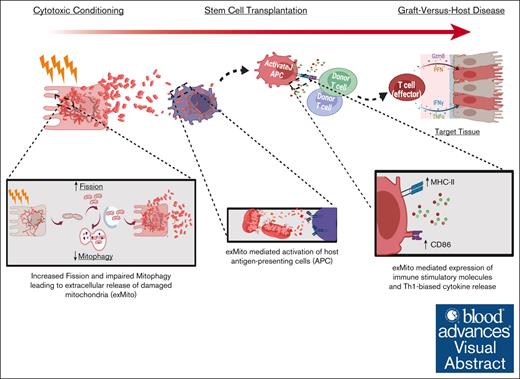
Key Points
Pretransplant conditioning regimens induces release of damaged extracellular mitochondria independent of cell death.
Presence of circulating extracellular mitochondria activates host antigen-presenting cells and aggravates graft-versus-host disease.
Visual Abstract
Despite therapeutic advancements, graft-versus-host disease (GVHD) is a major complication of hematopoietic stem cell transplantation (HSCT). In current models of GVHD, tissue injury induced by cytotoxic conditioning regimens, along with translocation of microbes expressing pathogen-associated molecular patterns, result in activation of host antigen-presenting cells (APCs) to stimulate alloreactive donor T lymphocytes. Recent studies have demonstrated that in many pathologic states, tissue injury results in the release of mitochondria from the cytoplasm to the extracellular space. We hypothesized that extracellular mitochondria, which are related to archaebacteria, could also trigger GVHD by stimulation of host APCs. We found that clinically relevant doses of radiation or busulfan induced extracellular release of mitochondria by various cell types, including cultured intestinal epithelial cells. Conditioning-mediated mitochondrial release was associated with mitochondrial damage and impaired quality control but did not affect the viability of the cells. Extracellular mitochondria directly stimulated host APCs to express higher levels of major histocompatibility complex II (MHC-II), costimulatory CD86, and proinflammatory cytokines, resulting in increased donor T-cell activation, and proliferation in mixed lymphocyte reactions. Analyses of plasma from both experimental mice and a cohort of children undergoing HSCT demonstrated that conditioning induced extracellular mitochondrial release in vivo. In mice undergoing MHC-mismatched HSCT, administration of purified syngeneic extracellular mitochondria increased host APC activation and exacerbated GVHD. Our data suggest that pre-HSCT conditioning results in extracellular release of damaged mitochondria, which increase alloreactivity and exacerbate GVHD. Therefore, decreasing the extracellular release of damaged mitochondria after conditioning could serve as a novel strategy for GVHD prevention.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a therapeutic strategy to treat various oncologic, hematologic, immune, and metabolic disorders by replacement of a patient’s blood-forming stem cells with those of a healthy donor.1 The broad application of allogeneic HSCT is limited by risks of several complications including graft-versus-host disease (GVHD), the leading cause of nonrelapse mortality after HSCT.2 GVHD occurs when alloreactive donor T lymphocytes recognize and direct an immune response against host cells.3,4 Current prevention and treatment strategies for GVHD rely on immunosuppressive medications that largely target the T cells, which mediate the disease.5 However, therapies targeting the upstream mechanisms that initiate the GVHD immune response are limited.
Tissue damage from cytotoxic conditioning given before HSCT significantly contributes to the onset of GVHD. One explanation for the interrelationship between tissue damage and GVHD is the local or systemic release of microbe- and tissue-derived byproducts (pathogen-associated molecular patterns and damage-associated molecular patterns [DAMPs]) respectively), which prime host antigen presenting cells (APCs).6,7 Recently, extracellular mitochondria (exMito) have been identified as a link between tissue damage and inflammation in a variety of critical illnesses.8-12 Because of their archaebacterial ancestry, mitochondria retain molecular motifs that are recognized by pattern recognition receptors of the innate and adaptive immune system.8,11,12
Although extracellular release of mitochondrial content was previously thought to be a passive process secondary to cell death, recent discoveries suggest that it can be an alternative mechanism for mitochondrial quality control, especially in settings in which the canonical mitochondrial quality control system is impaired.13,14 Our group, as well as others, have demonstrated that alterations in mitochondrial dynamics, in particular excessive mitochondrial fission, can mediate the extracellular release of damaged mitochondria after inflammatory insults.15,16 However, the impact of cytotoxic conditioning therapies on mitochondrial dynamics and the extracellular release of mitochondria have not been explored. In this article, we demonstrate that doses of irradiation or chemotherapy relevant to HSCT conditioning significantly increased mitochondrial fission and release of damaged mitochondria in various cell types, including intestinal epithelial cells. Furthermore, using an in vivo model of HSCT, we show that exMito increase the incidence and severity of GVHD. The preclinical findings are corroborated by an association between increased exMito content in circulation after conditioning and incidence of acute GVHD (aGVHD) in a cohort of children undergoing HSCT.
Methods
Cell culture and murine strains
The human colon epithelial cell-line, Caco-2, human embryonic kidney (HEK293), and human umbilical vein endothelial cells (HUVEC; American Type Culture Collection) were used to evaluate the impact of cytotoxic conditioning on mitochondrial morphology and function and exMito release. Caco-2 and HEK293 cells were grown in Dulbecco modified Eagle medium containing 4 mM L-glutamine, 4.5 g/L glucose, 1 mM sodium pyruvate (Corning), 10% fetal bovine serum (Genesee Scientific), and 1% penicillin and streptomycin. HUVECs were grown in endothelial cell basal medium containing endothelial cell growth supplement (Promocell). All cells were maintained at 37°C in an incubator with 5% CO2 and 100% humidity. All chemicals and reagents, unless specified, were purchased from Sigma Aldrich.
BALB/c and C57BL6/J mice were acquired from The Jackson Laboratories. All animals had at least a 1-week acclimation period before initiation of experiments. All animal experiments were carried out under the protocol (no. 34455) approved by the Institutional Animal Care and Use Committee of Stanford University.
Cytotoxic pre-HSCT conditioning
Cell lines were treated with clinically relevant doses of pre-HSCT conditioning regimens (irradiation [5 Gy and 10 Gy] or busulfan [3 μg/mL and 10 μg/mL]). Similarly, BALB/c mice were treated with varying doses of total body irradiation (TBI; 4.4 Gy and 8.8 Gy) or busulfan (20 mg/kg per day intraperitoneally for 2 doses).17 C57BL6/J mice were treated with 10 Gy of TBI. After treatment, changes in mitochondrial morphology and function and extracellular release of mitochondria was evaluated as detailed in supplemental Methods.
