Key Points
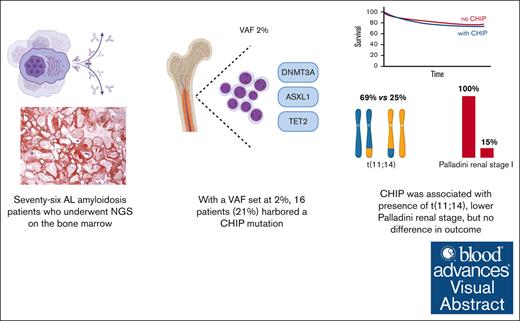
CHIP is more prevalent in patients with AL amyloidosis than in the general population.
CHIP presence was associated with the presence of t(11;14) and a lower Palladini renal stage in patients with renal involvement.
Visual Abstract
Immunoglobulin light-chain (AL) amyloidosis is characterized by the deposition of misfolded monoclonal free light chains, with cardiac complications accounting for patient mortality. Clonal hematopoiesis of indeterminate potential (CHIP) has been associated with worse cardiovascular outcomes in the general population. Its significance in AL amyloidosis remains unclear. We collected clinical information and outcome data on 76 patients with a diagnosis of AL amyloidosis who underwent deep targeted sequencing for myeloid neoplasia–associated mutations between April 2018 and August 2023. Variant allele frequency was set at 2% to call CHIP-associated mutations. CHIP mutations were present in patients with AL amyloidosis at a higher frequency compared with age-matched control individuals. Sixteen patients (21%) had at least 1 CHIP mutation. DNMT3A was the most frequent mutation (7/16; 44%). Compared with patients without CHIP, patients with CHIP had a higher prevalence of t(11;14) translocation (69% vs 25%, respectively; P = .004). Furthermore, among patients with renal involvement, those with CHIP had a lower Palladini renal stage (P = .001). At a median follow-up of 32.5 months, the presence of CHIP was not associated with worse overall survival or major organ dysfunction progression-free survival. Larger studies and longer follow-up are needed to better define the impact of CHIP in patients with AL amyloidosis.
Introduction
Immunoglobulin light-chain (AL) amyloidosis is a plasma cell (PC) dyscrasia whose hallmark is the production and secretion of misfolded, monoclonal immunoglobulin free light chains (FLC).1,2 The circulating FLC drives pathogenesis, causing direct cytotoxicity in its soluble form and disruption of target organ architecture upon deposition as insoluble fibrillary aggregates. It is thought that any organ/system in the body can be involved by AL amyloidosis deposition, with the heart and kidneys being the most commonly affected organs.3,4 The severity of cardiac involvement is the single most important prognostic factor in AL amyloidosis, as cardiac events represent the major cause of mortality.5,6 Renal involvement occurs in up to 70% of cases and is a key determinant of patient morbidity, decreased quality of life, and ineligibility for clinical trials.7,8 To aid in patients’ prognostication and management, several staging systems have been developed to evaluate cardiac involvement,9-12 whereas Palladini et al7 have introduced a score predicting the risk of progression to renal replacement therapy. Cytogenetic alterations also have prognostic implications and are an important predictor of response to antineoplastic therapies.13-16 Among those, t(11;14) is the most prevalent.16 It portends a lower response rate to bortezomib-based therapies13 and is a negative prognostic factor, being associated with decreased progression-free survival (PFS) compared with patients without any alteration.15 According to the recently published ANDROMEDA study, daratumumab seems to overcome the negative prognostic impact of t(11;14).8 Further, extrapolating from multiple myeloma (MM), the presence of t(11;14) is a biomarker for response to Bcl2 inhibitor venetoclax.4,8,17
Clonal hematopoiesis of indeterminate potential (CHIP) refers to the presence of clonal, somatic mutations of myeloid-related genes in the absence of overt myeloid neoplasia or cytopenia. The most commonly CHIP-associated mutations involve DNMT3A, TET2, and/or ASXL1 genes, commonly known under the acronym DTA.18 CHIP incidence increases with age, being most frequent in older populations and nearly absent before the age of 40 years.19-23 Its occurrence has been associated with a higher incidence of hematological malignancies19,20,22 and cardiovascular disease, and worse cardiovascular outcomes.22,24-27 In the context of hematological neoplasms, CHIP has been detected in 9.7% to 21.6% of patients with PC dyscrasia and 14% to 29% of patients with lymphoma.23,28-32 In patients with MM and lymphoma receiving autologous stem cell transplantation (ASCT), CHIP presence was identified as an adverse prognostic factor.28,31 Tahri et al29 also noted a higher risk of progression to Waldenström macroglobulinemia in patients with immunoglobulin M monoclonal gammopathy of undetermined significance or smoldering Waldenström macroglobulinemia carrying DTA mutations. Two studies previously reported on the incidence of CHIP in patients with AL amyloidosis. The incidence of CHIP was 15% (4 out of 27 patients) in 1 study and 21% (10 out of 47 patients) in the other and did not correlate with any specific clinical features. It is important to note that although the presence of CHIP was not found to have prognostic significance in these studies, the association between CHIP and cytogenetic alterations or cardiac involvement was not investigated.32,33
We were interested in exploring whether an association exists between CHIP and cardiac outcome, according to prior studies. We were also interested in understanding the coexistence of relevant disease characteristics with CHIP.
