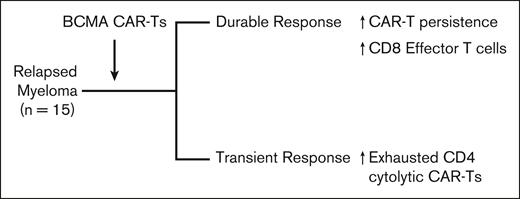
In this issue of Blood Advances, Ledergor et al1 describe the results of an elegant study using single-cell transcriptomics to study changes in immune cells in patients with multiple myeloma (MM) receiving chimeric antigen receptor (CAR) T cells (CAR-Ts) targeting B-cell maturation antigen (BCMA). Study population consisted of 15 patients with relapsed MM enrolled in clinical trials of 4 different CAR-Ts. In spite of these differences, patients with durable responses had greater persistence of infused CAR-Ts, whereas those with short-lived responses had increased frequencies of exhausted cytotoxic CD4+ CAR-Ts (see figure). These findings illustrate the power of single-cell approaches and provide insights into how the biology of CAR-Ts may affect outcomes in MM.
Correlates of outcome after BCMA CAR-T therapy in MM in the study by Ledergor et al.
Correlates of outcome after BCMA CAR-T therapy in MM in the study by Ledergor et al.
CAR-Ts have led to high rates of remissions in patients with MM. However, most patients still succumb to recurrent disease, underscoring the unmet need to improve the durability of response. Deeper understanding of the mechanisms that underlie durable regression is needed to achieve this goal. Data from Ledergor et al nicely complement that from other studies to illustrate the emerging picture that CAR-T therapy leads to dynamic cross talk between infused CAR-Ts, endogenous T cells, tumor cells, as well as other cells in the tumor microenvironment.2-4 Accordingly, outcomes after CAR-Ts may be affected by features intrinsic to CAR-Ts/infusion product, endogenous T cells, as well as those extrinsic to T cells, including cells in the tumor microenvironment, features of tumor cells, as well as host characteristics.5 Most of the attention has been on the properties of CAR-Ts and tumor cells. In some instances, the presence of less-differentiated T cells, CD4+ cytolytic CAR-Ts, or absence of CAR-Tregs and less exhausted T cells has been linked to outcome.6 Fitness of CAR-Ts may potentially affect their initial expansion as well as persistence. Infusion of CAR-Ts also leads to the expansion of endogenous T cells, and therefore, the properties of endogenous T-cell response, including T-cell receptor diversity, have also been linked to outcomes.2 Tumors seem to downregulate BCMA after exposure to BCMA CAR-Ts, prompting strategies to enhance BCMA expression. Persisting tumor cells that lack BCMA at these early time points have been correlated with early progression. In rare instances, antigen loss via biallelic loss of BCMA in tumor cells was linked to CAR-T resistance.6 Insights from the studies discussed above are already affecting the next generation of CAR-T studies, including targeting CAR-Ts earlier in the disease course to harness more “fit” T cells, altering manufacturing to infuse less-differentiated T cells, and multiepitope targeting of tumors.6
In addition to T cells and tumors, other cell types and immune-suppressive elements in the tumor microenvironment may also affect outcome. In this regard, Ledergor et al suggest an inhibitory role for myeloid cells in the tumor microenvironment, potentially mediated by the expression of transforming growth factor β. Other studies have similarly correlated a balance between myeloid cells expressing B-cell activating factor and dendritic cells with outcome.2 A deeper understanding of how features of tumor microenvironment and in particular extramedullary lesions affect CAR-T therapy may permit future rational combinations to improve outcomes.5 Adding to this complexity, it is possible and even likely that the relative importance of these factors may evolve over time, supporting a need for ongoing reassessment to improve outcomes. Biology of CAR-Ts in vivo and specific correlates of response may also depend in particular on the nature of the specific CAR-T product. A prominent example of this relates to major differences in CAR-T persistence for the 2 BCMA CAR-T products currently approved for MM.3,4 In this regard, clinical results with ciltacabtagene autoleucel are impressive in spite of a lack of CAR-T persistence, again emphasizing the potential role for endogenous immunity.
Studies by Ledergor et al illustrate that single-cell transcriptomics of blood or bone marrow aspirates can serve as a powerful new tool to gain insights into the potential mechanisms of response and resistance to CAR-T therapy. However, it is also useful to be aware of the potential limitations when interpreting data from these studies.7 It will be essential to validate transcriptome-based studies with functional data because gene signatures may not effectively predict functional properties of immune cells, particularly for features such as T-cell exhaustion. Analysis of data generated in small cohorts also carries potential pitfalls.7 For example, pooling of cells from several individuals when comparing gene expression carries the risk of false positives.7 We now have increasing appreciation of the spatial complexity of myeloma,8 which may affect infiltration and persistence of CAR-Ts, as well as its interactions with other cells in the tumor microenvironment. Finally, many aspects of the host and tumor, including the nature of systemic immunity, tumor genetics, and even environmental influences including the microbiome, may have an impact on outcomes after CAR-T therapy in MM.5 CAR-Ts were developed as a simple yet elegant tool to achieve T-cell redirection. Integrating this simplicity with the complexity of human immune response and cancer biology may be needed to optimize outcomes with this therapy.
Conflict-of-interest disclosure: M.V.D. has served on advisory boards for Lava Therapeutics, Bristol Myers Squibb, Sanofi, and Janssen and has no conflicts specific to this manuscript.