Allogeneic HSCT model
BALB/c mice (8-12 weeks old) were treated with fractionated TBI (2 doses of 4.4 Gy TBI on day 0, 8 hours apart) and subsequently received transplantation with 5 × 106 T-cell–depleted HSCs from the bone marrow (BM) alone, or with 4 × 106 splenocytes from C57BL6/J congenic mice expressing CD45.1+ allele to induce GVHD.18 Syngeneic damaged mitochondria along with BM and splenocytes were administered to a subset of recipient BALB/c mice to determine the impact of circulating mitochondria on the incidence of GVHD. Syngeneic mitochondria were collected from the heart, liver, and kidney by differential centrifugation15 and treated with 1 mM H202 for 1 hour. Subsequently, they were pelleted by centrifugation at 16000g for 20 minutes, resuspended in mannitol sucrose (MS) buffer, and the protein concentration was estimated by Bradford assay. Graded doses of damaged mitochondria (25 μg or 100 μg) were cotransplanted intravenously along with the BM cells with or without donor splenocytes to determine the presence of a dose-dependent response. Irradiated (8.8 Gy) mice receiving either phosphate-buffered saline, BM, or BM with mitochondria served as controls. Mice were monitored daily, and body weight and GVHD score were assessed twice weekly.19 A subset of animals was euthanized at day 3 after transplantation, and recipient (CD45.1 negative) cells from the spleen and mesenteric lymph nodes were analyzed by flow cytometry for changes in major histocompatibility complex II (MHC-II) expression. The gating strategy used for flow cytometry analysis excluded doublets, Zombie Yellow or Zombie Aqua (Biolegend) stained dead cells, and CD45.1 stained donor cells.
Clinical cohort
Blood samples obtained from a prospective cohort of children undergoing HSCT at Lucile Packard Children’s Hospital (protocol ID: IRB 47220) were processed to deplete the platelets per previous protocols.20 MS buffer (900 μL) was added to 100 μL of plasma and centrifuged at 16000g for 20 minutes. Supernatant was carefully removed and the mitochondrial pellet was resuspended in 100 μL of MS buffer. exMito abundance was quantified by flow cytometry using MitoTracker Green (MTG) staining (200 nM) as detailed in supplemental Methods. Abundance of exMito were evaluated before and after conditioning and compared with incidence of aGVHD.
Statistical analyses
Statistical analyses and graphing were performed using Prism 9.0 (Graphpad). Experimental results were evaluated based on the average of ≥3 independent experiments and calculated as mean values ± standard deviation. Data distribution was evaluated with kernel density plots and the Shapiro-Wilk test for normality. For continuous variables, differences across groups were compared by 1-way analysis of variance or Kruskal-Wallis test with Bonferroni correction for multiple comparisons. Cox proportional-hazards model was used to assess survival differences across groups. The nominal P value of <.05 was used as statistically significant threshold.
Blood samples obtained from a prospective cohort of children undergoing HSCT at Lucile Packard Children's Hospital were used in this study. This was approved by the Lucile Packard Children's Hospital’s institutional review board (protocol ID: IRB 47220). All animal experiment were approved by the institutional animal care and use committee under protocols (APLAC 33881 and APLAC 34455)
Results
Cytotoxic conditioning induces excessive mitochondrial fission and mitochondrial dysfunction in intestinal epithelial cells
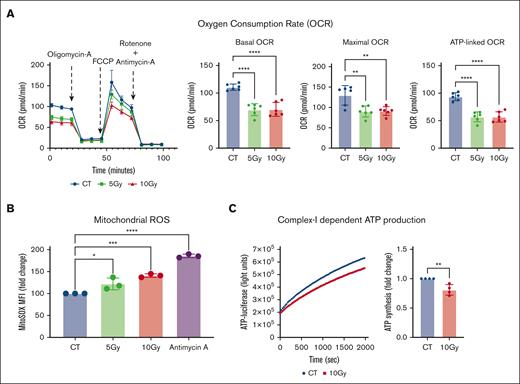
To determine the impact of pre-HSCT irradiation conditioning on mitochondrial function, we treated intestinal epithelial cells (Caco-2) with escalating doses of irradiation (5 Gy and 10 Gy) and characterized changes in bioenergetics, mitochondrial reactive oxygen species, and adenosine triphosphate (ATP) production. Irradiated Caco-2 cells exhibited a significant decrease in mitochondrial oxygen consumption rate (OCR; basal OCR: P = .008; ATP-linked OCR: P = .002; and maximal OCR: P = .03; Figure 1A) as well as basal glycolysis and glycolytic capacity (supplemental Figure 1). These impairments in cellular respiration were associated with a dose-dependent increase in mitochondrial oxidative stress (MitoSox median fluorescence intensity; 10 Gy fold change relative to control = 1.41 ± 0.04; P = .0006; Figure 1B) and a decrease in complex I–dependent ATP production (10 Gy fold change relative to control = 0.8 ± 0.09; P = .005; Figure 1C).
Irradiation leads to mitochondrial dysfunction in Caco-2 cells. Caco-2 cells were irradiated at the indicated doses, cultured for an additional 24 hours, and subjected to various mitochondrial functional assays. (A) A representative Seahorse plot of the Mito Stress assay and bar graphs of the respiratory parameters normalized within the experiment for difference in the total protein content between the groups (n = 6). (B) The median fluorescence intensity (MFI) of cells stained with MitoSOX and analyzed by flow cytometry is shown as relative fold inductions in relation to control nonirradiated cells (CT; n = 3). Cells treated with 5 μM antimycin-A for 3 hours served as a positive control for the assay. (C) ATP production in 1 μg of isolated mitochondria from nonirradiated (CT) and irradiated (10 Gy) Caco-2 cells when provided with pyruvate and malate as substrates. A representative plot of luciferase activity recorded over time (left) and the ATP concentration calculated are shown as relative fold change in comparison to CT (n = 4). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001; n, number of independent experiments.
Irradiation leads to mitochondrial dysfunction in Caco-2 cells. Caco-2 cells were irradiated at the indicated doses, cultured for an additional 24 hours, and subjected to various mitochondrial functional assays. (A) A representative Seahorse plot of the Mito Stress assay and bar graphs of the respiratory parameters normalized within the experiment for difference in the total protein content between the groups (n = 6). (B) The median fluorescence intensity (MFI) of cells stained with MitoSOX and analyzed by flow cytometry is shown as relative fold inductions in relation to control nonirradiated cells (CT; n = 3). Cells treated with 5 μM antimycin-A for 3 hours served as a positive control for the assay. (C) ATP production in 1 μg of isolated mitochondria from nonirradiated (CT) and irradiated (10 Gy) Caco-2 cells when provided with pyruvate and malate as substrates. A representative plot of luciferase activity recorded over time (left) and the ATP concentration calculated are shown as relative fold change in comparison to CT (n = 4). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001; n, number of independent experiments.
