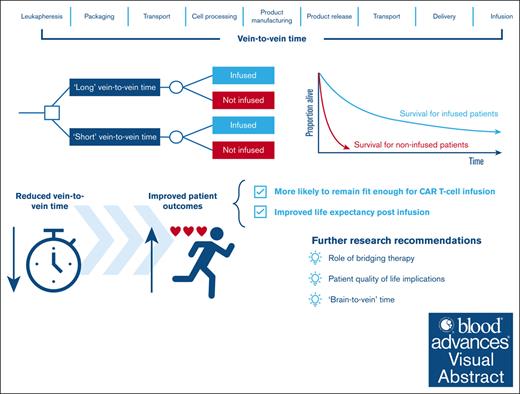
Key Points
This modeling study shows that V2VT is an important predictor of outcomes, and reducing V2VT can substantially improve life expectancy by up to 3.2 years, saving up to 18 875 lives in the US
More real-world data are needed on long-term outcomes associated with varying V2VT.
Visual Abstract
Chimeric antigen receptor T-cell therapy (CAR T) has revolutionized the treatment of hematological cancers. Its production requires a complex logistical process, and the time from leukapheresis to patient infusion (known as the vein-to-vein time [V2VT]) can be long during which a patients clinical condition may deteriorate. This study was designed to estimate the benefits of reduced V2VT for third-line or later (3L+) relapsed/refractory large B-cell lymphoma (R/R LBCL) patients treated with CAR T. A mathematical model was developed to estimate the lifetime outcomes of a hypothetical cohort of patients who had either a long or short V2VT. Life-years (LYs), quality-adjusted LYs (QALYs), and costs were estimated. Scenario analyses were performed to assess the robustness of results to key assumptions. The results of the model show that reducing V2VT from 54 days (tisa-cel median V2VT; JULIET) to 24 days (axi-cel median V2VT; ZUMA-1) led to a 3.2-year gain in life expectancy (4.2 vs 7.7 LYs), and 2.4 additional QALYs (3.2 vs 5.6) per patient. Furthermore, a shorter V2VT was shown to be cost-effective under conventional willingness-to-pay thresholds in the United States. Results are driven by a higher infusion rate and a better efficacy of CAR T for those infused. Scenario analyses using a smaller difference in V2VT (24 vs 36 days) produced consistent results. Our study is the first to quantify lifetime V2VT-related outcomes for 3L+ R/R LBCL patients treated with CAR T utilizing currently available evidence. Shorter V2VTs led to improved outcomes, demonstrating the importance of timely infusion achievable by faster manufacturing times and optimization of hospital delivery.
Introduction
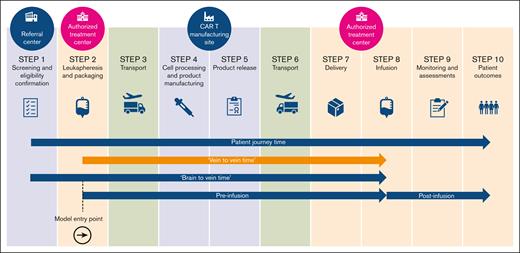
Chimeric antigen receptor T-cell therapies (CAR Ts) are those where genetically modified autologous T cells are programmed to express a CAR, to target and destroy cancer cells. They have revolutionized the treatment of certain hematological cancers.1 The production of CAR T cells requires a multistep process, including leukapheresis (ie, collection of white blood cells from the patient), manufacturing, bidirectional transport, and storage, before infusion.2Figure 1 depicts an overview of the patient journey undergoing CAR T.
Vein-to-vein time (V2VT), highlighted in the image, is observable and measured routinely in datasets and trials.3-5 While waiting for a CAR T-cell infusion, a patient’s condition may deteriorate; thus it is essential that the manufacturing process should be (1) rapid, because patients often have aggressive disease requiring prompt treatment; (2) robust and reproducible, because patients may be lymphopenic (ie, a lack of lymphocytes), and there may be variability in the starting leukapheresis material; and (3) reliable, to avoid the need to repeat leukapheresis.
Some patients who undergo leukapheresis ultimately may not receive a CAR T-cell infusion, highlighting the potential importance of minimizing avoidable delays in V2VT, for example, those owing to manufacturing failure and disease progression, for patient outcomes.6
Emergent research has attempted to identify a link between V2VT and short- to medium-term outcomes. Tully et al developed a discrete event simulation to investigate the relationship between CAR T wait times and 1-year mortality rate.7 Locke et al estimated the impact of V2VT on survival after axicabtagene ciloleucel treatment, using data collected in the Center for International Blood and Marrow Transplant Research registry.6 These 2 studies are currently the extent of the published research investigating the specific link between V2VT and long-term patient outcomes (ie, over a lifetime horizon). The estimation of long-term survival outcomes is important to fully understand the consequences of any potentially avoidable delays in V2VT.