Hence, we performed a single-center, retrospective cohort study including 76 consecutive patients with AL amyloidosis who were seen at Brigham and Women’s Hospital (BWH)/Dana-Farber Cancer Institute (DFCI) between April 2018 and August 2023 and had a bone marrow (BM) biopsy performed with a targeted myeloid mutation panel assessed.
To our knowledge, this is the largest study to date investigating broadly the prevalence and impact of the presence of CHIP in patients with AL amyloidosis.
Materials and methods
Patients
We identified patients seen at BWH/DFCI for a diagnosis of AL amyloidosis who underwent deep targeted sequencing for myeloid neoplasia–associated mutations between April 2018 and August 2023. We retrospectively collected clinical information, including age, sex, ethnicity, smoking status, FLC subtype, European Modification of 2004 Mayo stage, organ involvement, cytogenetic alterations detected by fluorescence in situ hybridization, left ventricular ejection fraction (LVEF), and Palladini renal stage for patients with renal involvement.7,9 Additionally, we assessed the type of antineoplastic treatment, depth of hematological response, and whether patients received an ASCT. We chose as a primary outcome the major organ dysfunction PFS (MOD-PFS) as defined by Kastritis et al8 and the overall survival (OS). We selected as a secondary outcome the cardiac-specific disease response and PFS (assessed at 6 and 12 months after commencement of therapy) as defined by Palladini et al.7 Next-generation sequencing on BM samples was performed using our custom validated assay, Rapid Heme Panel (RHP).34 Genes assessed for CHIP attribution included the following: JAK1, JAK3, PDGFRA, SFA3A1, DNMT3A, GNB1, CEBPA, SBDS, FLT3, KRAS, BCORL1, PIGA, SF3B1, ASXL1, CTCF, CSF3R, CUX1, NOTCH3, PPM1D, ZRSR2, ATM, CCND1, KMT2A, EP300, EZH2, SETD2, SH2B3, GNAS, GATA1, IKZF3, PRPF8, KIT, NOTCH2, WT1, TET2, PIK3CA, PTPN11, CREBBP, NOTCH1, BRCC3, DDX41, TP53, CALR, and LUC7L2. The median coverage obtained was 759 reads per base (interquartile range, 1131).
CHIP status was assigned to patient samples when putative driver lesions in genes associated with myeloid neoplasms were observed at variant allele frequency (VAF) >2%. Reported variants were then analyzed and filtered according to common practice standards through a semiautomatic pipeline.35-37 The age-specific CHIP rates reported by Jaiswal et al19 were used to calculate the standardized incidence rate.
This study was approved by the BWH/DFCI Institutional Review Board (approval number 2023P001501) and was conducted in accordance with the Declaration of Helsinki.
Statistical analysis
Statistical analyses were performed using Stata Statistical Software Release 17 (StataCorp LLC, College Station, TX) and R, version 4.2.3 (Shortstop Beagle). Normal distribution was visually assessed for all continuous variables. Data dispersion was assessed with standard deviation (SD) for normally distributed variables and with interquartile range for nonnormally distributed variables. Baseline demographics and disease characteristics were compared between patients with and without CHIP. Comparison was performed with the independent-samples t test for normally distributed variables and with the Wilcoxon rank-sum (Mann-Whitney U) test for nonnormally distributed variables. Comparison for categorical variables was performed with the χ2 test and Fisher's exact test. MOD-PFS and cardiac-specific PFS were measured from the time of diagnosis to the time of the first MOD-PFS/cardiac progression–defining event or were censored at the last follow-up. All reported P values were 2-sided, with a statistical significance set at <.05. We used the Kaplan-Meier method to estimate the survival curves for the OS and MOD-PFS, and the log-rank test to assess the difference between survival curves. We used a Cox regression and a stratified Cox regression model to assess the time-to-event outcome and calculate hazard ratios (HRs) with 95% confidence intervals (CIs). The stratified multivariate Cox regression model was built using the forward selection principle and following a 10:1 event-to-covariate ratio.
