TO THE EDITOR:
D-dimer is often increased in patients with or at risk of venous thromboembolism (VTE)1 and is decreased after starting anticoagulation.2 Patients with D-dimer above cutoff after stopping anticoagulation are at risk of recurrent VTE.3,4 Accordingly, high D-dimer is employed as a biomarker to guide decisions on extended anticoagulation with vitamin K antagonists (VKAs).5,6 This concept was introduced by the Prolong prospective trial5 that included patients with unprovoked VTE, who had been on VKAs for at least 3 months. D-dimer was measured 1 month after stopping anticoagulation. Patients with D-dimer below the cutoff mark stopped anticoagulation and those with D-dimer above cutoff were randomized to resume or stop anticoagulation. Patients were followed up for 1.5 years to record recurrent VTE. The analysis showed that patients with increased D-dimer who stopped VKAs, had increased rates of recurrent VTE when compared with patients resuming anticoagulation. Hence, the study concluded that patients presenting with D-dimer above cutoff after stopping VKAs are candidates for extended anticoagulation.5 Subsequent studies debated on whether D-dimer below cutoff can be taken as a biomarker to safely stop anticoagulation.7,8
Recently, a similar protocol (Apidulcis)8 was applied to patients, who after unprovoked VTE and treatment with any anticoagulant for at least 12 months, underwent D-dimer measurement during anticoagulation. If D-dimer level was below the cutoff, measurements were performed 15, 30, and 60 days after stopping anticoagulation. Patients with D-dimer levels below cutoff stopped anticoagulation and patients with the first D-dimer levels above cutoff were offered apixaban dose (2.5 mg, twice daily). Patients were followed up to 18 months to record recurrent VTE.7 The proportion of patients on direct oral anticoagulants (DOACs) included in the study was 91.3%. The study was prematurely interrupted because of excess primary outcomes in patients with D-dimer below cutoff who stopped anticoagulation. Hence, at variance with the Prolong, Apidulcis did not confirm the value of D-dimer measurement to stratify patients for extended anticoagulation.8
A reason that may account for the discrepancy could be the fact that Apidulcis was conducted during the COVID-19 pandemic. Hence, patients included in the study might have had increased VTE risk because of lockdown that limited physical activity.9 Herein, we propose another possible explanation. It could be that the type of anticoagulation (VKAs vs DOACs) may impact differently on D-dimer levels achieved during anticoagulation.
Comparisons of D-dimer levels for patients on VKAs vs those on DOACs are scanty. Furthermore, there is little information on comparisons of D-dimer for patients taking different DOACs. Finally, most studies investigated D-dimer for patients on DOACs either at peak or trough.
With this background, we undertook investigations aimed at comparing D-dimer during chronic anticoagulation for patients on VKAs vs DOACs. D-dimer for patients on DOACs was tested at peak and trough. The study was approved by the local ethics committee and all patients gave informed consent.
A total of 131 patients on DOACs and 33 on VKAs (Table 1) treated for a first VTE (80%) or atrial fibrillation, were included in a cross-sectional study. The patients’ age was comparable for all categories (median, 65-72 years), except for rivaroxaban (median, 55 years). However, linear regression analysis showed no significant association between age and D-dimer. VKA dosages were adjusted and aimed at an International normalized ratio (INR) 2.0-3.0 and DOACs were prescribed at dosages consistent with patients’ characteristics. Blood was collected at the time of regular INR monitoring (VKAs) or periodic clinical checks (DOACs). Samples were collected from patients during chronic anticoagulation (ie, at least 2 months from starting DOACs or 3 months from VKAs). Patients on DOACs were instructed to come to the clinic before taking the next dose. Blood was designated as trough. Samples were collected again from patients 2 hours after taking the drug (peak).
Median (IQR) D-dimer levels for patients on VKAs compared with those on DOACs
| Drugs . | D-dimer (μg/L) . | ||||
|---|---|---|---|---|---|
| VKAs (n = 33) . | Apixaban (n = 37) . | Rivaroxaban (n = 34) . | Dabigatran (n = 30) . | Edoxaban (n = 30) . | |
| Trough | 219 (174-385)† | 290 (163-383)†† | 201 (98-322) | 220 (158-449)† | |
| Peak | 225 (158-343)∗,† | 263 (200-430)†† | 169 (140-296) | 201 (152-445)∗,† | |
| VKAs | 149 (117-270) | ||||
| Drugs . | D-dimer (μg/L) . | ||||
|---|---|---|---|---|---|
| VKAs (n = 33) . | Apixaban (n = 37) . | Rivaroxaban (n = 34) . | Dabigatran (n = 30) . | Edoxaban (n = 30) . | |
| Trough | 219 (174-385)† | 290 (163-383)†† | 201 (98-322) | 220 (158-449)† | |
| Peak | 225 (158-343)∗,† | 263 (200-430)†† | 169 (140-296) | 201 (152-445)∗,† | |
| VKAs | 149 (117-270) | ||||
Blood samples were collected in 1:10 volume of trisodium citrate, centrifuged for 20 minutes at 3000g and plasma aliquoted in capped plastic tubes, immediately frozen and stored at −70°C for later testing that was performed within 6 months. To limit methodological variability, the same number of plasma samples from patients on different types of anticoagulants was included in each session. D-dimer was measured with HSD-dimer on ACLTOP (Werfen Bedford, MA).
IQR, interquartile range.
P < .05, trough-vs-peak (paired data [Wilcoxon].
P < .05.
P < .01, trough or peak-vs-VKAs [unpaired data, Mann-Whitney]).
