of the N/P ratio before treatment initiation and N fraction after 1 course of chemotherapy was also useful for prognostic estimation in the subgroup of patients aged ≤70 years, generally considered transplant candidates31,32 (supplemental Figure 5C-D), and patients with a treatment response of PR or better, providing additional information of prognostication for responding cases (supplemental Figure 5E-F).
N fraction–based parameters and survival of aggressive ATL. (A) OS of 2 groups of pretreatment patients with aggressive ATL separated by an N/P ratio of 12.5 (n = 49). (B-C) OS (B) and PFS (C) of 2 groups of patients with aggressive ATL after 1 course of chemotherapy separated by an N fraction of 85% (n = 49). (D-E) OS (D) and PFS (E) of 2 groups of pretransplant patients with aggressive ATL separated by an N fraction of 40% (n = 33). All P values were calculated by log-rank test.
N fraction–based parameters and survival of aggressive ATL. (A) OS of 2 groups of pretreatment patients with aggressive ATL separated by an N/P ratio of 12.5 (n = 49). (B-C) OS (B) and PFS (C) of 2 groups of patients with aggressive ATL after 1 course of chemotherapy separated by an N fraction of 85% (n = 49). (D-E) OS (D) and PFS (E) of 2 groups of pretransplant patients with aggressive ATL separated by an N fraction of 40% (n = 33). All P values were calculated by log-rank test.
Univariate and multivariate analysis: effects of each values on OS/PFS
| . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) . | P value . | Hazard ratio (95% CI) . | P value . | |
| Effects of pretreatment values on OS | ||||
| Age (≥65 vs <65), y | 0.93 (0.39-2.26) | .881 | ||
| Sex (male vs female) | 3.09 (1.26-7.58) | .014 | 3.47 (1.26-9.57) | .016 |
| sIL-2R (≥10 000 vs <10 000) | 1.61 (0.63-4.03) | .313 | ||
| LDH (≥1.5 times UNL vs <1.5 times UNL) | 1.81 (0.60-5.42) | .293 | ||
| N/P (≥12.5 vs <12.5) | 3.75 (1.43-9.87) | .007 | 4.35 (1.23-15.44) | .023 |
| Effects of values after 1 course treatment on OS | ||||
| Age (≥65 vs <65) | 0.90 (0.37-2.17) | .814 | ||
| Sex (male vs female) | 3.15 (1.29-7.73) | .012 | 4.12 (1.63-10.42) | .003 |
| sIL-2R (≥5 000 vs <5 000) | 2.64 (1.09-6.42) | .032 | ||
| LDH (≥UNL vs <UNL) | 1.70 (0.57-5.08) | .345 | ||
| N fraction (≥85 vs <85), % | 4.74 (1.86-12.11) | .001 | 5.37 (1.71-16.86) | .004 |
| Effects of values after 1 course treatment on PFS | ||||
| Age (≥65 vs <65) | 0.61 (0.28-1.33) | .217 | ||
| Sex (male vs female) | 2.22 (1.00-4.93) | .049 | 3.29 (1.33-8.11) | .01 |
| sIL-2R (≥5000 vs <5000) | 1.33 (0.61-2.91) | .472 | ||
| LDH (≥UNL vs <UNL) | 1.06 (0.44-2.54) | .895 | ||
| N fraction (≥85 vs <85), % | 3.97 (1.64-9.65) | .002 | 5.90 (1.92-18.10) | .002 |
| Effects of pretransplant values on OS | ||||
| Age (≥65 vs <65) | 1.00 (0.39-2.60) | .999 | ||
| Sex (male vs female) | 3.81 (1.24-11.70) | .02 | 5.54 (1.58-19.43) | .007 |
| sIL-2R (≥2000 vs <2000) | 0.79 (0.28-2.24) | .655 | ||
| LDH (≥UNL vs <UNL) | 1.20 (0.46-3.12) | .706 | ||
| N fraction (%) (≥40 vs <40) | 2.65 (0.99-7.10) | .053 | 3.36 (1.10-10.26) | .033 |