Results
CHIP is present at a higher prevalence in patients with AL amyloidosis compared with a healthy population
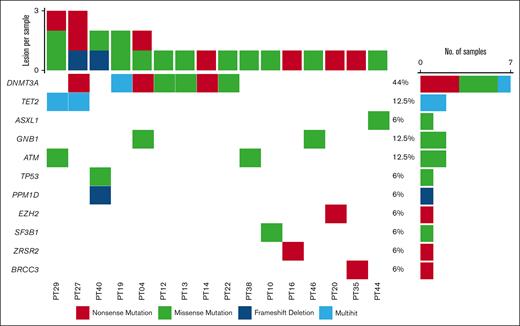
We identified a total of 76 patients. Sixteen patients (21%) had at least 1 CHIP mutation. Figure 1 shows the detected mutational profile. DNMT3A was the most frequently involved gene (7/16; 44%), followed by TET2, GNB1, and ATM (each 2/16; 12.5%), and SF3B1, TP53, ZRSR2, EZH2, BRCC3, PPM1D, and ASXL1 (6%). DNMT3A variants included 6 missense mutations (2 of which were in the same patient) at the R882, R736, Y735, I780, and F755 residues, and 3 nonsense mutations. TET2 lesions included 3 stop codons and 1 variant in the catalytic domain (residues 1843-2002). ASXL1, GNB1, and ATM-reported variants included known missense hotspots. Of note, 5 samples carried >1 CHIP-defining lesion. The median VAF of CHIP-associated mutations was 0.036. A subset of 4 cases carried CHIP variants with an allele frequency ≥10%, suggesting the presence of a larger clone. Based on the age distribution of our patients, the age-standardized incidence rate of CHIP would be 6%.
Oncoplot displaying CHIP results. Oncoplot showing the relative genetic contribution to CHIP in the cohort (76 patients, of whom 16 are carrying CHIP-defining lesions). Mutation types are color-coded according to legend. The right bar chart shows the number of samples carrying a specific genetic variant, whereas the top bar chart recapitulates the number of variants per sample. DTA lesions are shown in the first 3 lines followed by other genes involved in hematologic neoplasms.
Oncoplot displaying CHIP results. Oncoplot showing the relative genetic contribution to CHIP in the cohort (76 patients, of whom 16 are carrying CHIP-defining lesions). Mutation types are color-coded according to legend. The right bar chart shows the number of samples carrying a specific genetic variant, whereas the top bar chart recapitulates the number of variants per sample. DTA lesions are shown in the first 3 lines followed by other genes involved in hematologic neoplasms.
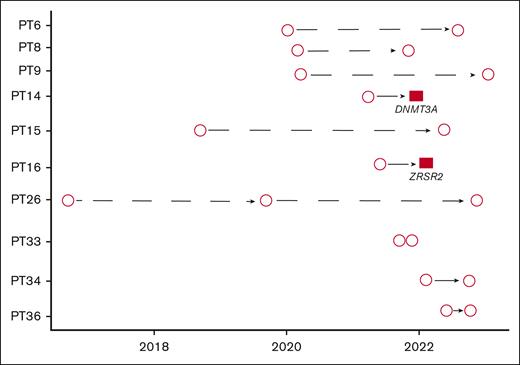
Ten patients (13%) had >1 sample available. Eight out of 10 patients with >1 BM biopsy available had no evidence of CHIP on either biopsy. In contrast, the other 2 patients (20%) were initially negative and subsequently had a biopsy positive for CHIP (DNMT3A, ZRSR2). There were no patients with CHIP on a first biopsy who were CHIP-negative on a subsequent biopsy (Figure 2).
Longitudinal data. Plot showing patients with longitudinal samples available. Circles represent RHP not showing any CHIP, whereas red squares represent RHP where CHIP was detected. Two patients (PT 14 and PT 16) who had no CHIP at the initial RHP were later found to harbor 1 CHIP (DNMT3A and ZRSR2, respectively). In contrast, no CHIP was found in the repeated RHP of the remaining 8 patients.
Longitudinal data. Plot showing patients with longitudinal samples available. Circles represent RHP not showing any CHIP, whereas red squares represent RHP where CHIP was detected. Two patients (PT 14 and PT 16) who had no CHIP at the initial RHP were later found to harbor 1 CHIP (DNMT3A and ZRSR2, respectively). In contrast, no CHIP was found in the repeated RHP of the remaining 8 patients.