To validate our observations in vivo, we evaluated the effects of cytotoxic conditioning on mitochondrial dynamics and function by treating BALB/c mice with standard conditioning doses of irradiation (TBI of 8.8 Gy). Changes in mitochondrial architecture and function were then evaluated in intestinal cells as a target organ for GVHD. Electron microscopy of intestinal epithelial cells revealed mitochondrial damage with increased fission at 72 hours, followed by a loss of cristae density as well as outer mitochondrial membrane integrity at 120 hours (Figure 2A). Changes in mitochondrial morphology were quantified by a decrease in mitochondrial aspect ratio (Control = 1.93 ± 0.71 vs 8.8 Gy = 0.8 ± 0.6; P = .001) and form factor (Control = 1.54 ± 0.4 vs 8.8 Gy = 0.51 ± 0.32; P < .001; supplemental Figure 2A-B). Western blot analyses of isolated mitochondrial fractions from the bowel showed an eightfold increase in mitochondrial translocation of dynamin-related protein 1 (P = .02), a key protein responsible for mitochondrial fission, in animals that received irradiation (Figure 2B). Additionally, protein carbonylation assay of the mitochondrial fraction from the small bowel showed a 1.8-fold increase in oxidative posttranslational modifications (protein carbonylation; Control = 0.95 ± 0.09 vs 8.8 Gy = 1.8 ± 0.47; P = .01) after irradiation, suggesting an increase in oxidative stress (Figure 2C).
TBI induces mitochondrial fission and oxidative stress in the intestinal epithelium. The small bowel of BALB/c mice treated with TBI was resected at the indicated time points and evaluated for changes in mitochondrial architecture. (A) Representative electron micrograph for each time point is shown. (B-C) Enriched mitochondrial fractions isolated 24 hours after TBI from the small bowel of mice. (B) A representative western blot probed with antibodies against dynamin-related protein 1 (DRP1) for mitochondrial fission, and VDAC-1 for total mitochondrial content (left) and densitometric protein quantification (n = 3; right) is shown. (C) A representative western blot derivatized with dinitrophenylhydrazine (DNP) and probed with antibodies against DNP for protein carbonylation and VDAC-1 for total mitochondrial content (left) and densitometric protein quantification (n = 3; right) is shown. CT, control (nonirradiated) mice; ∗P < .05, and ∗∗P < .01; n, number of mice). VDAC-1, Voltage-dependent anion-selective channel 1.
TBI induces mitochondrial fission and oxidative stress in the intestinal epithelium. The small bowel of BALB/c mice treated with TBI was resected at the indicated time points and evaluated for changes in mitochondrial architecture. (A) Representative electron micrograph for each time point is shown. (B-C) Enriched mitochondrial fractions isolated 24 hours after TBI from the small bowel of mice. (B) A representative western blot probed with antibodies against dynamin-related protein 1 (DRP1) for mitochondrial fission, and VDAC-1 for total mitochondrial content (left) and densitometric protein quantification (n = 3; right) is shown. (C) A representative western blot derivatized with dinitrophenylhydrazine (DNP) and probed with antibodies against DNP for protein carbonylation and VDAC-1 for total mitochondrial content (left) and densitometric protein quantification (n = 3; right) is shown. CT, control (nonirradiated) mice; ∗P < .05, and ∗∗P < .01; n, number of mice). VDAC-1, Voltage-dependent anion-selective channel 1.
Under physiologic conditions, mitochondrial damage leads to the induction of mitophagy, the cellular quality control response for selective removal of damaged mitochondria. Analyses of intracellular mitochondrial content in total tissue lysates of the small bowel showed no significant differences in the abundance of mitochondrial outer, inner membrane, and matrix proteins between control and irradiated mice (supplemental Figure 3A-B), indicating that dysfunctional mitochondria did not accumulate within the cells. However, western blot analyses of bowel tissue revealed that LC3-II levels (the lipidated form of LC3) in total tissue lysates (supplemental Figure 3A-B) and the polyubiquitination status of mitochondrial proteins (supplemental Figure 3C-D) were not changed after irradiation, indicating no significant induction of either autophagy or mitophagy after irradiation. Absence of Pink-1 stabilization and Parkin translocation in the mitochondrial fractions further corroborated the lack of mitophagy induction by pre-HSCT radiation (supplemental Figure 3E-F). Altogether, these results suggest an alternate mechanism for clearance of dysfunctional mitochondria.
Cytotoxic conditioning induces extracellular release of damaged mitochondria
Besides mitophagy, extracellular release of mitochondria has been proposed to be an alternate mechanism of mitochondrial quality control after cellular stress.13,14 Because irradiation did not induce mitophagy, we hypothesized that pre-HSCT conditioning led to the extracellular release of damaged mitochondria. To test this hypothesis, we examined both mitochondrial release into the supernatant from cultured intestinal epithelial cells and into plasma from mice treated with pre-HSCT irradiation. For the in vitro analyses, equal volumes of the cell culture supernatants from control and irradiated cells were subjected to differential centrifugation and the pellet was analyzed for mitochondrial presence by flow cytometry. Multiple mitochondrial markers including the mitochondria-specific stain MTG, mitochondrial membrane potential dye TMRM (tetramethylrhodamine, methyl ester), and mitochondrial outer membrane protein (TOM20) were used to confirm that the yield from the differential centrifugation was indeed mitochondria (supplemental Figure 4A). In addition, exMito fractions were devoid of other associated organelles, including lysosomes, endoplasmic reticulum, and proteasome, as evaluated by western blot using antibodies against the respective organelle-specific markers (supplemental Figure 4B)
Comparison of supernatants from control and irradiated Caco-2 cells revealed that irradiation caused an ∼50% increase in abundance of exMito (fold change in MTG-positive events; 10 Gy = 1.45 ± 0.22; P = .0002; Figure 3A). Similarly, western blot analyses using antibodies against the complexes of the electron transport chain indicated increased levels of mitochondrial proteins in the extracellular milieu of irradiated cells (supplemental Figure 5A). The increased release of mitochondria was independent of cell death, as measured by lack of significant difference in lactate dehydrogenase release (Figure 3B), suggesting that the expulsion of damaged mitochondria after irradiation was mediated by viable cells.
Irradiation leads to release of damaged exMito. exMito isolated from cell-culture supernatants of nonirradiated (CT) and irradiated (10 Gy) Caco-2 cells was subjected to (A) MTG staining and analyzed by flow cytometry. The relative MTG-positive events are shown as fold induction (n = 7). (B) Cell culture supernatants from nonirradiated (CT) and irradiated (10 Gy) Caco-2 cells cultured for 24 hours were subjected to lactate dehydrogenase (LDH) assay and the results are represented as percent (%) cytotoxicity. Staurosporine (STS; 1 μM) treatment for 12 hours was used as a positive control for the experiment (n = 3). (C) Complex-I–dependent ATP-production in 1 μg of isolated mitochondria. A representative plot of luciferase activity recorded over time is shown. (D) exMito content in the plasma of BALB/c mice 24 hours after TBI (8.8 Gy) was stained with MTG and was analyzed by flow cytometry. The number of MTG-positive events detected in 60 μL of plasma is shown; CT, nonirradiated control mice (n = 7). (E) exMito isolated from the plasma of BALB/C mice after 24 hours after TBI (8.8 Gy) was subjected to retramethylrhodamine, methyl ester (TMRM) staining in the presence or absence of complex-I substrates (pyruvate and malate) and analyzed by flow cytometry. The MFI of TMRM calculated is represented as relative fold induction (n = 4). (F) Mitochondria isolated from the plasma of BALB/C mice 24 hours after TBI (8.8 Gy) or no irradiation (CT) was subjected to complex-I–dependent ATP production (n = 3). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001; n, number of independent experiments.