| Effects of pretransplant values on PFS | ||||
| Age (≥65 vs <65) | 0.87 (0.35-2.12) | .75 | ||
| Sex (male vs female) | 2.58 (1.02-6.53) | .045 | 6.41 (1.99-20.70) | .002 |
| sIL-2R (≥2000 vs <2000) | 1.25 (0.50-3.13) | .637 | ||
| LDH (≥UNL vs <UNL) | 1.26 (0.52-3.04) | .602 | ||
| N fraction (≥40 vs <40), % | 3.64 (1.40-9.44) | .008 | 5.03 (1.82-13.91) | .002 |
| . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) . | P value . | Hazard ratio (95% CI) . | P value . | |
| Effects of pretreatment values on OS | ||||
| Age (≥65 vs <65), y | 0.93 (0.39-2.26) | .881 | ||
| Sex (male vs female) | 3.09 (1.26-7.58) | .014 | 3.47 (1.26-9.57) | .016 |
| sIL-2R (≥10 000 vs <10 000) | 1.61 (0.63-4.03) | .313 | ||
| LDH (≥1.5 times UNL vs <1.5 times UNL) | 1.81 (0.60-5.42) | .293 | ||
| N/P (≥12.5 vs <12.5) | 3.75 (1.43-9.87) | .007 | 4.35 (1.23-15.44) | .023 |
| Effects of values after 1 course treatment on OS | ||||
| Age (≥65 vs <65) | 0.90 (0.37-2.17) | .814 | ||
| Sex (male vs female) | 3.15 (1.29-7.73) | .012 | 4.12 (1.63-10.42) | .003 |
| sIL-2R (≥5 000 vs <5 000) | 2.64 (1.09-6.42) | .032 | ||
| LDH (≥UNL vs <UNL) | 1.70 (0.57-5.08) | .345 | ||
| N fraction (≥85 vs <85), % | 4.74 (1.86-12.11) | .001 | 5.37 (1.71-16.86) | .004 |
| Effects of values after 1 course treatment on PFS | ||||
| Age (≥65 vs <65) | 0.61 (0.28-1.33) | .217 | ||
| Sex (male vs female) | 2.22 (1.00-4.93) | .049 | 3.29 (1.33-8.11) | .01 |
| sIL-2R (≥5000 vs <5000) | 1.33 (0.61-2.91) | .472 | ||
| LDH (≥UNL vs <UNL) | 1.06 (0.44-2.54) | .895 | ||
| N fraction (≥85 vs <85), % | 3.97 (1.64-9.65) | .002 | 5.90 (1.92-18.10) | .002 |
| Effects of pretransplant values on OS | ||||
| Age (≥65 vs <65) | 1.00 (0.39-2.60) | .999 | ||
| Sex (male vs female) | 3.81 (1.24-11.70) | .02 | 5.54 (1.58-19.43) | .007 |
| sIL-2R (≥2000 vs <2000) | 0.79 (0.28-2.24) | .655 | ||
| LDH (≥UNL vs <UNL) | 1.20 (0.46-3.12) | .706 | ||
| N fraction (%) (≥40 vs <40) | 2.65 (0.99-7.10) | .053 | 3.36 (1.10-10.26) | .033 |
| Effects of pretransplant values on PFS | ||||
| Age (≥65 vs <65) | 0.87 (0.35-2.12) | .75 | ||
| Sex (male vs female) | 2.58 (1.02-6.53) | .045 | 6.41 (1.99-20.70) | .002 |
| sIL-2R (≥2000 vs <2000) | 1.25 (0.50-3.13) | .637 | ||
| LDH (≥UNL vs <UNL) | 1.26 (0.52-3.04) | .602 | ||
| N fraction (≥40 vs <40), % | 3.64 (1.40-9.44) | .008 | 5.03 (1.82-13.91) | .002 |
UNL, upper normal limit.
There was no consensus as to the appropriate level of disease control in ATL before allo-HCT. Therefore, we examined 33 cases whose samples were available for flow cytometry just before allo-HCT and evaluated their effects on survival (supplemental Table 5). Patients with N fraction ≥40% before allo-HCT had significantly worse OS and PFS than those with N fraction <40% (OS, P = .045; PFS, P = .005) (Figure 2D-E). In a multivariate analysis, N fraction ≥40% before allo-HCT was a significant prognostic factor for shorter OS and PFS (OS, P = .033; HR, 3.36 [95% CI, 1.10-10.26]; PFS, P = .002; HR, 5.03 [95% CI, 1.82-13.91]; Table 3), along with being male.