Clinical characteristics
Baseline demographics are shown in Table 1. The mean age of our cohort was 63 years (range, 44-85). Thirty-two patients (42%) were female, 6 (8%) were Black, 2 (3%) were Asian, 1 (1%) was Middle Eastern, 2 (3%) self-reported as other, and 65 (85%) were White. No significant difference was noted in epidemiologic characteristics between patients harboring a CHIP mutation and those without it (P > .05). Sixty-nine percent of patients with CHIP and 67% of patients without CHIP (P = 1) were treatment-naive at the time when the RHP was obtained. Among patients with CHIP, 2 (12.5%), 5 (31%), 7 (44%), and 2 (12.5%) had a Mayo stage of I, II, IIIA, and IIIB, respectively. Among those without CHIP, 19 (32%), 13 (22%), 14 (24%), and 13 (24%) had a Mayo stage of I, II, IIIA, and IIIB, respectively. There was no statistically significant difference in the distribution of Mayo stages between the 2 groups. When focusing on patients with histopathology-proven or clinically determined renal involvement, patients with CHIP were more likely to have a lower Palladini renal stage7 (P = .001). No significant difference was noted regarding the frequency of organ involvement (ie, heart, kidneys, liver, lung, autonomic nervous system, peripheral nervous system, gastrointestinal tract, and soft tissue) and the total number of organs affected. LVEF and the presence of anginal symptoms were also evaluated: the mean LVEF was 55% (SD, 6.4%) for patients with CHIP and 53% (SD, 10.2%) for those without. None of the CHIP patients had an LVEF ≤40% as opposed to 7 (12%) non-CHIP patients, but this difference was not statistically significant (P = .34). Two patients with CHIP (13%) reported anginal symptoms, as opposed to 4 patients without CHIP (7%), but this difference was not statistically significant (P = .60). We then assessed the cytogenetic profile of our cohort (Table 1). A total of 54 patients could be evaluated with fluorescence in situ hybridization: 13 with CHIP (81%) and 41 without CHIP (68%). Among those with CHIP, 11 of 13 (85%) were found to harbor the t(11;14) as opposed to 15 of 41 (37%) without CHIP (P = .004). To exclude a possible confounding effect of age on the association between CHIP and the t(11;14), we performed a multivariate logistic regression to assess this association while keeping age constant. Even after adjusting for age, the association between CHIP and t(11;14) remained statistically significant (adjusted odds ratio, 10.92; CI, 1.9-62.31; P = .007). No other cytogenetic abnormality was found to be significantly associated with CHIP. Regarding the treatment received, 35 (46%) patients received the CyBorD (cyclophosphamide, bortezomib, and dexamethasone) combination, 28 (37%) received Dara-CyBorD (daratumumab and CyBorD), and 12 (16%) received other regimens (VD [bortezomib and dexamethasone], RVD [lenalidomide, bortezomib, and dexamethasone], Dara-VD [daratumumab and VD], and a combination of melphalan and dexamethasone). Ten (13%) patients received ASCT during their disease course, including 2 (12.5%) patients with CHIP and 8 (13%) without.
Demographics of patients with AL amyloidosis with and without CHIP
| Demographics . | Total (n = 76) . | With CHIP (n = 16) . | Without CHIP (n = 60) . | P value (CHIP vs no CHIP) . |
|---|---|---|---|---|
| Age (mean ±SD) | 63 ± 9.9 | 66 ± 10.1 | 63 ± 9.8 | .28∗ |
| Sex | ||||
| Male | 44 (57.9%) | 7 (43.7%) | 37 (61.7%) | .26† |
| Female | 32 (42.1%) | 9 (56.3%) | 23 (38.3) | |
| Ethnicity | ||||
| White | 65 (85%) | 15 (94%) | 50 (84%) | .83† |
| Black | 6 (8%) | 1 (6%) | 5 (8%) | |
| Asian | 2 (3%) | 0 | 2 (3%) | |
| Middle Eastern | 1 (1%) | 0 | 1 (2%) | |