Irradiation leads to release of damaged exMito. exMito isolated from cell-culture supernatants of nonirradiated (CT) and irradiated (10 Gy) Caco-2 cells was subjected to (A) MTG staining and analyzed by flow cytometry. The relative MTG-positive events are shown as fold induction (n = 7). (B) Cell culture supernatants from nonirradiated (CT) and irradiated (10 Gy) Caco-2 cells cultured for 24 hours were subjected to lactate dehydrogenase (LDH) assay and the results are represented as percent (%) cytotoxicity. Staurosporine (STS; 1 μM) treatment for 12 hours was used as a positive control for the experiment (n = 3). (C) Complex-I–dependent ATP-production in 1 μg of isolated mitochondria. A representative plot of luciferase activity recorded over time is shown. (D) exMito content in the plasma of BALB/c mice 24 hours after TBI (8.8 Gy) was stained with MTG and was analyzed by flow cytometry. The number of MTG-positive events detected in 60 μL of plasma is shown; CT, nonirradiated control mice (n = 7). (E) exMito isolated from the plasma of BALB/C mice after 24 hours after TBI (8.8 Gy) was subjected to retramethylrhodamine, methyl ester (TMRM) staining in the presence or absence of complex-I substrates (pyruvate and malate) and analyzed by flow cytometry. The MFI of TMRM calculated is represented as relative fold induction (n = 4). (F) Mitochondria isolated from the plasma of BALB/C mice 24 hours after TBI (8.8 Gy) or no irradiation (CT) was subjected to complex-I–dependent ATP production (n = 3). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001; n, number of independent experiments.
When compared with isolated intracellular mitochondria, exMito were noted to be dysfunctional, represented by lower complex-I–dependent ATP production (ATP [μM] per 1 μg isolated mitochondria; intracellular mitochondria = 19.31 ± 3.74 μM vs exMito = 6.34 ± 3.5 μM, P = .0006; supplemental Figure 5B). Interestingly, the exMito released after irradiation demonstrated further impairment in ATP production (fold-change in ATP [μM] per 1 μg isolated mitochondria relative to control; 10 Gy = 0.65 ± 0.24, P = .006; Figure 3C; supplemental Figure 5C) as well as oxidative stress (dinitrophenylhydrazine/Succinate Dehydrogenase Complex Iron Sulfur Subunit B fold change; 10 Gy = 2.1 ± 0.3, P = .0003; supplemental Figure 5D).
To determine the impact of cytotoxic conditioning on exMito content in vivo, platelet-depleted plasma from control and irradiated BALB/c mice was collected. Flow cytometry of plasma with MTG stain revealed a significant increase in circulating cell-free mitochondria 24 hours after irradiation (MTG positive events/60 μL plasma; Control = 6321 ± 4543 vs 8.8 Gy = 56 349 ± 28 824; P = .0007), which persisted up to day 5 of analysis after conditioning (Figure 3D; supplemental Figure 6A). The increase in cell-free mitochondria in plasma after irradiation was confirmed by western blot analysis of various mitochondrial proteins such as cytochrome c, and ATP5A1 (supplemental Figure 6B). Next, we evaluated the functional competency of exMito by testing their ability to hyperpolarize the mitochondrial membrane potential in response to complex-I substrates (pyruvate and malate). Addition of complex-I substrates to exMito isolated from plasma of nonirradiated mice significantly increased the resting membrane potential, quantified by median fluorescence intensity of TMRM. However, exMito from plasma of irradiated mice exhibited diminished hyperpolarization (fold change; Control = 5.7 ± 1.2 vs 8.8 Gy = 2.3 ± 0.55; P < .0001; Figure 3E), indicating that exMito from irradiated mice have impairments in the production or maintenance of the proton motive force. This finding was further confirmed by reduced complex-I–dependent ATP production from exMito of irradiated mice compared with nonirradiated controls (ATP [μM] per 1 μg isolated mitochondria; Control = 2.6 ± 0.35 μM vs 8.8G y = 0.35 ± 0.26 μM, P = .001; Figure 3F; supplemental Figure 6C).
To confirm that this observation is not specific to BALB/c strain, we collected platelet-depleted plasma from control and irradiated C57BL6/J mice and demonstrated significant increase in circulating cell-free mitochondria 24 hours after irradiation (MTG-positive events/60 μL plasma; Control = 27 796 ± 15 574 vs 10 Gy = 51 638 ± 14 632; P = .008; supplemental Figure 7A). Furthermore, to evaluate that these observations are also applicable to chemotherapy-based conditioning regimens, we treated Caco-2 cells with graded doses of busulfan and subsequently quantified exMito in the supernatant at 24 hours after treatment. Results demonstrated that busulfan treatment resulted in a 30% increase in exMito at 24 hours (P = .001; supplemental Figure 7B). Similarly, in vivo, mice treated with myeloablative doses of busulfan (20 mg/kg intraperitoneal × 2 doses)17 resulted in a significant increase in exMito, 24 hours after treatment (MTG-positive events/50 μL plasma; Control = 14 978 ± 16 262 vs busulfan 20 mg/kg intraperitoneal = 30 639 ± 11 541; P = .03; supplemental Figure 7C).
