Key Points
Both myeloablative and nonmyeloablative consolidation had encouraging efficacy and safety in patients with PCNSL aged 18 to 75 years.
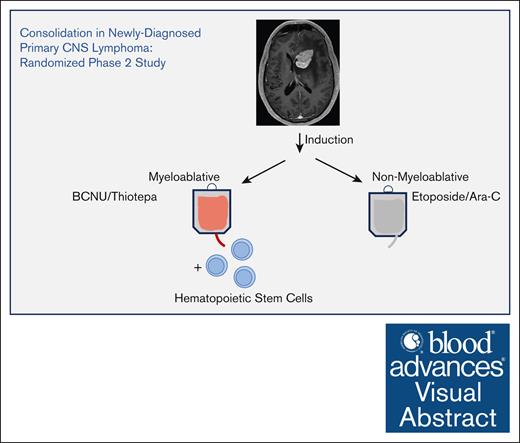
Visual Abstract
Although it is evident that standard-dose whole-brain radiotherapy as consolidation is associated with significant neurotoxicity, the optimal consolidative strategy for primary central nervous system lymphoma (PCNSL) is not defined. We performed a randomized phase 2 clinical trial via the US Alliance cancer cooperative group to compare myeloablative consolidation supported by autologous stem cell transplantation with nonmyeloablative consolidation after induction therapy for PCNSL. To our knowledge, this is the first randomized trial to be initiated that eliminates whole-brain radiotherapy as a consolidative approach in newly diagnosed PCNSL. Patients aged 18 to 75 years were randomly assigned in a 1:1 manner to induction therapy (methotrexate, temozolomide, rituximab, and cytarabine) followed by consolidation with either thiotepa plus carmustine and autologous stem cell rescue vs induction followed by nonmyeloablative, infusional etoposide plus cytarabine. The primary end point was progression-free survival (PFS). A total of 113 patients were randomized, and 108 (54 in each arm) were evaluable. More patients in the nonmyeloablative arm experienced progressive disease or death during induction (28% vs 11%; P = .05). Thirty-six patients received autologous stem cell transplant, and 34 received nonmyeloablative consolidation. The estimated 2-year PFS was higher in the myeloablative vs nonmyeloablative arm (73% vs 51%; P = .02). However, a planned secondary analysis, landmarked at start of the consolidation, revealed that the estimated 2-year PFS in those who completed consolidation therapy was not significantly different between the arms (86% vs 71%; P = .21). Both consolidative strategies yielded encouraging efficacy and similar toxicity profiles. This trial was registered at www.clininicals.gov as #NCT01511562.
Introduction
Primary central nervous system lymphoma (PCNSL) is associated with outcomes inferior to systemic large B-cell lymphoma and is refractory to CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone).1,2 Although the combination of methotrexate, temozolomide, and rituximab (MTR) has been applied with significant efficacy as induction therapy in multicenter investigations in newly diagnosed PCNSL,3,4 it is highly unlikely that methotrexate-based induction strategies alone are curative for a significant fraction of patients.5,6 Standard-dose (36-45 Gy) whole-brain radiotherapy (WBRT) is associated with severe neurotoxicity, particularly in patients aged >60 years.7,8 Although reduced-dose WBRT (23 Gy) has been studied as an alternative to standard-dose WBRT, long-term follow-up is lacking.9 To date, no studies have compared nonradiation-based, dose-intensive chemotherapy consolidation strategies in PCNSL.