This study aims to compare potential lifetime outcomes of a hypothetical cohort of relapsed/refractory (R/R) large B-cell lymphoma (LBCL) patients treated with CAR T at third-line or later (3L+) with differing V2VTs.
Methods
To accomplish the aim of this study, we estimated expected future life-years (LYs) and quality-adjusted LYs (QALYs) for 2 groups with different V2VTs being compared ("long" vs "short" V2VT; see “Data inputs” for more details) over a lifetime horizon using a mathematical model. These measures (LYs and QALYs) represent remaining life expectancy (ie, LYs) and remaining health-related quality of life adjusted LYs (ie, QALY). LYs and QALYs are measures of health (used by the Institute of Clinical and Economic Review [ICER] and others) to measure health benefit in health technology assessments. Importantly, for a life-extending treatment, it is necessary to estimate both over a lifetime horizon to fully understand potential implications, which requires extrapolation of incomplete survival data. To give a sense of economic as well as clinical implications, total lifetime cost implications of V2VT delays were also estimated.
Model overview
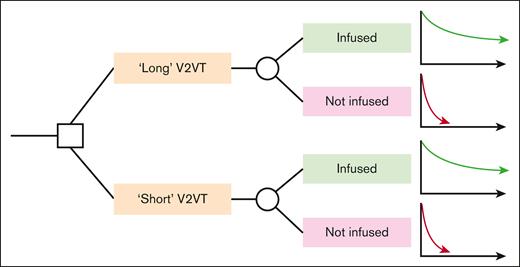
A cohort-level decision-analytic model was developed in Microsoft Excel to map the consequences associated with a long or short V2VT, for R/R LBCL patients intended to be treated with CAR T.8 A schematic of the model is provided in Figure 2.
Simple model schematic. A square node represents a decision node, whereas a circle node represents a probability node. Graphs represent modelled long-term survival functions for each sub-cohort.
Simple model schematic. A square node represents a decision node, whereas a circle node represents a probability node. Graphs represent modelled long-term survival functions for each sub-cohort.
A hypothetical cohort of eligible patients entered the model at leukapheresis and were assigned either a long or short V2VT. The probability of successful infusion was estimated as a function of V2VT, as described later; in this way the assigned V2VT determines the probability of infusion success. The subcohort predicted to be successfully infused in each case arm followed one survival projection, and those predicted to not be infused followed a different (poorer) survival projection. The extrapolation portion of the model is comparable with a typical partitioned survival analysis commonly used in health technology assessment for cancer treatments, though with only 2 health states: alive and dead.
Data inputs
The study model was established based on published clinical evidence for (1) V2VT from registrational studies of CAR T in R/R LBCL and (2) analyses of outcomes for similar patients in routine clinical practice, some of whom received CAR T. The analysis was built around evidence from these clinical studies, supplemented by targeted searches for cost, patient health–related quality of life and other data described throughout this section.
V2VT and infusion success
We used data from the following 3 pivotal clinical trials of populations with 3L+ R/R LBCL: (1) ZUMA-1: a phase 1/2 study of axicabtagene ciloleucel (Yescarta, axi-cel) in refractory LBCL (NCT02348216)4; (2) JULIET: a phase 2 study of tisagenlecleucel (Kymriah, tisa-cel) for patients with R/R diffuse LBCL (NCT02445248)3; (3) TRANSCEND-NHL-001: a phase 1 study of lisocabtagene maraleucel (Breyanzi, liso-cel) for patients with R/R LBCL (NCT02631044).5
From these studies, we extracted published data on numbers of patients enrolled and infused and V2VT among those infused; these data are shown in Table 1. The additional information in Table 1 is the reported V2VT dispersion data.
Available data from pivotal clinical trials of populations with 3L+ R/R LBCL regarding V2VT
| Study . | N . | Infused, n (%) . | Median V2VT . | Additional information . |
|---|---|---|---|---|
| ZUMA-14 | 111 | 101 (91) | 24 d | V2VT range, 16-73 d |
| JULIET3 | 165 | 111 (67) | 54 d | 90% of patients infused between 30-92 d |
| TRANSCEND-NHL-0015 | 344 | 269 (78) | 37 d | V2VT range, 27-224 d |
To estimate the probability of infusion success, a range of statistical models were fitted to the data summarized in Table 1, to estimate the potential relationship between V2VT, and the likelihood of patients being ultimately infused. Of note, the regression analysis assumed that the attrition of patients from enrollment to infusion serves as a proxy for infusion success. This is because of a lack of reported data specifically for the number of patients that undergo leukapheresis to inform the regression analysis.