| Other | 2 (3%) | 0 | 2 (3%) | |
| Smoking status | ||||
| Smoker | 43 (58%) | 7 (44%) | 24 (41%) | .54† |
| Nonsmoker | 31 (42%) | 9 (56%) | 34 (59%) | |
| Other neoplasm | ||||
| Yes | 13 (17%) | 3 (19%) | 10 (17%) | .55† |
| MM | 9 (12%) | 3 (19%) | 6 (10%) | |
| SMM | 2 (3%) | 0 | 2 (3%) | |
| WM | 1 (1%) | 0 | 1 (2%) | |
| CML | 1 (1%) | 0 | 1 (2%) | |
| No | 63 (83%) | 13 (81%) | 50 (83%) | |
| Type of FLC | ||||
| Lambda | 63 (82.9%) | 12 (75%) | 51 (85%) | .45† |
| Kappa | 13 (17.1%) | 4 (25%) | 9 (15%) | |
| Treatment status at CHIP evaluation | ||||
| Newly diagnosed | 51 (67%) | 11 (69%) | 40 (67%) | 1† |
| Rel/Ref | 25 (33%) | 5 (31%) | 20 (33%) | |
| Median time in months from diagnosis | 1 (0-159) | 1 (0-41) | 1 (0-159) | .73‡ |
| Median no. of lines of therapy (range) | 1 (1-3) | 1 (1-3) | 1.5 (1-2) | .64‡ |
| Mayo stage | ||||
| I | 21 (28%) | 2 (12.5%) | 19 (32%) | .44‡ |
| II | 18 (24%) | 5 (31%) | 13 (22%) | |
| IIIA | 21 (28%) | 7 (44%) | 14 (24%) | |
| IIIB | 15 (20%) | 2 (12.5%) | 13 (22%) | |
| Mayo stage IIIA-IIIB | 36 (48%) | 9 (56%) | 27 (46%) | .58 |
| Palladini kidney stage (if kidney involvement) | .001‡ | |||
| I | 9 (28%) | 5 (100%) | 4 (15%) | |
| II | 14 (44%) | 0 | 14 (52%) | |
| III | 9 (28%) | 0 | 9 (33%) | |
| No. of organs involved (median, range) | 3 (1-7) | 3 (1-5) | 3 (1-7) | .43‡ |
| Sites | ||||
| Heart | 60 (79%) | 15 (94%) | 45 (75%) | N.S. |
| Renal | 32 (42%) | 5 (31%) | 27 (45%) | |
| ANS | 23 (30%) | 8 (50%) | 15 (25%) | |
| PNS | 19 (25%) | 3 (19%) | 16 (27%) | |
| GI | 36 (47%) | 8 (50%) | 28 (47%) | |
| Soft tissue/skin | 32 (42%) | 7 (44%) | 25 (42%) | |
| Liver | 3 (4%) | 1 (6%) | 2 (3%) | N.As. |
| Lungs/pleura | 4 (5%) | 0 | 4 (7%) | |
| Lymph node | 2 (3%) | 0 | 2 (3%) | |
| LVEF (mean ± SD) | 53 ± 9.5 | 55 ± 6.4 | 53 ± 10.2 | .28∗ |
| ≤40% | 9.5% | 0 | 12% | .34† |
| ≤50% | 28% | 13% | 33% | .13† |
| CAD | ||||
| Yes | 6 (8%) | 2 (12%) | 4 (7%) | .6† |
| No | 70 (92%) | 14 (88%) | 56 (93%) | |
| Del 17p | ||||
| Yes | 36 (13%) | 0 (13%) | 1 (13%) | 1† |
| No | 18 (58%) | 13 (69%) | 40 (55%) | |
| N.A. | 22 (29%) | 3 (19%) | 19 (32%) | |
| t(4;14) | ||||
| Yes | 1 (1%) | 0 | 1 (2%) | 1† |
| No | 53 (70%) | 13 (81%) | 40 (67%) | |
| N.A. | 22 (29%) | 3 (19%) | 19 (32%) | |
| Monosomy 13 | ||||
| Yes | 5 (7%) | 0 | 5 (8%) | .32† |
| No | 49 (64%) | 13 (81%) | 36 (60%) | |
| N.A. | 22 (29%) | 3 (19%) | 19 (32%) | |
| Gain 1q | ||||
| Yes | 6 (8%) | 2 (12%) | 4 (7%) | |
| No | 48 (63%) | 11 (69%) | 37 (61%) | .62† |
| N.A. | 22 (29%) | 3 (19%) | 19 (32%) | |
| ≥2 trisomies | ||||
| Yes | 3 (4%) | 0 | 3 (5%) | 1† |
| No | 51 (67%) | 13 (81%) | 38 (63%) | |
| N.A. | 22 (29%) | 3 (19%) | 19 (32%) | |
| t(11;14) | ||||
| Yes | 26 (34%) | 11 (69%) | 15 (25%) | .004† |
| No | 28 (37%) | 2 (12%) | 26 (43%) | |
| N.A. | 22 (29%) | 3 (19%) | 19 (32%) | |
| BM PC percentage (median, IQR) | 15% (7%-20%) | 17% (10%-22%) | 15% (7%-20%) | .08‡ |
| First-line treatment | ||||
| CyBorD | 35 (46%) | 5 (31%) | 30 (50%) | .33† |
| Dara-CyBorD | 28 (37%) | 8 (50%) | 20 (33%) | |
| Other§ | 12 (16%) | 3 (19%) | 9 (15%) | |
| None | 1 (1%) | 0 | 1 (2%) | |
| ASCT | ||||
| Yes | 10 (13%) | 2 (12.5%) | 8 (13%) | 1† |
| No | 66 (87%) | 14 (87.5%) | 52 (87%) |
| Demographics . | Total (n = 76) . | With CHIP (n = 16) . | Without CHIP (n = 60) . | P value (CHIP vs no CHIP) . |
|---|---|---|---|---|
| Age (mean ±SD) | 63 ± 9.9 | 66 ± 10.1 | 63 ± 9.8 | .28∗ |
| Sex | ||||
| Male | 44 (57.9%) | 7 (43.7%) | 37 (61.7%) | .26† |
| Female | 32 (42.1%) | 9 (56.3%) | 23 (38.3) | |
| Ethnicity | ||||
| White | 65 (85%) | 15 (94%) | 50 (84%) | .83† |
| Black | 6 (8%) | 1 (6%) | 5 (8%) | |