exMito increase activation of APC, induce T-cell proliferation, and increase the incidence and severity of GVHD in murine models
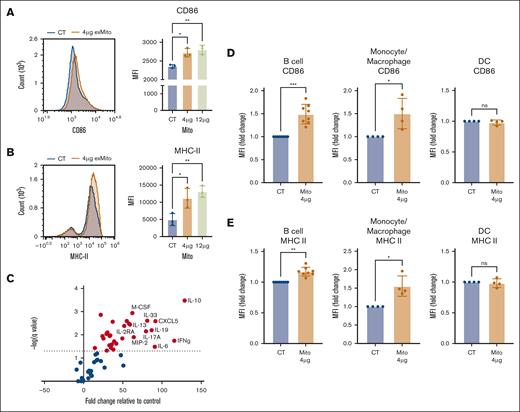
Activated host APCs play a crucial role in promoting the alloreactivity of donor T-cells after HSCT. To test the hypothesis that exMito stimulate host APCs to increase the allogeneic response of donor T-cells, we used an in vitro mixed lymphocyte reaction (MLR) with responder T cells from C57BL/6J mice and stimulator T-cell–depleted splenocytes from BALB/c mice. To evaluate the impact of exMito, the stimulator T-cell–depleted (BALB/c) splenocytes were incubated with and without exMito for 24 hours before MLR. Stimulation with exMito induced a dose-dependent increase in the surface expression of the costimulatory molecule CD86 and MHC-II without change in MHC-I expression (Figure 4A-B; supplemental Figure 8A), indicating an increased state of APC activation. Consistent with the altered expression of stimulatory molecules, the supernatants from the cultured splenocytes had significantly higher levels of cytokines and chemokines. Notably, cytokines relevant to GVHD that were induced by exMito included interferon gamma, interleukin-10 (IL-10), IL-33, IL-6, macrophage inflammatory protein 2, IL-17, and IL-2 receptor α21-28 (Figure 4C; supplemental Table 1). Further evaluation of APC subpopulations demonstrated that B cells and monocyte/macrophages have the most significant increase in the surface expression of the costimulatory molecule CD86 and MHC-II after exposure to exMito whereas dendritic cells have minimal response (Figure 4D-E; supplemental Figure 8B). Together, the data demonstrate that exMito activate APCs to express both surface and secreted proteins that are predicted to lead to greater T-cell activation, differentiation, and expansion.
exMito activates APCs in vitro. T-cell–depleted BALB/c splenocytes were treated with 4 μg or 12 μg of mitochondria for 24 hours; cells were stained with (A) CD86 and (B) MHC-II antibodies, respectively. A representative histogram and the calculated MFI analyzed by flow cytometry is shown (n = 3). (C) Changes in cytokine levels in cell culture supernatants between control and 4 μg of mitochondria-treated splenocytes is shown in volcano plot. Fold change relative to control demonstrated on x-axis and inverse log P value demonstrated on y-axis. Chemokines and cytokines highlighted in red were noted to be significantly different (n = 6). (D-E) The impact of exMito on different APC subpopulations including B cell, macrophages/monocytes, and dendritic cells were further characterized (gating protocol described in supplemental Figure 8B). Expression of (D) CD86 and (E) MHC-II quantified as fold change in MFI is shown (n = 4-8). ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001; n, number of independent experiments.
exMito activates APCs in vitro. T-cell–depleted BALB/c splenocytes were treated with 4 μg or 12 μg of mitochondria for 24 hours; cells were stained with (A) CD86 and (B) MHC-II antibodies, respectively. A representative histogram and the calculated MFI analyzed by flow cytometry is shown (n = 3). (C) Changes in cytokine levels in cell culture supernatants between control and 4 μg of mitochondria-treated splenocytes is shown in volcano plot. Fold change relative to control demonstrated on x-axis and inverse log P value demonstrated on y-axis. Chemokines and cytokines highlighted in red were noted to be significantly different (n = 6). (D-E) The impact of exMito on different APC subpopulations including B cell, macrophages/monocytes, and dendritic cells were further characterized (gating protocol described in supplemental Figure 8B). Expression of (D) CD86 and (E) MHC-II quantified as fold change in MFI is shown (n = 4-8). ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001; n, number of independent experiments.
To test the functional consequences of APC activation by exMito on interacting T lymphocytes, we evaluated the donor T-cell activation and proliferation in the aforementioned MLR experiments. exMito-treated BALB/c stimulators significantly increased the surface expression of the early activation marker CD69 on the allogeneic responder T cells (Figure 5A). Similarly, exMito-treated stimulator splenocytes increased proliferation of responder T cells in comparison with untreated stimulators (Figure 5B). Further characterization of the T-cell populations at 48 hours of MLR demonstrated that exMito-treated splenocytes increased CD25 expression on both CD4+ and CD8+ T cells (Figure 5C). However, no significant differences were observed at 48 hours between the groups in the differentiation of CD4+ and CD8+ T cells to central memory and effector cell populations as analyzed by the expression of CD44 and CD62L (Figure 5D). Additionally, exMito-treated stimulators did not alter the abundance of regulatory T cells in culture (supplemental Figure 9) indicating that the increased proliferation of conventional T cells was not due to a reduction in regulatory T-cell abundance. Altogether, these data indicate that exMito stimulation of recipient APCs leads to increased activation donor T cells in vitro, an effect that was predicted to exacerbate GVHD in vivo.
exMito treated APCs increase allogeneic T-cell activation and proliferation in MLR. In vitro MLR between C57BL/6J responder T cells and BALB/c T-cell–depleted splenocytes was set up as described in “Methods.” (A) Cells were stained with a CD69 antibody and representative dot plots for the expression of CD69 on green fluorescent protein (GFP)-expressing responder T cells after 48 hours of MLR (S/R: 0.5 to 1; left) and the mean percentage CD69+ T cells (right) are shown. (B) Representative histograms after 120 hours of MLR with the indicated treatments showing the percentage of proliferate responder T cells (left) and relative proliferation index (RPI) calculated using the equation: RPI = (stimulated T cells with or without mitochondrial treatment − nonstimulated T cells)/stimulated T cells without mitochondrial treatment (right) is shown (n = 3). (C) CD25 expression on CD4+ and CD8+ allogeneic T cells after 48 hours presented as MFI relative fold change (n = 6). (D) Percent of naïve (CD44−CD62L+), central memory (CM; CD44+CD62L+), and effector (CD44+CD62L−) CD4+ and CD8+ T cells after 48 hours in a MLR (n = 4-6). ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001; n, number of independent experiments. S/R, Stimulator/Responder.
exMito treated APCs increase allogeneic T-cell activation and proliferation in MLR. In vitro MLR between C57BL/6J responder T cells and BALB/c T-cell–depleted splenocytes was set up as described in “Methods.” (A) Cells were stained with a CD69 antibody and representative dot plots for the expression of CD69 on green fluorescent protein (GFP)-expressing responder T cells after 48 hours of MLR (S/R: 0.5 to 1; left) and the mean percentage CD69+ T cells (right) are shown. (B) Representative histograms after 120 hours of MLR with the indicated treatments showing the percentage of proliferate responder T cells (left) and relative proliferation index (RPI) calculated using the equation: RPI = (stimulated T cells with or without mitochondrial treatment − nonstimulated T cells)/stimulated T cells without mitochondrial treatment (right) is shown (n = 3). (C) CD25 expression on CD4+ and CD8+ allogeneic T cells after 48 hours presented as MFI relative fold change (n = 6). (D) Percent of naïve (CD44−CD62L+), central memory (CM; CD44+CD62L+), and effector (CD44+CD62L−) CD4+ and CD8+ T cells after 48 hours in a MLR (n = 4-6). ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001; n, number of independent experiments. S/R, Stimulator/Responder.