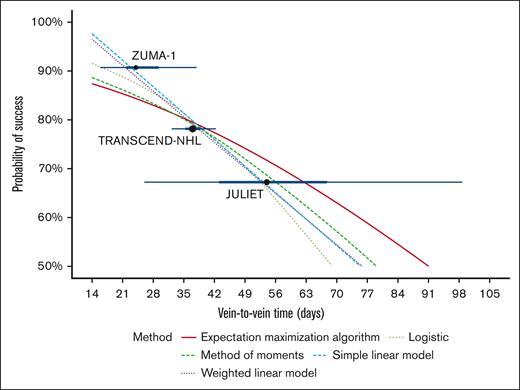
Figure 3 shows the estimated relationship between V2VT and probability of infusion success, across a range of statistical methods. The methods explored ranged from simple regression on the median to methods that estimate the underlying V2VT distribution. Of note, Figure 3 presents estimated interquartile ranges (heavier, darker blue range), and estimated 95% ranges (lighter, blue range); estimated to aid with the regression model fitting because these data were not reported for all studies. For simplicity, the base-case analysis used results from the simple linear model, with alternative models tested in sensitivity analyses.
The relationship between V2VT and probability of infusion based on ZUMA-1, TRANSCEND-NHL, and JULIET using a variety of regression models. Lighter blue horizontal range indicates estimated 95% range; heavier, darker blue range indicates estimated interquartile range; point size proportionate to sample size.
The relationship between V2VT and probability of infusion based on ZUMA-1, TRANSCEND-NHL, and JULIET using a variety of regression models. Lighter blue horizontal range indicates estimated 95% range; heavier, darker blue range indicates estimated interquartile range; point size proportionate to sample size.
The data in Table 1 also define the difference between short and long V2VT in the analysis. The median V2VT in ZUMA-1, 24 days, was assumed to represent a short V2VT, whereas the median V2VT in the JULIET study, 54 days, was used as proxy for long V2VT. Alternative V2VT definitions were explored in scenario analyses. The distinction between short and long V2VT drives differences in outcomes across arms of the analysis, through the predicted difference in infusion success chance, as described in this subsection, and further consequences described in the remainder of this section.
Survival for non-infused and infused patients
Once the cohort was partitioned into “infused” and “not infused” subcohorts, each subcohort was assumed to follow an infusion-outcome–dependent survival projection for the remainder of the lifetime horizon.
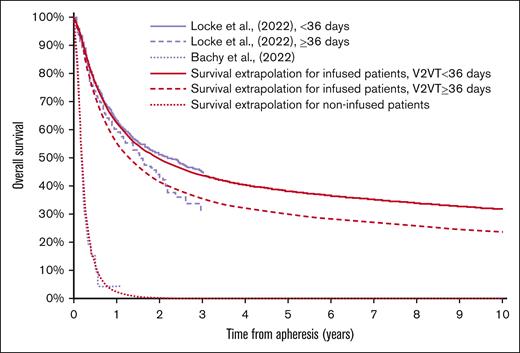
For the postinfusion component of the model, the following data from 3 recent publications were harnessed: (1) Bachy et al reported overall survival (OS) survival projections separately for 3L+ LBCL patients with a CAR T-cell product order for those who did not proceed to infusion, from the point of order and for those who did proceed to infusion, from the point of infusion.9 The analyses are based on data from the French DESCAR-T registry, and include patients with axi-cel and tisagenlecleucel (tisa-cel) orders between December 2019 and October 2021. (2) Similarly, Kuhnl et al reported OS Kaplan-Meier data for 3L+ LBCL patients approved for CAR T-cell treatment by the National CAR-T Clinical Panel for England for those who did not proceed to infusion, from the point of approval and for those who did proceed to infusion, from the point of infusion.10 The analyses are based on data from patients submitted for National CAR T Clinical Panel (axi-cel or tisa-cel) approval between December 2018 and November 2020. (3) Locke et al reported OS projections for 3L+ LBCL patients who received axi-cel commercially in the United States between October 2017 and August 2020, using data from Center for International Blood and Marrow Transplant Research.6 Unlike the previous 2 studies, Locke et al6 explicitly sought to evaluate the effect of V2VT upon patient outcomes, and present survival projections as outputs from multivariate logistic and Cox regression analyses. Specifically, Locke et al6 present OS projections from point of infusion stratified by V2VT categories, and hazard ratios associated with different categories.