| Asian | 2 (3%) | 0 | 2 (3%) | |
| Middle Eastern | 1 (1%) | 0 | 1 (2%) | |
| Other | 2 (3%) | 0 | 2 (3%) | |
| Smoking status | ||||
| Smoker | 43 (58%) | 7 (44%) | 24 (41%) | .54† |
| Nonsmoker | 31 (42%) | 9 (56%) | 34 (59%) | |
| Other neoplasm | ||||
| Yes | 13 (17%) | 3 (19%) | 10 (17%) | .55† |
| MM | 9 (12%) | 3 (19%) | 6 (10%) | |
| SMM | 2 (3%) | 0 | 2 (3%) | |
| WM | 1 (1%) | 0 | 1 (2%) | |
| CML | 1 (1%) | 0 | 1 (2%) | |
| No | 63 (83%) | 13 (81%) | 50 (83%) | |
| Type of FLC | ||||
| Lambda | 63 (82.9%) | 12 (75%) | 51 (85%) | .45† |
| Kappa | 13 (17.1%) | 4 (25%) | 9 (15%) | |
| Treatment status at CHIP evaluation | ||||
| Newly diagnosed | 51 (67%) | 11 (69%) | 40 (67%) | 1† |
| Rel/Ref | 25 (33%) | 5 (31%) | 20 (33%) | |
| Median time in months from diagnosis | 1 (0-159) | 1 (0-41) | 1 (0-159) | .73‡ |
| Median no. of lines of therapy (range) | 1 (1-3) | 1 (1-3) | 1.5 (1-2) | .64‡ |
| Mayo stage | ||||
| I | 21 (28%) | 2 (12.5%) | 19 (32%) | .44‡ |
| II | 18 (24%) | 5 (31%) | 13 (22%) | |
| IIIA | 21 (28%) | 7 (44%) | 14 (24%) | |
| IIIB | 15 (20%) | 2 (12.5%) | 13 (22%) | |
| Mayo stage IIIA-IIIB | 36 (48%) | 9 (56%) | 27 (46%) | .58 |
| Palladini kidney stage (if kidney involvement) | .001‡ | |||
| I | 9 (28%) | 5 (100%) | 4 (15%) | |
| II | 14 (44%) | 0 | 14 (52%) | |
| III | 9 (28%) | 0 | 9 (33%) | |
| No. of organs involved (median, range) | 3 (1-7) | 3 (1-5) | 3 (1-7) | .43‡ |
| Sites | ||||
| Heart | 60 (79%) | 15 (94%) | 45 (75%) | N.S. |
| Renal | 32 (42%) | 5 (31%) | 27 (45%) | |
| ANS | 23 (30%) | 8 (50%) | 15 (25%) | |
| PNS | 19 (25%) | 3 (19%) | 16 (27%) | |
| GI | 36 (47%) | 8 (50%) | 28 (47%) | |
| Soft tissue/skin | 32 (42%) | 7 (44%) | 25 (42%) | |
| Liver | 3 (4%) | 1 (6%) | 2 (3%) | N.As. |
| Lungs/pleura | 4 (5%) | 0 | 4 (7%) | |
| Lymph node | 2 (3%) | 0 | 2 (3%) | |
| LVEF (mean ± SD) | 53 ± 9.5 | 55 ± 6.4 | 53 ± 10.2 | .28∗ |
| ≤40% | 9.5% | 0 | 12% | .34† |
| ≤50% | 28% | 13% | 33% | .13† |
| CAD | ||||
| Yes | 6 (8%) | 2 (12%) | 4 (7%) | .6† |
| No | 70 (92%) | 14 (88%) | 56 (93%) | |
| Del 17p | ||||
| Yes | 36 (13%) | 0 (13%) | 1 (13%) | 1† |
| No | 18 (58%) | 13 (69%) | 40 (55%) | |
| N.A. | 22 (29%) | 3 (19%) | 19 (32%) | |
| t(4;14) | ||||
| Yes | 1 (1%) | 0 | 1 (2%) | 1† |
| No | 53 (70%) | 13 (81%) | 40 (67%) | |
| N.A. | 22 (29%) | 3 (19%) | 19 (32%) | |
| Monosomy 13 | ||||
| Yes | 5 (7%) | 0 | 5 (8%) | .32† |
| No | 49 (64%) | 13 (81%) | 36 (60%) | |
| N.A. | 22 (29%) | 3 (19%) | 19 (32%) | |
| Gain 1q | ||||
| Yes | 6 (8%) | 2 (12%) | 4 (7%) | |
| No | 48 (63%) | 11 (69%) | 37 (61%) | .62† |
| N.A. | 22 (29%) | 3 (19%) | 19 (32%) | |
| ≥2 trisomies | ||||
| Yes | 3 (4%) | 0 | 3 (5%) | 1† |
| No | 51 (67%) | 13 (81%) | 38 (63%) | |
| N.A. | 22 (29%) | 3 (19%) | 19 (32%) | |
| t(11;14) | ||||
| Yes | 26 (34%) | 11 (69%) | 15 (25%) | .004† |
| No | 28 (37%) | 2 (12%) | 26 (43%) | |
| N.A. | 22 (29%) | 3 (19%) | 19 (32%) | |
| BM PC percentage (median, IQR) | 15% (7%-20%) | 17% (10%-22%) | 15% (7%-20%) | .08‡ |
| First-line treatment | ||||
| CyBorD | 35 (46%) | 5 (31%) | 30 (50%) | .33† |
| Dara-CyBorD | 28 (37%) | 8 (50%) | 20 (33%) | |
| Other§ | 12 (16%) | 3 (19%) | 9 (15%) | |
| None | 1 (1%) | 0 | 1 (2%) | |
| ASCT | ||||
| Yes | 10 (13%) | 2 (12.5%) | 8 (13%) | 1† |
| No | 66 (87%) | 14 (87.5%) | 52 (87%) |
Bold values indicate statistical significance.
ANS, autonomic nervous system; CML, chronic myeloid leukemia; GI, gastrointestinal tract; N.A., not available; N.As., statistical significance not assessed; N.S., non-statistically significant; PNS, peripheral nervous system; rel/ref, relapsed/refractory; SMM, smoldering MM; WM, Waldenström macroglobulinemia.