In the phase 2 Cancer and Leukemia Group B (CALGB) 50202 study, dose-intensive chemotherapy consolidation after MTR induction consisted of nonmyeloablative, infusional etoposide plus high-dose cytarabine (EA).4,10 In this study, EA consolidation was evaluated only in patients who achieved a complete response (CR) to induction, was determined to be well tolerated in the multicenter setting, and resulted in a 2-year progression-free survival (PFS) of 57%, at least similar to that reported for reduced-dose WBRT.4
Several clinical trials have demonstrated the feasibility and efficacy of consolidation in PCNSL with myeloablative high-dose chemotherapy followed by autologous stem cell transplant (ASCT). Two randomized phase 2 trials demonstrated that high-dose chemotherapy/ASCT is associated with promising 2-year PFS at least comparable with consolidative WBRT but with a reduced frequency of clinical neurotoxicity.11 In particular, the carmustine/thiotepa combination has been applied as a conditioning regimen with encouraging efficacy and safety in newly diagnosed PCNSL.12,13
In this trial, CALGB 51101 (NCT01511562), we compared the outcomes and toxicities of myeloablative consolidation and ASCT using the carmustine/thiotepa conditioning regimen vs those of nonmyeloablative consolidation using dose-intensive EA chemotherapy and tested the hypothesis that myeloablative consolidation would be superior to the nonmyeloablative regimen. Distinct from CALGB 50202, in CALGB 51101, these 2 consolidation approaches were evaluated in patients with PCNSL who achieved stable disease or better with MTR induction followed by single administration of high-dose cytarabine.4
Methods
Study Design
CALGB (Alliance) 51101 is a randomized, open-label, phase 2, multicenter trial. The study was conducted in the Alliance cooperative group in 27 hospitals in the United States. All participating hospitals received approval from their respective institutional review boards.
Patients
Patients with newly diagnosed PCNSL were the target population for this clinical trial. Key inclusion criteria included pathological diagnosis of diffuse large B-cell lymphoma, no concurrent or prior systemic lymphoma, age 18 to 75 years, Karnofsky performance status (KPS) ≥30 in patients aged ≤69 years or ≥50 in patients aged 70 to 75 years, negative serology for human immunodeficiency virus, and no history of organ transplantation. Gender was self-reported by patients as male or female. All patients or legally authorized representatives signed informed consent approved by the institutional review board of each enrolling institution. The study was performed in accordance with the Declaration of Helsinki and the International Conference on Harmonization/Good Clinical Practice.
Randomization
Patients were randomized in a 1:1 allocation to receive induction chemotherapy followed by myeloablative chemotherapy vs induction therapy followed by nonmyeloablative chemotherapy. Patient randomization was performed at the time of registration through the Alliance Registration and Randomization Office.
Randomization was stratified by a composite of age and KPS (age <51 years vs age ≥51 years and KPS ≥70 vs age ≥51 years and KPS < 70) using dynamic allocation.
Procedures
All patients received the same induction therapy consisting of IV methotrexate (8 g/m2 on days 1 and 15 of cycles 1 and 2); temozolomide (150 mg/m2, days 7-11 of cycle 1; and 200 mg/m2, days 7-11 on cycle 2); rituximab (375 mg/m2, days 3, 10, 17, and 24 of cycle 1 and days 3 and 10 on cycle 2). This was followed by methotrexate (8 g/m2, days 1 and 15) and temozolomide (200 mg/m2, days 7-11 for cycles 3 and 4), followed by IV cytarabine (2 g/m2 over 2 hours every 12 hours for 48 hours) for cycle 5. After these 5 cycles, patients in the myeloablative arm underwent stem cell mobilization with granulocyte colony-stimulating factor followed by stem cell collection. This was followed by IV carmustine 400 mg/m2 on day –6, IV thiotepa 5 mg/kg every 12 hours on days –5 and –4, followed by stem cell infusion on day 0 with granulocyte colony-stimulating factor 5 μg/kg per day, days +4, and beyond until absolute neutrophil count was >1500. Patients in the nonmyeloablative arm received IV cytarabine 2 g/m2 over 2 hours every 12 hours for 8 doses (total dose, 16 g/m2) and etoposide 5 mg/kg, administered IV over 96 hours (total dose, 40 mg/kg4) (Figure 1A).