Though the data from Bachy et al9 and Kunhl et al10 report outcomes stratified by different CAR T-cell product, for simplicity we assumed no differences in efficacy between axi-cel and other CAR Ts, which can be considered a conservative assumption.9-15
As a first step in harnessing the published survival data, survival plots in each study were digitized to create pseudo–patient-level data, using the WebPlotDigitizer software and the recreation algorithm of Guyot et al.16,17 Parametric survival models were then fitted to recreated patient-level data. A range of parametric models were considered as per National Institute for Health and Care Excellence (NICE) Decision Support Unit Technical Support Document 21 guidelines, including standard parametric models, restricted cubic spline–based models (or flexible parametric models), and mixture-cure models.18
In the base-case analysis, as the sample of patients recruited in Bachy et al9 represent a more recent cohort than those recruited in Kuhnl et al10 (∼1 year difference in enrollment periods), survival for non-infused patients (from CAR T approval) was based on recreated data from Bachy et al9 with use of recreated data from Kunhl et al,10 tested in scenario analysis. Survival from infusion for infused patients was based on data from Locke et al6 in the base case, given the ability to link V2VT to postinfusion outcomes with this source. Specifically, recreated data from Locke et al projections for OS for patients with V2VT <36 days were used alongside an HR from the same study to produce survival projections for patients with V2VT ≥36 days.6 For robustness, an alternative V2VT categorization approach from Locke et al6 was explored in scenario analysis.
A log-normal model was assumed for survival outcomes for noninfused patients in the base-case analysis, based on Bayesian Information Criterion goodness-of-fit statistics across tested models. For successfully infused patients, a mixture-cure survival model structure was assumed, in line with expectations that a proportion of patients may achieve long-term survivorship comparable with the age-adjusted, disease-free population, owing to the curative potential of CAR T in this setting. Specifically, a log-normal mixture-cure model was assumed, for consistency in structural assumptions across non-infused patient outcomes and outcomes for the uncured fraction of infused patients. For the fraction of infused patients estimated to be cured, US age- and sex–matched general population survival data from the Human Mortality Database were used.19
The base-case survival projections for infused/non-infused patients are presented in Figure 4. Alternative structural assumptions were tested in scenario analyses and the analytic model retained the functionality to test the range of survival models fitted to each data set.
Base-case survival extrapolations for infused and non-infused patients.
Health-related quality of life
To predict expected patient QALYs in addition to LYs, patient utility is defined as a measure of value a patient derives from their health-related quality of life, where a utility of 1 is associated with full health, and a utility of 0 is associated with death. An average lifetime utility value for the modeled cohort was estimated using data reported by Lin et al.20 In this study, utility values of 0.782 and 0.729 for patients with and without disease progression (respectively) were reported. To retain simplicity of the model, we did not partition patients by progression status. As such, an average of these values (0.756) was assumed to apply across the model’s lifetime horizon as a measure of the average utility experienced by patients. This is expected to represent an underestimate of the average utility value, because patients are expected to spend more time without disease progression than with disease progression.
Costs
Exploratory cost and cost-effectiveness analyses took a 2022 US health care payer perspective. The cost assumed for CAR T-cell acquisition is $462 000, based on Kite pricing at time of writing. The cost assumed for leukapheresis and hospitalization was $54 450.47, based on the ICER review of axi-cel and tisa-cel, uplifted to 2022 prices using US Bureau of Statistics Consumer Price Index data.21,22
Aside from CAR T-cell acquisition, leukapheresis, and hospitalization costs, ongoing health care costs were considered. An estimate of $11 890 health care costs per month for diffuse LBCL patients was reported by a burden-of-illness study that analyzed costs from diagnosis onwards.23 In this indicative analysis, after uplifting the $11 890 monthly estimate to 2022 prices to $14 791.75, we assumed this cost applies in full only to patients who are not infused. For patients who are infused, we assumed 50% of this monthly estimate ($7395.88 per month) for the first year, then 25% ($3697.94 per month) for the next 3 years, then 5% ($739.59 per month) from 5 years postinfusion onwards. Additional costs, such as the cost of waiting for patients that are not infused and end-of-life care were not included. However, the omission of these costs indicates that current estimates of cost-effectiveness are likely conservative.
Key settings and other assumptions
We assumed a baseline age of 60 years and a time horizon of 40 years, tracking the cohort to an upper limit of age 100 years in monthly model cycles. In presentation of LY and QALY results in isolation, the analysis assumed no time-preference, discounting future costs and health outcomes, to provide accurate differences in lifetime patient mortality and QALYs. However, in the exploratory cost-effectiveness analysis, a 3% per annum discount rate was assumed for cost and health outcomes, to fully capture the opportunity costs of longer V2VTs , in line with ICER methods.
For reference and clarity, base-case settings and assumptions are provided (supplemental Material), alongside population, incidence and eligibility assumptions used to estimate the number of US 3L+ R/R LBCL patients likely to receive CAR T-cell treatment in a given year.