Using 2-sample independent t test.
Using Fisher's exact test.
Using Wilcoxon rank-sum test.
Other regimens were bortezomib-dexamethasone, lenalidomide-bortezomib-dexamethasone, daratumumab-bortezomib-dexamethasone, and melphalan-dexamethasone.
The presence of CHIP does not impact MOD-PFS or OS
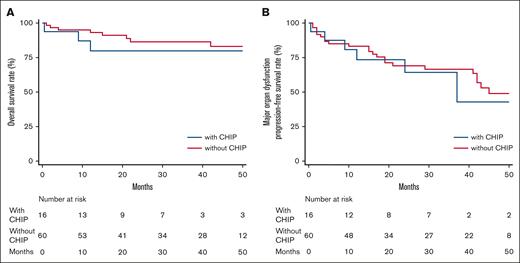
After a median follow-up from diagnosis of 32.5 months (range, 0.5-168), 11 patients (14%) died and 30 (39%) had a MOD-PFS defining event including death. The presence of CHIP was not associated with lower OS (P = .483) (Figure 3A) or with lower MOD-PFS (P = .815) (Figure 3B). In the univariate Cox proportional hazard model, variables associated with an increased hazard of mortality were age (HR, 1.12; 95% CI, 1.04-1.21; P = .003), a Mayo stage >2 (HR, 5.74; 95% CI, 1.22-26.93; P = .027), LVEF ≤40% (HR, 4.11; 95% CI, 1.09-15.53; P = .037), and a co-occurring diagnosis of coronary artery disease (CAD) (HR, 4.94; 95% CI, 1.31-18.63; P = .018). In this cohort, CHIP was not associated with an increased hazard of mortality.
OS and MOD-PFS. Kaplan-Meier curves showing OS (A) and MOD-PFS (B) expressed in months among patients with AL amyloidosis with (blue line) and without (red line) CHIP.
OS and MOD-PFS. Kaplan-Meier curves showing OS (A) and MOD-PFS (B) expressed in months among patients with AL amyloidosis with (blue line) and without (red line) CHIP.
We then assessed MOD-PFS, and the variables associated with an increased hazard of MOD-defining events were Mayo stage IIIA or IIIB (HR, 4.68; 95% CI, 1.94-11.29; P = .001), an LVEF ≤40% (HR, 3.09; 95% CI, 1.15-8.34; P = .026), a diagnosis of CAD (HR, 5.43; 95% CI, 2.13-13.88; P < .001), and the presence of orthostatic hypotension requiring treatment (HR, 2.35; 95% CI, 1.08-5.10; P = .030). CHIP was not associated with an increased hazard of MOD-defining events.
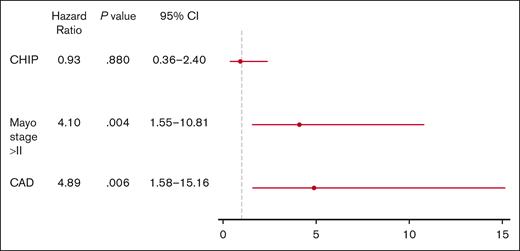
A multivariate Cox regression model stratified by age (44-54, 55-64, 65-71, >71) for MOD-PFS was constructed, which included CAD and Mayo stage IIIA or IIIB, in addition to CHIP status. Mayo stage IIIA or IIIB (HR, 4.10; 95% CI, 1.55-10.81; P = .004) and the presence of CAD (HR, 4.89; 95% CI, 1.58-15.16; P = .006) were associated with an increased hazard of MOD-defining events (Figure 4). CHIP was not associated with an increased hazard of MOD-defining events in the multivariate models.
Multivariate Cox regression model. Forest plot recapitulating MOD-PFS determinants in terms of HRs. Mayo stage >II and the presence of CAD were found to be statistically significant negative MOD-PFS predictors (P = .004 and P = .006, respectively).
Multivariate Cox regression model. Forest plot recapitulating MOD-PFS determinants in terms of HRs. Mayo stage >II and the presence of CAD were found to be statistically significant negative MOD-PFS predictors (P = .004 and P = .006, respectively).
We then focused solely on cardiac-specific outcomes assessed at 6 and 12 months. Among patients with cardiac involvement at diagnosis (n = 58), patients harboring CHIP had a lower rate of cardiac organ response than those without CHIP, but this difference was not statistically significant (7/14 [50%] vs 28/44 [64%], respectively; P = .532). Cardiac-specific disease progression was observed in a total of 29 patients (38%), including 8 patients with CHIP (50%) and 21 patients without CHIP (35%). CHIP presence was not associated with a higher hazard of cardiac-specific disease progression in a univariate Cox regression analysis (HR, 1.66; 95% CI, 0.70-3.94; P = .250).