These results suggest that N fraction parameters in CD4+ T cells may serve as prognostic indicators at 3 different time points: before treatment, after 1 course of chemotherapy, and before allo-HCT in the aggressive ATL clinical course.
Flow cytometric data after mogamulizumab administration
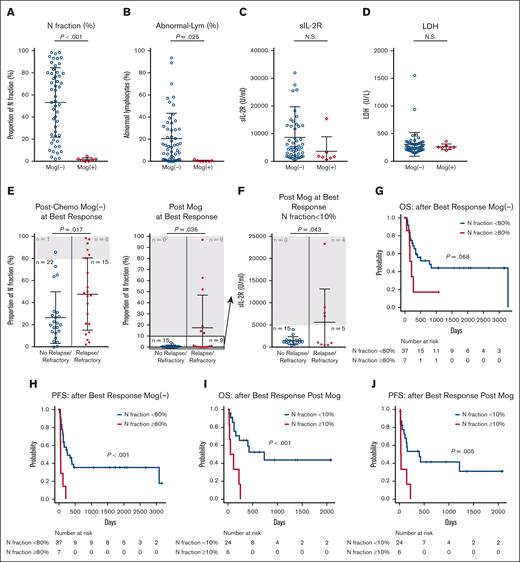
Mogamulizumab, the anti-CCR4 monoclonal antibody, is highly effective against ATL, especially for peripheral blood lesions.33,34 The study analyzed samples after 1 course of chemotherapy (supplemental Table 1). The percentage of N fraction and visually defined abnormal lymphocytes was significantly lower after chemotherapy, including mogamulizumab, than those without mogamulizumab (Figure 3A-B). In contrast, the same analysis showed no significant differences in sIL-2R and LDH (Figure 3C-D). This indicates a potent response to mogamulizumab, specifically in peripheral blood lesions, as previously reported.33,34 These results suggest that the N fraction after mogamulizumab administration may not correctly reflect the disease activity of ATL. Thus, we examined the performance of the N fraction as a prognostic factor after mogamulizumab administration. For this purpose, we compared the proportion of N fraction at the time of best response after chemotherapy, including with mogamulizumab (n = 30) or without mogamulizumab (n = 44). In both groups, the proportion of N fraction was significantly higher in the relapsed-refractory cases than in the nonrelapsed/refractory cases (Figure 3E). All patients in the mogamulizumab group with N fraction ≥10% at best response turned out relapsed/refractory ATL, including lymph node and mass lesions (Figure 3E; supplemental Table 6). In comparison, 6 of 7 patients (88%) with N fraction ≥80% in the non-mogamulizumab group turned out relapse/refractory (Figure 3E), indicating a substantial difference in the threshold for prediction of subsequent refractoriness between the 2 groups. Notably, a marked reduction of N fraction <10% at the best response does not assure freedom from relapse/refractoriness after the use of mogamulizumab. In fact, 9 of the 24 cases with N fraction <10% had relapse/refractoriness in this group (Figure 3F), and all of them had nodal lesions at the time of relapse/refractory (supplemental Table 7). In this post-mogamulizumab group, simultaneous evaluation of sIL-2R values is useful for predicting clinical outcomes; relapsed/refractory cases had significantly higher sIL-2R values than nonrelapse/refractory group (P = .043; Figure 3F). In addition, all but 1 of the 5 relapsed patients who had sIL-2R <5000 U/mL at best response became sIL-2R ≥5000 U/mL at later relapse.