Model outputs
The base-case analysis produced predicted probability of infusion success for long and short V2VTs, and total expected per-patient costs, QALYs and LYs associated with long and short V2VTs, respectively. These results were used to calculate incremental per-patient QALY and LY gains predicted to be associated with reducing V2VT from a long V2VT (54 days) to a short V2VT (24 days). The population-level analysis produced similar outputs to the base-case analysis, scaled up to the estimated annual CAR T–eligible 3L+ LBCL US population level.
The indicative cost-effectiveness analysis compared the cost-effectiveness of a short vs long V2VT and outputs total and incremental per-patient costs, QALYs and LYs, as per the base-case analysis, except with the inclusion of cost outputs and application of time-preference discounting assumptions described in “Data inputs.”
We perform numerous sensitivity and scenario analyses to test the impact upon headline results of different data and assumption choices to fully explore robustness of the results, as described throughout “Data inputs.”
Results
Base case results
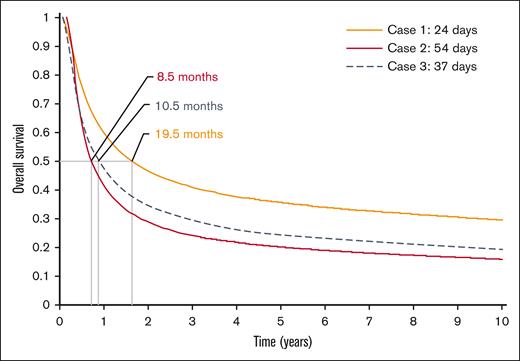
The modeled difference in V2VT led to a 3.2-year gain in life expectancy (4.2 vs 7.7 LYs), and an additional 2.4 undiscounted QALYs (3.2 vs 5.6) per patient. Based on the regression model, a reduction in V2VT from 54 to 24 days improved the probability of being successfully infused by 23.3% (from 66.6% to 89.8%). Using a smaller difference in V2VT (24 vs 37 days) produced 2.5 and 1.9 additional LYs and QALYs, respectively. The resultant survival extrapolations for these comparisons are provided in Figure 5.
Base-case survival extrapolations for all patients based on cohort average V2VT and median survival.
Base-case survival extrapolations for all patients based on cohort average V2VT and median survival.
The total population of US CAR T–eligible 3L+ R/R LBCL patients was estimated by ICER to be 5902 per year.21 Using the epidemiological model, if all patients in the United States were to receive a short V2VT compared with long V2VT, an additional 18 875 LYs and 14 260 additional QALYs would be generated every year. Using a smaller difference in V2VT (24 vs 37 days), the per-patients results equate to population level gains of 14 526 LYs and 10 974 QALYs. Equivalent results for smaller populations (eg, at a local hospital level), and/or to reflect smaller uptake, can be estimated by a simple multiplication of the per-patient results.
Sensitivity analyses
As described throughout the “Methods,” scenario analyses were used to test the sensitivity of results to various assumptions in the base case analysis. These scenario analyses and their results are summarized in Table 2. Across tested scenarios, shorter V2VT is associated with better health outcomes, though the magnitude of predicted health benefit varies with different assumptions. The predicted health benefit associated with a shorter V2VT is notably reduced if either the probability of successful infusion or the survival projection postinfusion is assumed to be uncorrelated with V2VT.