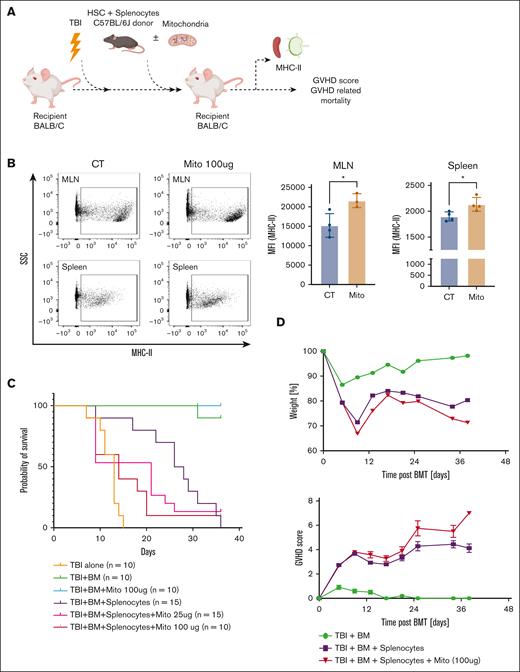
To validate our in vitro findings and determine the impact of exMito on incidence and severity of aGVHD, we used a well-characterized aGVHD model18 in which T-cell–depleted BM cells (5 × 106 cells per mouse) and splenocytes (4 × 106 cells per mouse) from C57BL6/J donors were transplanted into irradiated BALB/c recipient mice, which received increasing doses of damaged mitochondria (vehicle control, or 25 or 100 μg; Figure 6A). Dosing with isolated exMito in addition to TBI significantly increased the abundance of circulating cell-free mitochondria in comparison with mice that received TBI alone (supplemental Figure 10). Flow cytometric analysis at day 3 after transplantation revealed an increased expression of MHC-II in recipient cells isolated from the spleen and mesenteric lymph nodes of mice that were treated with mitochondria, confirming the observed effect of exMito on isolated splenocytes in vitro (Figure 6B). BALB/c mice that received transplantation with HSCs and splenocytes had a median survival of 31 days, which was worsened when cotreated with exMito, (median survival; BM + splenocyte + mitochondria (25 μg) = 21 days, P = .0042; BM + splenocyte + mitochondria (100 μg) = 14 days, P = .0014; Figure 6C-D). To confirm that the accelerated mortality after treatment with exMito is GVHD specific, we transplanted irradiated BALB/c recipient mice with T-cell–depleted BM cells (5 × 106 cells per mouse) from C57BL6/J donors with and without damaged mitochondria (100 μg) but without donor splenocytes. In the absence of donor splenocytes, exMito did not significantly increase mortality (Figure 6C). These findings, along with the MLR results (Figure 5A-C), suggest that the impact of damaged exMito is dependent on the presence of alloreactive T cells, which are necessary for GVHD.
exMito aggravate GVHD-induced mortality. HSCs from the BM (5 × 106 cells) and splenocyte (4 × 106 cells) from C57BL6/J congenic mice expressing CD45.1+ allele were transplanted into BALB/c CD45.2+ mice after TBI conditioning to model aGVHD. At the time of transplantation, the indicated doses of mitochondria were given along with the BM. (A) Graphical representation of the experimental setup. (B) Isolated recipient CD45.2+ cells from the spleen and mesenteric lymph nodes (MLNs) were analyzed for the expression of MHC-II. Donor cells were excluded from analysis using an antibody against CD45.1 Representative dot plots (left) and MFI (right) shown as bar graphs (Mito: mitochondria, 100 μg). (C) Survival curve and (D) illness severity, including weight loss, and GVHD scores. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001; n, number of animals.
exMito aggravate GVHD-induced mortality. HSCs from the BM (5 × 106 cells) and splenocyte (4 × 106 cells) from C57BL6/J congenic mice expressing CD45.1+ allele were transplanted into BALB/c CD45.2+ mice after TBI conditioning to model aGVHD. At the time of transplantation, the indicated doses of mitochondria were given along with the BM. (A) Graphical representation of the experimental setup. (B) Isolated recipient CD45.2+ cells from the spleen and mesenteric lymph nodes (MLNs) were analyzed for the expression of MHC-II. Donor cells were excluded from analysis using an antibody against CD45.1 Representative dot plots (left) and MFI (right) shown as bar graphs (Mito: mitochondria, 100 μg). (C) Survival curve and (D) illness severity, including weight loss, and GVHD scores. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001; n, number of animals.
Association of increased circulating mitochondria with conditioning and GVHD in children undergoing HSCT
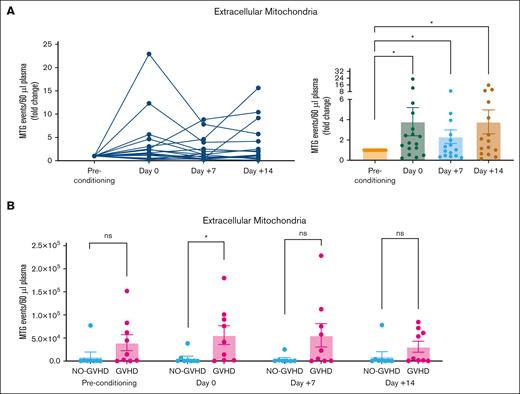
To validate the clinical relevance of our in vitro and in vivo findings, we evaluated changes in exMito content from platelet-depleted plasma samples of pediatric patients undergoing HSCT. Transplant indications for the cohort comprised hematologic cancers, nonhematologic cancers, and BM failure syndromes requiring diverse therapies before initiation of HSCT (Table 1). When stratified by incidence of GVHD, we did not observe any significant differences in patient demographics, indication for transplant, HLA molecular typing, or GVHD prophylaxis (Table 1). We observed an overall increase in exMito abundance after conditioning, which was most pronounced in the subset of patients who received TBI as a component of their conditioning regimen, (MTG-positive events per 60 μL plasma fold change from baseline; day 0 = 4.03 ± 1.5, P = .047; day 7 = 2.43 ± 0.71, P = .043; and day 14 = 3.7± 1.3, P = .016; Figure 7A). Compared with those who did not develop GVHD, the patients who later developed GVHD had a sixfold higher level of exMito content on day 0 (day of transplantation; MTG-positive events per 60 μL plasma; no GVHD = 6023 ± 13 239 vs GVHD = 56 222 ± 61 131, P = .04; Figure 7B).