Effects of N fraction after mogamulizumab administration. (A-D) Values of N fraction (%) (A), abnormal lymphocytes (%) (B), sIL-2R (C), and LDH (D) after 1 course of chemotherapy, comparison between with (n = 7) and without (n = 52) mogamulizumab administration. (E) Proportion of N fraction at the best response after chemotherapy with (left) and without (right) mogamulizumab administration is compared according to whether or not the patients subsequently relapsed/got refractory. (F) Values of sIL-2R at the best response after mogamulizumab administration among patients with N fraction ≤10% (n = 24) is compared according to whether or not the patients subsequently relapsed/got refractory. (G-H) OS (G) and PFS (F) of 2 groups of patients with aggressive ATL separated by N fraction of 80% at best response after chemotherapy without mogamulizumab (n = 44). (I-J) OS (I) and PFS (J) of 2 groups of patients with aggressive ATL separated by N fraction of 10% at the best response after chemotherapy, including mogamulizumab (n = 30). All data are shown as the mean ± standard deviation; P values were calculated by 2-tailed unpaired Student t test for panels A-F and log-rank test for panels G-J. Mog, mogamulizumab; N.S., not significant.
Effects of N fraction after mogamulizumab administration. (A-D) Values of N fraction (%) (A), abnormal lymphocytes (%) (B), sIL-2R (C), and LDH (D) after 1 course of chemotherapy, comparison between with (n = 7) and without (n = 52) mogamulizumab administration. (E) Proportion of N fraction at the best response after chemotherapy with (left) and without (right) mogamulizumab administration is compared according to whether or not the patients subsequently relapsed/got refractory. (F) Values of sIL-2R at the best response after mogamulizumab administration among patients with N fraction ≤10% (n = 24) is compared according to whether or not the patients subsequently relapsed/got refractory. (G-H) OS (G) and PFS (F) of 2 groups of patients with aggressive ATL separated by N fraction of 80% at best response after chemotherapy without mogamulizumab (n = 44). (I-J) OS (I) and PFS (J) of 2 groups of patients with aggressive ATL separated by N fraction of 10% at the best response after chemotherapy, including mogamulizumab (n = 30). All data are shown as the mean ± standard deviation; P values were calculated by 2-tailed unpaired Student t test for panels A-F and log-rank test for panels G-J. Mog, mogamulizumab; N.S., not significant.
We investigated the optimal N fraction threshold for predicting survival of patients who received mogamulizumab. We used the same thresholds that could predict relapse/refractoriness to evaluate their effects on survival. In the non-mogamulizumab group, patients with N fraction ≥80% at best response had marginally significantly shorter OS and significantly shorter PFS than those with N fraction <80% (OS, P = .068; HR, 2.47 [95% CI, 0.90-6.77]; PFS, P<.001; HR, 5.34 [95% CI, 2.11-13.55]; Figure 3G-H). In the mogamulizumab group, patients with N fraction ≥10% at best response had significantly shorter OS and PFS than those with N fraction <10% (OS, P<.001; HR, 5.65 [95% CI, 1.86-17.16]; PFS, P = .005; HR, 3.92 [95% CI, 1.40-10.97]; Figure 3I-J). These results suggest that although mogamulizumab markedly reduces N fraction, the proportion of N fraction at best response is a predictive indicator of relapse/refractory ATL and subsequent survival regardless of mogamulizumab administration. However, the threshold for predictive utility differed greatly between patients treated with and without mogamulizumab.
Discussions
Characterization of CD4+ T cells by flow cytometry using CADM1 vs CD7 plot has shown clinical utility, especially in HTLV-1 carriers and indolent ATL.24,27,28 It has been shown that HTLV-1 carriers or indolent ATL with high D+N fractions are at high risk for early progression to aggressive ATL, according to the previous report that focused on the D+N fraction.28 However, in this study, we focused on the quantity of N fractions that are assumed to represent the tumor burden of transformed ATL cells. We found that N fraction–based parameters function as a prognostic marker of the clinical course of ATL at both initial diagnosis and after treatment courses. The results suggest that the CADM1 vs CD7 plot is a consistently useful prognostic indicator from HTLV-1 carriers to aggressive ATL.