Results from scenario analyses (LYs gained)
| Scenario number and description . | Rationale . | Per patient . | US population . | |
|---|---|---|---|---|
| Base case | 3.20 | 18 875 | ||
| 1 | Probability of infusion not affected by V2VT | In this scenario, V2VT only impacts postinfusion survival (ie, not the proportion of patients that receive an infusion). | 1.98 | 11 706 |
| 2 | Postinfusion survival not affected by V2VT (Bachy et al9) | In this scenario, postinfusion survival is informed by Bachy et al9 which does not differentiate survival by V2VT. | 0.82 | 4826 |
| 3 | Switch non-infused survival source (Kuhnl et al8) | As above, except using an alternative source for postinfusion survival: Kuhnl et al9 | 3.19 | 18 832 |
| 4 | Switch HR cutoffs (<28 d vs 28-40 d vs ≥40 d) | In the base-case analysis, HR cutoffs of <36 and ≥36 d were used, as a simple means to dichotomize the Locke et al6 cohort in terms of their survival experience linked to V2VT. In this scenario, alternative cutoffs are used, which breaks the cohort into 3 groups instead of 2. | 3.47 | 20 500 |
| 5 | Change long V2VT to be 37 d | Alternative long V2VT specified to reflect a smaller reduction for the short V2VT group. | 2.46 | 14 526 |
| 6 | Change short V2VT to be 30 d | Alternative short V2VT specified to reflect a smaller reduction from the long V2VT group. | 2.82 | 16 661 |
| 7 | Assume half of the US population | Sensitivity of the population results stress-tested by assuming half of the estimated eligible cohort. | 3.20 | 9438 |
| 8 | Assume CIBMT registry population of 1294 patients | Sensitivity of the population results stress-tested by assuming same population per latest data from CIBMT registry. | 3.20 | 4138 |
| 9 | Postinfusion survival model: lognormal | 1.82 | 10 761 | |
| 10 | 1 knot(s) normal spline | 2.34 | 13 801 | |
| 11 | MCM: Weibull | Choice of an alternative survival extrapolation for patients that receive CAR T. | 3.53 | 20 813 |
| 12 | MCM: log-logistic | 3.29 | 19 435 | |
| 13 | Non-infused survival model | Choice of an alternative survival extrapolation for patients that do not receive CAR T. | 3.20 | 18 861 |
| 14 | Log-logistic | 3.20 | 18 865 | |
| 15 | 1 knot(s) odds spline | 3.06 | 18 042 | |
| 16 | MCM: lognormal MCM: log-logistic | 3.06 | 18 067 | |
| 17 | V2VT regression model: | Choice of an alternative regression model for estimating the proportion of patients who were infused, based on V2VT. | 3.14 | 18 529 |
| 18 | weighted-linear | 3.07 | 18 102 | |
| 19 | logistic | 2.68 | 15 802 | |
| 20 | method of moments Expectation maximization algorithm | 2.44 | 14 420 | |
| 21 | Iterative V2VT sampling | In the base-case analysis, all patients were assumed to have the same V2VT. In this scenario, V2VT is sampled from a distribution, with the mean results taken. Further details of this approach are provided in a supplemental Appendix. | 2.79 | 16 475 |
| Scenario number and description . | Rationale . | Per patient . | US population . | |
|---|---|---|---|---|
| Base case | 3.20 | 18 875 | ||
| 1 | Probability of infusion not affected by V2VT | In this scenario, V2VT only impacts postinfusion survival (ie, not the proportion of patients that receive an infusion). | 1.98 | 11 706 |
| 2 | Postinfusion survival not affected by V2VT (Bachy et al9) | In this scenario, postinfusion survival is informed by Bachy et al9 which does not differentiate survival by V2VT. | 0.82 | 4826 |
| 3 | Switch non-infused survival source (Kuhnl et al8) | As above, except using an alternative source for postinfusion survival: Kuhnl et al9 | 3.19 | 18 832 |
| 4 | Switch HR cutoffs (<28 d vs 28-40 d vs ≥40 d) | In the base-case analysis, HR cutoffs of <36 and ≥36 d were used, as a simple means to dichotomize the Locke et al6 cohort in terms of their survival experience linked to V2VT. In this scenario, alternative cutoffs are used, which breaks the cohort into 3 groups instead of 2. | 3.47 | 20 500 |
| 5 | Change long V2VT to be 37 d | Alternative long V2VT specified to reflect a smaller reduction for the short V2VT group. | 2.46 | 14 526 |
| 6 | Change short V2VT to be 30 d | Alternative short V2VT specified to reflect a smaller reduction from the long V2VT group. | 2.82 | 16 661 |
| 7 | Assume half of the US population | Sensitivity of the population results stress-tested by assuming half of the estimated eligible cohort. | 3.20 | 9438 |
| 8 | Assume CIBMT registry population of 1294 patients | Sensitivity of the population results stress-tested by assuming same population per latest data from CIBMT registry. | 3.20 | 4138 |
| 9 | Postinfusion survival model: lognormal | 1.82 | 10 761 | |
| 10 | 1 knot(s) normal spline | 2.34 | 13 801 | |
| 11 | MCM: Weibull | Choice of an alternative survival extrapolation for patients that receive CAR T. | 3.53 | 20 813 |
| 12 | MCM: log-logistic | 3.29 | 19 435 | |
| 13 | Non-infused survival model | Choice of an alternative survival extrapolation for patients that do not receive CAR T. | 3.20 | 18 861 |
| 14 | Log-logistic | 3.20 | 18 865 | |
| 15 | 1 knot(s) odds spline | 3.06 | 18 042 | |
| 16 | MCM: lognormal MCM: log-logistic | 3.06 | 18 067 | |
| 17 | V2VT regression model: | Choice of an alternative regression model for estimating the proportion of patients who were infused, based on V2VT. | 3.14 | 18 529 |
| 18 | weighted-linear | 3.07 | 18 102 | |
| 19 | logistic | 2.68 | 15 802 | |
| 20 | method of moments Expectation maximization algorithm | 2.44 | 14 420 | |
| 21 | Iterative V2VT sampling | In the base-case analysis, all patients were assumed to have the same V2VT. In this scenario, V2VT is sampled from a distribution, with the mean results taken. Further details of this approach are provided in a supplemental Appendix. | 2.79 | 16 475 |
CIBMT, Center for International Blood and Marrow Transplant Research; HR, hazard ratio.