Demographics for the cohort of patients who received HSCT; including primary diagnosis, HLA molecular typing, transplant conditioning regimen, and GVHD prophylaxis
| . | Total . | GVHD . | No GVHD . | P . |
|---|---|---|---|---|
| No. | 17 | 9 | 8 | |
| Age at transplant, y | ||||
| Median (q25-q75) | 11 (7.5-14.5) | 11 (6.5-14.5) | 13 (8.7-16.25) | .41 |
| Sex (M, %) | 8 (47.1) | 4 (44) | 4 (50) | .97 |
| Primary diagnosis | ||||
| ALL (%) | 6 (35) | 5 (55.6) | 1 (12.5) | .19 |
| AML (%) | 5 (29.5) | 2 (22.2) | 3 (37.5) | .45 |
| MDS (%) | 2 (11.7) | 1 (11.1) | 1 (12.5) | .99 |
| Severe aplastic anemia (%) | 2 (11.7) | 0 (0) | 2 (25) | .17 |
| Other (%) | 2 (11.7) | 1 (11.1) | 1 (12.5) | .93 |
| HLA molecular typing | ||||
| Matched related | 3 (17.6) | 3 (33.3) | 0 (0) | .16 |
| Matched unrelated | 0 (0) | 0 (0) | 0 (0) | NA |
| Mismatched related | 9 (53) | 4 (44.4) | 5 (62.5) | .89 |
| Mismatched unrelated | 5 (29.4) | 2 (22.2) | 3 (37.5) | .76 |
| Patient CMV serostatus | ||||
| Positive (%) | 2 (11.7) | 2 (22.2) | 0 (0) | .39 |
| GVHD prophylaxis | ||||
| αβ T-cell depletion | 8 (47.1) | 4 (44.4) | 4 (50) | .97 |
| Tacro, MTX | 3 (17.6) | 3 (33.3) | 0 | .21 |
| Tacro, MMF | 5 (29.4) | 2 (22.2) | 3 (37.5) | .22 |
| CsA, Tacro, MMF | 1 (5.9) | 0 | 1 (12.5) | .58 |
| Conditioning regimen | ||||
| Low-dose TBI (≤4 Gy) | 7 | 3 (33.3) | 4 (50) | .8 |
| High-dose TBI (≥10 Gy) | 10 | 6 (66.6) | 4 (50) | .78 |
| rATG | 12 | 6 (66.6) | 6 (75) | .93 |
| Thiotepa/fludarabine | 9 | 5 (55.6) | 4 (50) | .58 |
| Melphalan/clofarabine | 2 | 2 (22.2) | 0 | NA |
| Busulfan | 1 | 1 (11.1) | 0 | NA |
| Fludarabine/cyclophosphamide | 3 | 0 | 3 (37.5) | NA |
| Melphalan/thiotepa/clofarabine | 1 | 0 | 1 (12.5) | NA |
| . | Total . | GVHD . | No GVHD . | P . |
|---|---|---|---|---|
| No. | 17 | 9 | 8 | |
| Age at transplant, y | ||||
| Median (q25-q75) | 11 (7.5-14.5) | 11 (6.5-14.5) | 13 (8.7-16.25) | .41 |
| Sex (M, %) | 8 (47.1) | 4 (44) | 4 (50) | .97 |
| Primary diagnosis | ||||
| ALL (%) | 6 (35) | 5 (55.6) | 1 (12.5) | .19 |
| AML (%) | 5 (29.5) | 2 (22.2) | 3 (37.5) | .45 |
| MDS (%) | 2 (11.7) | 1 (11.1) | 1 (12.5) | .99 |
| Severe aplastic anemia (%) | 2 (11.7) | 0 (0) | 2 (25) | .17 |
| Other (%) | 2 (11.7) | 1 (11.1) | 1 (12.5) | .93 |
| HLA molecular typing | ||||
| Matched related | 3 (17.6) | 3 (33.3) | 0 (0) | .16 |
| Matched unrelated | 0 (0) | 0 (0) | 0 (0) | NA |
| Mismatched related | 9 (53) | 4 (44.4) | 5 (62.5) | .89 |
| Mismatched unrelated | 5 (29.4) | 2 (22.2) | 3 (37.5) | .76 |
| Patient CMV serostatus | ||||
| Positive (%) | 2 (11.7) | 2 (22.2) | 0 (0) | .39 |
| GVHD prophylaxis | ||||
| αβ T-cell depletion | 8 (47.1) | 4 (44.4) | 4 (50) | .97 |
| Tacro, MTX | 3 (17.6) | 3 (33.3) | 0 | .21 |
| Tacro, MMF | 5 (29.4) | 2 (22.2) | 3 (37.5) | .22 |
| CsA, Tacro, MMF | 1 (5.9) | 0 | 1 (12.5) | .58 |
| Conditioning regimen | ||||
| Low-dose TBI (≤4 Gy) | 7 | 3 (33.3) | 4 (50) | .8 |
| High-dose TBI (≥10 Gy) | 10 | 6 (66.6) | 4 (50) | .78 |
| rATG | 12 | 6 (66.6) | 6 (75) | .93 |
| Thiotepa/fludarabine | 9 | 5 (55.6) | 4 (50) | .58 |
| Melphalan/clofarabine | 2 | 2 (22.2) | 0 | NA |
| Busulfan | 1 | 1 (11.1) | 0 | NA |
| Fludarabine/cyclophosphamide | 3 | 0 | 3 (37.5) | NA |
| Melphalan/thiotepa/clofarabine | 1 | 0 | 1 (12.5) | NA |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CMV, cytomegalovirus; CsA, cyclosporin A; M, male; MMF, Mycophenolate mofetil; MDS, myelodysplastic syndrome; MTX, Methotrexate; rATG, rabbit anti-thymocyte globulin; q25-q75, (25th-75th quartile); Tacro, Tacrolimus.
TBI conditioning increases circulating exMito in patients undergoing HSCT. Serial samples collected from patients undergoing HSCT before initiation of conditioning regimen (preconditioning), day of transplant (day 0), and days 7 (+7) and 14 (+14) after HSCT was evaluated for abundance of exMito in circulation. (A) MTG-positive events represented as fold induction relative to the preconditioning sample of each patient. (B) Comparison of mitochondrial levels in samples collected at the indicated times between patients with and without GVHD; ∗P < .05.
TBI conditioning increases circulating exMito in patients undergoing HSCT. Serial samples collected from patients undergoing HSCT before initiation of conditioning regimen (preconditioning), day of transplant (day 0), and days 7 (+7) and 14 (+14) after HSCT was evaluated for abundance of exMito in circulation. (A) MTG-positive events represented as fold induction relative to the preconditioning sample of each patient. (B) Comparison of mitochondrial levels in samples collected at the indicated times between patients with and without GVHD; ∗P < .05.
Discussion
Although allogeneic T cells have been long understood to be a prerequisite for GVHD, the initiation of GVHD depends on tissue damage, typically resulting from the cytotoxic therapies given as pretransplant conditioning to ensure HSC engraftment.1-4 Conditioning-induced damage to mucosa, including the intestine, allows translocation of bacteria that express pathogen-associated molecular patterns while simultaneously leading to the release of endogenously derived DAMPs.6,7 Whereas previous studies have linked GVHD severity with the release of these danger signals, the role of exMito in the pathogenesis of GVHD has not been previously characterized. Here, we demonstrate that clinically relevant doses of radiation and chemotherapy, which are used as pre-HSCT conditioning, cause mitochondrial dysfunction and alteration in mitochondrial dynamics, resulting in exMito release both in vitro (Figures 1 and 3A) and in vivo (Figures 2 and 3D). The presence of circulating exMito exacerbates GVHD-associated mortality (Figures 4-6). The clinical relevance of these observations is highlighted by the demonstration that TBI-based conditioning increased the level of circulating cell-free mitochondria in patients undergoing HSCT and correlated with the incidence of aGVHD after transplant (Figure 7).