Discussion
Herein, we describe the prevalence, clinical characteristics, and outcome implications of CHIP presence in a cohort of 76 consecutive patients with AL amyloidosis seen at our center. We noted that CHIP occurs at a higher frequency (21%) than expected for an age-matched healthy population (5%-10% depending on the studies).19,20 The median VAF of CHIP-associated mutations was 0.036, lower than what was previously reported in patients with AL amyloidosis but comparable to patients with MM.31,32 We also report on an association between CHIP and the presence of the prognostically adverse t(11;14) translocation. Harboring CHIP was not associated with decreased OS, MOD-PFS, or cardiac PFS, and, although a lower rate of cardiac response was observed, this difference was not statistically significant. The presence of orthostatic hypotension requiring treatment with midodrine was found to be associated with worse MOD-PFS in a univariate analysis but was not included in the multivariate model. Conversely, an association between an underlying diagnosis of CAD and a Mayo stage IIIA or IIIB and decreased MOD-PFS was detected in a multivariate model.
The prevalence of CHIP that we observed in this study (21%) is consistent with the available literature for patients with hematological malignancies, wherein CHIP driven by DTA genes was identified in 15% to 21% of patients.32,33 In our cohort, DNMT3A was the most frequently mutated gene, followed by TET2, which appears consistent with previous studies on patients with PC dyscrasia.32,33
Consistent with prior data in AL patients, CHIP status did not correlate with age or smoking status.32 This is different from what others have observed in the general population and in the context of MM.20,31,38
Furthermore, our study included longitudinal data for a subset of patients. Interestingly, 2 patients (aged 59 and 80 years at the time of diagnosis) with no CHIP detected at the initial RHP evaluation were subsequently found to have a CHIP (DNMT3A and ZRSR2, respectively) after only a few cycles of therapy.
The association between lower Palladini renal stage7 and the presence of CHIP was unexpected, as was the association between CHIP and the presence of the t(11;14). Age was found not to be a significant confounder in the association between t(11;14) and CHIP, making this association even more intriguing. However, in this cohort of patients, it is impossible to ascertain whether CHIP followed or preceded a diagnosis of t(11;14) PC disorder, and the biological significance of this association still needs to be elucidated. No difference in kidney-specific outcome was noted between patients with and without CHIP (data not shown), but given the low number of patients, no adjustment could be performed for the renal involvement at diagnosis or the Palladini renal stage7 in patients with known renal involvement. Larger prospective studies are needed to clarify the significance of these preliminary findings and the potential cause–effect relationship.
In our study, the presence of CHIP was not associated with all-cause mortality or MOD-PFS. Although this finding seems in contrast to previous studies on MM and lymphomas undergoing ASCT,28,31 it is consistent with what was observed in patients with AL amyloidosis in other studies.32,33 However, other possible explanations for the absence of an association between CHIP and worse cardiac outcomes may be the small sample size and the limited follow-up of our series. Furthermore, there may have been a selection bias in the patients enrolled in our study, as critically ill patients who could not travel to our center for care or who died before commencing PC-directed therapy were not included, potentially confounding the association with CHIP.
Interestingly, we identified an association between the presence of orthostatic hypotension requiring treatment and reduced MOD-PFS. Although we chose not to include the variable of orthostatic hypotension requiring treatment in the final multivariate analysis to avoid overfitting the model, the association between this condition and worse outcome persisted when it was included in the multivariate analysis (data not shown). This has not been previously reported and warrants further evaluation.
There are several limitations to our study. First, given the limited sample size, the power of our analysis is significantly reduced, with wide CIs. Second, because of the short duration of follow-up, a low number of events was detected, reducing our capacity to fit more variables into our regression model. Lastly, in our series, t(11;14) did not emerge as a negative prognostic factor and was therefore not included in the multivariate model. This could be due to the use of Dara-CyBorD in over a third of the patients or possibly to the short duration of follow-up and low incidence of events.
In conclusion, we showed that CHIP mutations are frequent in what is, to our knowledge, the largest cohort of patients with AL amyloidosis analyzed to date. We demonstrated an association between CHIP and a lower Palladini renal stage7 at diagnosis and the presence of t(11;14). No impact on OS or MOD-PFS was observed for patients with CHIP compared with patients without CHIP. Larger prospective studies and a more prolonged follow-up are needed to better elucidate the impact of CHIP in patients with AL amyloidosis and to establish a cause-effect relationship with the observed associations.
Acknowledgments
N.B. was funded by the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant agreement number 817997), and the Fondazione AIRC per la Ricerca sul Cancro (IG25739). G.B. thanks the Demarest Lloyd Jr Foundation, the Appleby Cardiac Amyloidosis Fund, and the Darling Fund for their support of the Amyloidosis Program.
The visual abstract was created with BioRender.com.
Authorship
Contribution: G.B. and N.B. designed the study and secured funding for research; P.L. and G.B. wrote the manuscript; P.L. and A.M. performed the analysis; B.E., T.C., M.M., and S.B. collected the data; and all authors critically revised the manuscript and agreed on the final version for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Giada Bianchi, Harvard Institute of Medicine, 4 Blackfan Cir, Boston, MA 02115; email: gbianchi1@bwh.harvard.edu.
References
Author notes
B.E. and A.M. contributed equally to this study.
Original data are available on request from the corresponding author, Giada Bianchi (gbianchi1@bwh.harvard.edu).