Outcomes
The primary end point of the study was PFS, defined as the time from randomization until disease progression or death from any cause, censoring patients alive and disease free at the date of last disease assessment. Secondary end points included response to induction, event-free survival (EFS), overall survival (OS), assessment of adverse events (AEs) and tolerability, and neurocognition as measured by the Mini-Mental State Examination (MMSE). Response was evaluated using modified International PCNSL Collaborative Group criteria. EFS was defined from randomization until the first disease progression, start of alternative therapy, or death from any cause, censoring event-free patients at last disease assessment. OS was defined as the time from randomization until death from any cause, censoring patients alive at last follow-up.14 AEs were graded using National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events version 4.
Statistical analysis
Initially, the study was designed to test for an improvement in PFS corresponding to an improvement in 2-year PFS from 50% to 70% for patients randomized to the myeloablative vs nonmyeloablative arms. With 95 events in 160 patients, there was 90% power using a 1-sided log-rank test and alpha of 10%, assuming a median PFS of 3 months for patients who develop progression during induction therapy for each arm. Due to the lower-than-expected accrual, the design was modified to provide 84% power with 64 events in 110 patients to detect an improvement in PFS corresponding to an improvement in the 2-year PFS rates from 50% vs 73% in the nonmyeloablative vs myeloablative arms, respectively. Finally, with few events but mature follow-up, the protocol was amended to allow for the primary end point analysis after all patients had been followed for 3 years.
All randomized eligible patients who started induction therapy were included in the primary end point analysis (modified intent-to-treat population). Secondary analyses included the subset of patients who completed consolidation, with time-to-event analyses landmarked at the start of consolidation. PFS, EFS, and OS distributions were estimated using the Kaplan-Meier method. Per protocol, PFS distributions for the primary analysis were compared using a log-rank test; a stratified log-rank test was used for a sensitivity analysis and for other comparisons of time-to-event end points. Medians and 2-year estimates were calculated using Kaplan-Meier methods along with corresponding 95% confidence intervals (CIs). Response rates were estimated along with corresponding exact binomial 95% CIs and compared between arms using Fisher exact tests. Frequency tables of AEs were summarized by maximum severity and types of AEs, and differences in severe AEs (grade 3+) were compared between arms using Fisher exact tests. Similarly, the proportions of patients who required dose modifications, delays, or treatment holds were also summarized. The influence of factors (eg, baseline neurocognitive impairment) on PFS, EFS, and OS were evaluated using multivariable Cox regression models. Unless otherwise specified, all reported P value are 2-sided, and statistical significance was declared for P value <.05. All data collection and analyses were performed by the Alliance Statistics and Data Management Center using SAS version 9.4. Data quality was ensured by review of data by the Alliance Statistics and Data Management Center and by the study chairperson following Alliance policies. Data were locked as of 20 January 2021.
Role of the funding source
This study was conducted through the Alliance cooperative group, which is funded by the NCI. The NCI provided oversight for the design and implementation of this trial but not in the data collection, analysis, interpretation of the results, or the writing of the report.
The study was approved by the institutional review board of each of the participating institutions.
Results
Of a total of 113 patients registered between 17 November 2012 and 2 May 2017, a total of 57 were randomized to receive myeloablative treatment, and 56 were randomized to receive nonmyeloablative treatment across 27 centers. Five patients were ineligible or did not start protocol treatment. A total of 108 patients who were eligible and started treatment comprise the modified intent-to-treat population and are included in the primary end point analysis (54 in each arm; Figure 1B).
Table 1 shows the distributions of baseline characteristics by treatment arm for 108 eligible patients who started induction therapy. There were no significant differences between the 2 arms with respect to the following prognostic variables: age, KPS, ophthalmologic involvement by lymphoma, involvement of cerebrospinal fluid (CSF) by lymphoma, or deep brain location.