sIL-2R and LDH are widely used as indicators of disease status in the clinical course of ATL.7 The study showed that sIL-2R, LDH, and visually defined abnormal lymphocytes were significantly correlated with the proportion and absolute number of N fractions and N/P ratio. These parameters were able to significantly separate the clinical course of ATL (PR/CR, SD, and PD). However, these parameters have shortcomings predicted by their properties. For example, sIL-2R can be influenced by graft-versus-host disease, inflammation, and other malignancies,19 and LDH can also be affected by tissue damage, typically liver injury, hemolysis, and hematopoietic recovery after chemotherapy.20 Flow cytometric data using the N fraction shown in this study precisely reflect the amount of transformed tumor cells and are not susceptible to other conditions, unlike morphologically defined abnormal lymphocytes, which are affected by lack of objectivity and prone to interobserver differences. Meanwhile, flow cytometric data have the disadvantage of reflecting only tumor cells in the peripheral blood. This shortcoming can be a more serious concern regarding the role of N fractions after mogamulizumab administration, which has a specific effect on reducing tumor cells in the peripheral blood. Specifically, we speculated that the N fraction after mogamulizumab administration could not be used to predict clinical outcomes. However, the proportion of N fraction was still useful in predicting relapse/refractoriness even after mogamulizumab administration. Because the N fraction was greatly reduced after mogamulizumab treatment for the entire cohort, we could highlight those high-risk cases in which the N fraction remained high even after mogamulizumab treatment. However, even though high specificity is achieved, N fraction data alone cannot be sensitive enough to distinguish subsequent recurrence/refractoriness. Given the shortcomings of these parameters, it is expected that sIL-2R, LDH, and the number of tumor cells in peripheral blood can be integrated in a complementary manner to assess the disease status of ATL more accurately and estimate therapeutic efficacy, as was exemplified in the response prediction of post-mogamulizumab cases (Figure 3E-F). In addition, as previously reported,25 a certain number of cases in this study showed a discrepancy between the percentage of N fraction and visually defined abnormal lymphocytes (Figures 1D; supplemental Figure 4). This suggests that the accurate quantification of tumor cells by visual inspection is difficult in some cases, and flow cytometry is the preferred method for accurately assessing tumor cells in peripheral blood.
ATL is a poor prognosis disease; prognostic prediction at various points in the clinical course is vital for planning the subsequent treatment strategy. Therefore, various risk classifications have been proposed for indolent ATL, pretreatment, or pretransplant aggressive ATL.8-13,16-18 The study showed that CADM1 and CD7 plots of CD4+ T cells by flow cytometry are important prognostic indicators of aggressive ATL at 4 different time points: before treatment, after 1 course of chemotherapy, at best response after chemotherapy, and before allo-HCT. These indicators are also useful in HTLV-1 asymptomatic carriers and indolent ATL.28 The N/P ratio from the plot stratified the prognosis of aggressive ATL before treatment. Unlike information on the N fraction alone, the N/P ratio includes information on HTLV-1–uninfected intact CD4+ T cells and tumor cells. Cellular immunodeficiency is a crucial issue in ATL, and some patients have fatal infections such as pneumocystis pneumonia and cytomegalovirus infections, which might be derived from a deficiency of normal immunity.35 Given these considerations, the N/P ratio measured early in the clinical course may be a value that encompasses both tumor burden and cellular immunity, allowing for more accurate prognostic evaluation. Actually, before treatment, the N/P ratio was a more useful prognostic indicator than the N fraction alone, and infection was a problem in cases with a high N/P ratio relative to the N fraction due to low P fraction (Figures 2A; Table 3; supplemental Figure 5A; supplemental Tables 2-3). In contrast, the proportion of N fraction after 1 course of chemotherapy, at before allo-HCT or best response after chemotherapy also predicted subsequent prognosis and relapse/refractory ATL. These results suggest that simple tumor volume may be a more important indicator in the assessment after treatment initiation. Given complex and continuous genetic/epigenetic changes from asymptomatic carrier to aggressive ATL cells of HTLV-1–infected cells,36 it is difficult to define genetic/epigenetic markers that represent aggressive tumor burden. In this context, the identification of the practical markers that mirrors clinically significant residual disease is significantly useful.