Indicative cost-effectiveness results
Using annual discount rates of 3% for costs and outcomes, reducing V2VT from 54 to 24 days leads to improved health outcomes at an anticipated cost of $92 587 for every QALY gained. The increase costs are due to a higher proportion of patients receiving CAR T as typically CAR T costs are billed after a successful infusion. These results are below the ICER threshold range of $100 000 to $150 000 per QALY gained, suggesting such an improvement in V2VT is expected to be cost-effective in the US setting.21
Discussion
In clinical practice, there are multiple factors that can impact V2VT for patients receiving CAR T, and delays during this multistep process may impact patient outcomes. To our knowledge, our study model is the first to quantify the potential lifetime health consequences of reducing V2VT for 3L+ R/R LBCL patients intended to be treated with CAR T. Within this, we believe this is also the first study to estimate a formal relationship between study-level V2VT and infusion success. Further contributions from this study include the harnessing of recently published outcomes evidence, estimation of the impact of reducing V2VT upon expected patient quality of life-adjusted survival and derivation of cost-effectiveness implications.
The design of the decision-analytic model underling this study is intentionally simple and its description herein is intended to be transparent, serving as a foundation from which further work can be conducted; for instance, in assessing the expected benefits of newer experimental products with the potential to dramatically reduce expected time from apheresis to infusion. A modular approach was taken to identify and incorporate input data from a range of sources, which means it is possible to investigate uncertainty easily for specific aspects of the model. This is because it is not possible for a single study to provide all the necessary data to inform this type of analysis (as doing so would require designing a study with intentionally delayed time to administration of treatment, which introduces a number of ethical issues). Moreover, should further data be later made available, such sources can readily be included within the analysis (without requiring other components of the model to be re-analyzed).
We identified cost inputs from published literature as well as reporting produced by health technology assessment bodies, such as NICE and ICER. Morrison et al23 found that costs decreased after the first year following diagnosis, and so use of this cost without accounting for changes over time may lead to an overestimate for 3L+ LBCL patients. Further, ongoing costs post CAR T infusion have been estimated to be low, across NICE appraisals of CAR T therapies in 3L+ LBCL and in the ICER review of axi-cel and tisa-cel.21,24,25 Specifically, the ICER modeling group assumed ongoing medical management costs decreased in stages, first upon assessment of CAR T response, then at 1 year following response assessment, then at 5 years following response assessment, from which point only minimal ongoing costs are assumed. Similarly, NICE appraisals of axi-cel and tisa-cel assumed minimal ongoing health care costs after 5 years, from which point patients are effectively assumed to be cured. This mirrors the approach taken in our study (to capture decreasing costs over time), but is nevertheless an area of uncertainty within our costing analysis.
Relatedly, our model assumes that all CAR T administration takes place in an inpatient setting. In reality, some patients could be infused with some CAR Ts in an outpatient setting, which is expected to be less costly. Therefore, all other things held equal, the incremental costs projected by our modeling associated with V2VT would reduce if a proportion of patients are assumed to be treated in an outpatient rather than an inpatient setting.
We have undertaken extensive sensitivity analyses to contextualize the base-case analysis results in the context of limited data. Specifically, we have explored alternative regression analyses for V2VT vs infusion probability, various parametric survival models for survival for both infused and non-infused patients and tested different data sources. These sensitivity analyses demonstrate a consistent benefit associated with reduced V2VT, supporting our headline results.
Key limitations include the limited granularity of data to fully interrogate relationship between time elapsed before infusion and survival, and reliance on data from a range of sources, each associated with its own limitations. There would clearly be ethical issues in purposefully delaying infusion to investigate the relationship between V2VT and survival in a controlled setting, and so studies such as this will likely always need to rely on real-world analyses.
We believe our results support a call for increased clinical and research attention on “brain-to-vein” time (ie, the time from referral to infusion); delays from referral to CAR T order will logically have similar implications to delays from order to infusion. Ultimately, the results of our analysis demonstrate that outcomes for non-infused patients are substantially poorer than those for infused patients, and so infusion success is of critical importance for survival outcomes. Median estimates of survival for non-infused patients used to inform the model were in the region of 2 to 3 months, compared with 6.3 months in the historical SCHOLAR-1 cohort study; in other words, those patients who are not infused, have a worse outcome compared with the historical standard of care in the pre–CAR-T era.