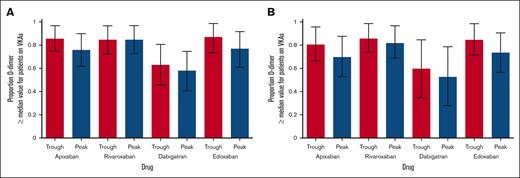
Patients on VKAs had lower median D-dimer levels than any of the DOACs (P < .05; Table 1). The median differences between VKAs and DOACs were the smallest for dabigatran and the greatest for rivaroxaban. When analyses were restricted to DOACs, there were marginal differences in peak vs trough for the 4 drugs (less pronounced for apixaban and edoxaban, and more for rivaroxaban and dabigatran). The proportion of patients on DOACs having higher D-dimer levels than the median value of patients on VKAs was considerably higher than the expected 0.50 (Figure 1). There was no correlation between INR and D-dimer levels or the concentration of DOACs and D-dimers.
Proportion of patients on DOACs who had D-dimer levels higher than the median value observed for patients on VKAs. (A) All patients (treated for atrial fibrillation + venous thromboembolism). (B) Patients treated for venous thromboembolism only. Vertical bars represent 95% confidence interval (CI) calculated by the one-sample proportion procedure.
Proportion of patients on DOACs who had D-dimer levels higher than the median value observed for patients on VKAs. (A) All patients (treated for atrial fibrillation + venous thromboembolism). (B) Patients treated for venous thromboembolism only. Vertical bars represent 95% confidence interval (CI) calculated by the one-sample proportion procedure.
For the purpose of this study, the condition is set that D-dimer can be taken as an index of hypocoagulability achieved during anticoagulation. This is based on the following pathophysiological considerations. Anticoagulation may result in clinical hypocoagulability, detectable as an increased bleeding risk and/or biochemical hypocoagulability, detectable by laboratory methods. D-dimer increases in hypercoagulability1 and decreases in hypocoagulability.2 Hence, we assume that D-dimer is a reliable biochemical marker of hypocoagulability achieved during anticoagulation. Accordingly, the results of the study allow for the following considerations:
The results indicate that the level of hypocoagulability achieved following drug intake is more pronounced for VKAs than DOACs. Whether this effect pertains to the relative efficacy/safety of the 2 types of drugs, remains undetermined. Interestingly, clinical trials reported that the risk of intracranial bleeding is higher for patients on VKAs than DOACs.10 It is tempting to speculate that the higher risk observed for patients on VKAs might be due to a greater degree of hypocoagulability achieved in patients on VKAs, as shown by the relative levels of D-dimer.
D-dimer for patients on DOACs was in general greater for trough than peak, but differences were marginal. It is debatable whether DOACs are effective/safe during their entire course of daily treatment, when levels are higher or lower in the period close to or far from peak. The results of the study would be reassuring, as there were marginal differences in the hypocoagulability achieved in these patients during the course of daily treatment.
There are no obvious differences between peak vs trough for the drugs taken once daily or twice daily, suggesting that DOAC efficacy/safety is unlikely to be related to numbers of daily administrations.
There are differences between D-dimer observed for different DOACs. The hypocoagulability achieved during anticoagulation is more pronounced for dabigatran and edoxaban than for rivaroxaban and apixaban. To our knowledge, there is no head-to-head comparison between DOACs with respect to efficacy/safety. Hence, no inference can be drawn between the association of D-dimer and efficacy/safety of different DOACs.
The study confirms and extends previous findings showing that VKAs are more effective than DOACs in achieving hypocoagulability as indicated by D-dimer levels in patients on anticoagulation.11,12 Another study reported no differences in D-dimer levels for patients with acute pulmonary embolism on different DOACs.13 The setting and the acute-phase of the disease may account for the different results.
The explanations for the differences between VKA and DOAC D-dimer levels are unknown and probably pertain to the mode of action of these anticoagulants. Perhaps, VKAs are more effective than DOACs to induce hypocoagulability because, in contrast to DOACs, VKAs inhibit more than 1 coagulation factor. Other explanations could pertain to the relatively different half-lives of D-dimers compared with DOACs or D-dimers compared with VKAs.
Based on these results, one might speculate that the poor value of D-dimer to make decision on extended anticoagulation in patients on DOACs as observed in Apidulcis8 is due to the poor sensitivity of this biomarker to detect biochemical signs of hypocoagulability achieved by DOACs. Whatever the reason, the results of this study and previous studies indicate that D-dimer is less effective to stratify patients for extended anticoagulation when they are on DOACs. Large clinical trials with a design similar to that of the Prolong and Apidulcis are warranted to resolve this issue definitively.
Acknowledgment: This work was partially supported by the Italian Ministry of Health (Ricerca Corrente 2023).
Contribution: A.T. conceived the study, reviewed data, and wrote the manuscript; M.C. and B.S. enrolled patients; E.S. and M.C. performed laboratory analyses; and all the other authors reviewed the data and approved the final version of the manuscript.
Conflict-of-interest disclosure: A.T. received speakers’ fees from Stago, Roche, BioMarin, and Werfen. P.A. received honoraria for participating as a speaker at educational meetings organized by Sanofi. The remaining authors declare no competing financial interests.
Correspondence: Armando Tripodi, IRCCS Ca’ Granda Maggiore Hospital Policlinico Foundation, Angelo Bianchi Bonomi Hemophilia and Thrombosis Centre, Via Pace 9, 20122 Milan, Italy; email: armando.tripodi@unimi.it.
References
Author notes
Data are available on request from the corresponding author, Armando Tripodi (armando.tripodi@unimi.it).