Immunomodulatory role of extracellular released mitochondria
Mitochondria contain byproducts that can induce immune cell activation via multiple mechanisms, including but not limited to, mitochondrial DNA–mediated Toll-like receptor 9 signaling, N-formyl peptide–mediated receptor activation, cardiolipin-mediated proinflammatory activation, and ATP-mediated signaling through purinergic receptors.11,29,30 Specific to GVHD, Koehn et al demonstrated that extracellular ATP exacerbates GVHD-associated mortality in preclinical models by impairing the anti-inflammatory effect of donor myeloid-derived suppressor cells.31 Our results demonstrate that release of mitochondria to the extracellular space can also stimulate host APCs (Figure 4), resulting in activation and proliferation of donor T cells (Figure 5).
A wide array of cytokines, which are known contributors and/or markers of GVHD,21-28 were induced upon exMito stimulation (Figure 4C; supplemental Table 1). There was also upregulation of cytokines or chemokines, such as IL-13, Colony-stimulating factor (CSF)-1, and C-X-C motif chemokine ligand 5 (CXCL5), which may have mixed roles in GVHD pathogenesis.32-36 Some molecules, such as IL-19, have not been specifically studied in GVHD but are associated with autoimmune diseases such as psoriasis37 (Figure 4C; supplemental Table 1). Much of our current knowledge on the immune stimulation by mitochondria comes from studying specific mitochondrial byproducts (mitochondrial DAMPs) in isolation. With the recent discoveries that intact cell-free mitochondria can be found in circulation,38,39 future multiomic and single-cell approaches are needed to comprehensively characterize the many potential immunostimulatory mitochondrial molecules that are released in the circulation and their impact on the immune phenotypes under physiologic and pathologic conditions.
Mitochondrial dynamics and extracellular release
Mitochondrial dynamics, that is, the balance between mitochondrial fusion, fission, biogenesis, and mitophagy, play an essential role in mitochondrial quality control.40 Once damaged, mitochondria are segregated from the network by dynamin-related protein 1–dependent mitochondrial fission and subsequently, removed by mitochondrial autophagy.41 Similar to our findings, previous studies have demonstrated mitochondrial damage, represented by early impairment in both oxidative phosphorylation and glycolysis shortly after irradiation (Figure 1; supplemental Figure 1),42 with subsequent increase in glycolysis as a compensatory mechanism. We demonstrate that this impairment in mitochondrial function is coupled with alterations in mitochondrial dynamics, in particular, increased mitochondrial fission (Figure 2). However, we did not observe changes in mitophagy or accumulation of intracellular mitochondrial content after conditioning (supplemental Figure 3). Together, this suggests that fragmented mitochondria might be cleared by an alternate mechanism.
Recent publications have suggested that extracellular release of damaged mitochondria is an alternate mechanism by which cells maintain quality control, especially when canonical mechanisms of mitochondrial dynamics are impaired.13,43 Our data demonstrating an increase in damaged exMito after TBI independent of cell death (Figure 3), suggest that this mechanism plays an important role in the clearance of damaged mitochondria after conditioning. The underlying mechanism for the extracellular release of mitochondria has been a topic of intense investigation in recent years. Although the consensus is that cell-free mitochondria are a byproduct of intracellular mitochondrial quality control, various mechanisms leading to their generation and release have been proposed.43,44 Work from our group has shown that excessive mitochondrial fission is associated with exMito release in preclinical models of sepsis and neuroinflammation.15,16 Independently, other studies have reported that impairments in the classical autophagy pathway contribute to the release of mitochondria.13,43 Hence, we propose that pharmacologic agents, which block excessive mitochondrial fission or induce mitophagy, might abrogate the extracellular release of mitochondria and reduce GVHD. Supporting this notion, a recent study reported that baicalin, a flavonoid purified from the plant Scutellaria baicalensis, induced autophagy and preserved mitochondrial architecture in the intestinal epithelium and reduced intestinal aGVHD.45 However, the extracellular release of mitochondria was not explored in this study and requires future investigation.
Although our results are focused on mitochondrial damage and extracellular release in intestinal epithelial cells, it is highly likely that other cell types in various end-organs are sensitive to conditioning-mediated mitochondrial damage and therefore contribute to the exMito content in the circulation. This is supported by our observations of impaired bioenergetics and oxidative stress in both HEK cells and HUVECs after irradiation (supplemental Figure 11).
In conclusion, this study introduces a new pathogenic mechanism for GVHD and suggests potential novel approaches to GVHD prevention, raising several questions that need to be addressed in future investigations. Our results have shown that exMito increase the incidence and severity of aGVHD. However, the molecular mechanism of exMito-mediated host APC activation require further investigation. Additionally, although our results suggest that both CD4+ and CD8+ T cells are activated in response to exMito (Figure 5), further characterization of the T-cell subsets, in both murine and human models would further the understanding of this novel mechanism of GVHD pathogenesis. Finally, our clinical observations need to be validated in a larger cohort of patients to determine the effects of underlying diagnosis and conditioning methods on exMito levels and GVHD.
Current GVHD prophylaxis strategies consist of globally immune suppressive drugs that are prone to complications including opportunistic infections, viral reactivation, as well as engraftment failure. Future studies aiming to block the release of mitochondria into the extracellular milieu or scavenge existing exMito from circulation as a prophylaxis before HSCT are warranted to determine whether exMito could serve as a clinically useful target for prevention of GVHD, possibly without risk of immune suppression.
Authorship
Contribution: V.V., H.Y., K.W., and B.H. contributed to manuscript preparation; V.V., K.W., and B.H. generated the hypothesis; R.S.N., A.B., and D.M.-R. aided with experimental design; V.V., H.Y., B.H., J.K.L., K.A.P., A.E., K.P., and J.B. conducted the in vitro and animal experiments and analysis; V.V., R.V.P., G.B., and I.L. collected and conducted the analysis on the clinical samples; N.P.O. preformed the blinded imaging analysis; and all authors edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bereketeab Haileselassie, Division of Critical Care Medicine, Department of Pediatrics, Stanford University School of Medicine, 725 Welch Rd, Palo Alto, Stanford, CA; email: bhailes3@stanford.edu.
References
Author notes
V.V. and H.Y. are joint first authors.
Data are available on request from the corresponding author, Bereketeab Haileselassie (bhailes3@stanford.edu).
The full-text version of this article contains a data supplement.