In some countries (eg, the United Kingdom), there is a relatively high uptake of bridging therapy as a debulking strategy before CAR T infusion. For example, Kuhnl et al reported that 86.7% of patients received bridging therapy.10 Similarly, in Bachy et al reported 82.7% of patients receiving bridging therapy.9 This is understood to be driven by the infusion date being intentionally delayed to maximize the effect of the bridging therapy before infusion. Such intentional delays are different to the avoidable delays that comprise the focus of our analysis. The potential role of bridging therapy and its associated impact on the results of our analysis are unclear, though this limitation was mitigated somewhat by considering a sensitivity analysis only from the point of infusion.
Our analysis assumes the same efficacy for all CAR T-cell products, because the focus of our study was on the impact of V2VT. In reality, it is expected that there may be some differences in outcomes that arise as a function of both V2VT and different efficacy for specific products. For example, one real-world comparison by Bachy et al suggested differences in efficacy and safety between axi-cel and tisa-cel.9 CAR T efficacy may be influenced by a multitude of factors, such as viral vector, culture, novel activation domains, bicistronic constructs, etc.; however, these were not explored in this study owing to a lack of current or anticipated future head-to-head studies comparing different CAR T-cell products. It remains challenging to disentangle the effects of V2VT and the specific CAR T-cell product on postinfusion survival.
Conclusions
We find that V2VT may be an important predictor of outcomes and aiming for short manufacturing, product release, shipping, and infusion times may be key to further improve outcomes for patients treated with CAR T. We predicted life expectancy gains in the region of 3 years associated with shortening V2VT. At a population level, up to 18 875 LYs could be gained each year if all 3L+ R/R LBCL CAR T–intended patients in the United States received a short V2VT compared with the longer dates modeled in this study. Furthermore, indicative economic results show reducing V2VT to be a cost-effective treatment strategy, in the US setting.
Data on the relationship between V2VT and long-term patient outcomes are sparse. Further data collection and reporting for V2VT in general would aid additional research, including proxy measures for patients who are not infused. This would allow for specific investigations to be undertaken, including the reasons why V2VT can vary across individuals, regions, and the impact of bridging strategies.
Authorship
Contribution: S.V. conceived the research, designed the model, and provided study management; R.T.M., G.C., M.C.P., M.R., Z.-H.H., H.S., and S.V. contributed to aspects of the concept/design of the study and data interpretation, and critically reviewed, revised, and approved the manuscript content for publication; M.R., Z.H., and H.S. provided data needed for the model; A.B. and W.S. drafted the manuscript and constructed the model used to inform the analysis; and M.E.-J. developed the regression analysis used to relate V2VT to the probability of infusion.
Conflict-of-interest disclosure: R.T.M. is an adviser or consultant for AlloVir, Artiva, CRISPR Therapeutics, Incyte, and Novartis; reports honoraria from Bristol Myers Squibb/Celgene, Incyte, Intellia, and Kite; received research support from AlloVir and Novartis; participates in data and safety monitoring boards for Athersys, Novartis, Century Therapeutics, and VorPharma; and has a patent with Athersys. A.B., W.S., and M.E-.J. were all employees of Delta Hat at time of manuscript development. Delta Hat received consulting fees for the development of the model and associated analyses described within this manuscript. S.V., Z.-H.H., M.R., and H.S. are employees of and stockholders of Kite, a Gilead company. G.C. received consulting fees and honoraria from Roche, Bristol Myers Squibb, Onwards Therapeutics, MedxCell, EmerCell, MabQ, Sanofi, AbbVie, Takeda, Janssen, Novartis, and Myltenyi. M.L. received honoraria or travel grants from Pfizer, Novartis, Gilead, and Bristol Myers Squibb. M.C.P. has received honoraria from Pfizer and consulting fees from MediGene. M.R. has received research support from Seres Therapeutics; has consulted, received honorarium from, or participated in advisory boards for Seres Therapeutics, Flagship Ventures, Novartis, Evelo, Jazz Pharmaceuticals, Therakos, Amgen, Merck & Co, Acute Leukemia Forum, and DKMS Medical Council (board); and has intellectual property licensing with Seres Therapeutics and Juno Therapeutics.
Correspondence: Sachin Vadgama, Kite, A Gilead company, HEOR, 2 Round Wood Ave, Stockley Park, Uxbridge, UB11 1AF, United Kingdom; email: sachin.vadgama@gilead.com.
References
Author notes
Data are available on request from the corresponding author, Sachin Vadgama sachin.vadgama@gilead.com).
The full-text version of this article contains a data supplement.