Allo-HCT constitutes an essential treatment for aggressive ATL.6,7 Indisputably, achieving CR is ideal before receiving allo-HCT, but such favorable responses are not always obtained due to the intractable nature of aggressive ATL.37 The tolerable level of disease control before allo-HCT is a matter of great interest, but no consensus has been reached. Several reports have indicated pretransplant treatment strategies. Factors such as time from diagnosis of aggressive ATL to allo-HCT,15 pretransplant sIL-2R levels,16-18 and use of mogamulizumab before transplant38 have influenced treatment strategies as prognostic factors after allo-HCT. This study provides additional data for appropriate ATL disease control before allo-HCT. The proportion of the N fraction significantly stratified the prognosis of allo-HCT cases, along with sex, as previously reported.14 In contrast to the previous report that identified pretransplant sIL-2R <2000 U/mL as a prognostic marker after allo-HCT,16,17 this threshold did not predict outcomes in this cohort. Comparison of these markers is an issue to be addressed in the future. Although there have been no reports on peripheral blood tumor burden and prognosis of allo-HCT for ATL, this study suggests that quantifying peripheral blood tumor burden by flow cytometry may be a novel prognostic factor. Therefore, it is suggested that following the kinetics of peripheral blood N fraction may allow us to estimate a more appropriate pretransplant ATL disease status and contribute to an improved prognosis of allo-HCT for ATL.
Various gating strategies have been shown to detect ATL cells by flow cytometry, and their clinical usefulness has been reported.22-26 In this study, we evaluated the usefulness of the N fraction in aggressive ATL clinical practice for 2 reasons: (1) the specificity of CADM1 for HTLV-1–infected cells24 (supplemental Figure 2A); and (2) the CADM1-CD7 plot as an indicator of ATL clonal progression.24 We detected CADM1 by a secondary fluorescent antibody method using biotin-streptavidin in this study, because it is more sensitive than using a primary antibody. However, the secondary fluorescent antibody method has the disadvantage of adding 1 more staining step, which takes about 15 minutes. In addition, although we showed that high N fraction is specific for ATL, the lower threshold of the N fraction that borders HTLV-1–infected cases from healthy individuals has not been adequately determined (supplemental Figure 2A-B). Therefore, it should be recognized that although the test is useful in detecting high-risk cases, it is not suitable for the evaluation of small amount of residual tumor burden. Detection of ATL cells by different gating strategy may also be useful as a prognostic indicator of aggressive ATL and is an issue for future investigation.
This study has several limitations. First, the number of patients and samples is small, and it is a single-center, retrospective study. A prospective study with a large population for validation is warranted. Second, only 4 lymphoma-type ATL cases were included in this study. Although the lymphoma type is defined to have ≤1% atypical lymphocytes in the peripheral blood, it is noteworthy that all of the lymphoma-type cases in this cohort had 20.45% (range, 3.3%-25.1%) of N fraction (supplemental Figure 2A). Thus, it is meaningful to investigate the significance of the N fraction using a larger cohort of this subtype as well. Third, we could not compare our data with widely used ATL-PI due to a lack of performance status data. Investigation of the clinical significance of HAS-Flow data, including these indices, is an important issue for future study.
Results of this flow cytometry–based study found that the amount or ratio of CADM1+ and CD7– cells in CD4+ T cells can be used as prognostic indicators. These indicators can be applied dynamically at several time points during treatment courses. Further examination is needed to prove the validity of the results.
Acknowledgments
The authors thank all the physicians and staff at the hospital especially in Department of Hematology/Oncology and Department of Laboratory Medicine, the laboratory members in Division of Hematopoietic Disease Control, and IMSUT Clinical Flow Cytometry Laboratory (The Institute of Medical Science, The University of Tokyo) for their help in this study. This work was supported by grants-in-aid from Japan Society for the Promotion of Science (22K16317).
Authorship
Contribution: K.J. collected the clinical data, analyzed and interpreted the data, and wrote the manuscript; T.K., Y.I., A.I., K.Y., A.S., and T.F. contributed to the collection of clinical data; K.U. planned and guided the research and interpreted the data; Y.N. planned and guided the research, analyzed and interpreted the data, and wrote the manuscript; and all authors approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yasuhito Nannya, Department of Hematology/Oncology, Research Hospital, The Institute of Medical Science, The University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108-8639, Japan; email: ynanya@g.ecc.u-tokyo.ac.jp.
References
Author notes
Individual participant data will not be shared.
The full-text version of this article contains a data supplement.