Patient characteristics
| Characteristic . | All patients (N = 108) . | Myeloablative (n = 54) . | Nonmyeloablative (n = 54) . |
|---|---|---|---|
| Age, y | |||
| Median (IQR) | 61 (54-67) | 61 (52-67) | 61 (55-68) |
| Range | 33-75 | 34-74 | 33-75 |
| KPS | |||
| Median (IQR) | 80 (70-90) | 80 (70-90) | 70 (60-90) |
| Range | 30-100 | 30-100 | 30-100 |
| Age/KPS risk groups, n (%) | |||
| Age <51 y, any KPS | 20 (19) | 10 (19) | 10 (19) |
| Age ≥51 y, KPS ≥70 | 65 (60) | 33 (61) | 32 (59) |
| Age ≥51 y, KPS <70 | 23 (21) | 11 (20) | 12 (22) |
| Female, n (%) | 47 (44) | 22 (41) | 25 (46) |
| Elevated LDH, n (%) | 26 (25) | 13 (25) | 13 (24) |
| Deep brain involvement, n (%) | 46 (43) | 24 (44) | 22 (41) |
| Slit lamp result, n (%) | |||
| Normal | 90 (87) | 43 (83) | 47 (90) |
| Minor RPE abnormality | 7 (7) | 3 (6) | 4 (8) |
| Decrease in vitreous cells of retinal infiltrate | 1 (1) | 1 (2) | 0 (0) |
| Recurrent or new disease | 6 (6) | 5 (10) | 1 (2) |
| CSF Cytology, n (%) | |||
| Negative | 67 (76) | 35 (80) | 32 (73) |
| Atypical or suspicious | 13 (15) | 6 (14) | 7 (16) |
| Positive | 8 (9) | 3 (7) | 5 (11) |
| Characteristic . | All patients (N = 108) . | Myeloablative (n = 54) . | Nonmyeloablative (n = 54) . |
|---|---|---|---|
| Age, y | |||
| Median (IQR) | 61 (54-67) | 61 (52-67) | 61 (55-68) |
| Range | 33-75 | 34-74 | 33-75 |
| KPS | |||
| Median (IQR) | 80 (70-90) | 80 (70-90) | 70 (60-90) |
| Range | 30-100 | 30-100 | 30-100 |
| Age/KPS risk groups, n (%) | |||
| Age <51 y, any KPS | 20 (19) | 10 (19) | 10 (19) |
| Age ≥51 y, KPS ≥70 | 65 (60) | 33 (61) | 32 (59) |
| Age ≥51 y, KPS <70 | 23 (21) | 11 (20) | 12 (22) |
| Female, n (%) | 47 (44) | 22 (41) | 25 (46) |
| Elevated LDH, n (%) | 26 (25) | 13 (25) | 13 (24) |
| Deep brain involvement, n (%) | 46 (43) | 24 (44) | 22 (41) |
| Slit lamp result, n (%) | |||
| Normal | 90 (87) | 43 (83) | 47 (90) |
| Minor RPE abnormality | 7 (7) | 3 (6) | 4 (8) |
| Decrease in vitreous cells of retinal infiltrate | 1 (1) | 1 (2) | 0 (0) |
| Recurrent or new disease | 6 (6) | 5 (10) | 1 (2) |
| CSF Cytology, n (%) | |||
| Negative | 67 (76) | 35 (80) | 32 (73) |
| Atypical or suspicious | 13 (15) | 6 (14) | 7 (16) |
| Positive | 8 (9) | 3 (7) | 5 (11) |
IQR, interquartile range; LDH, lactate dehydrogenase; RPE, retinal pigment epithelium.
There were 6 grade 5 AEs during induction, including 4 in the nonmyeloablative arm (7.4%; sepsis, acute kidney injury, death not otherwise specified, and neoplasms benign/malignancy/other) and 2 in the myeloablative arm (3.7%; sudden death not otherwise specified and other malignancy). There were no grade 5 AEs reported during consolidation therapy. One patient in the myeloablative arm experienced a grade 5 AE, a depression/suicide event that occurred 16 months after the end of treatment.
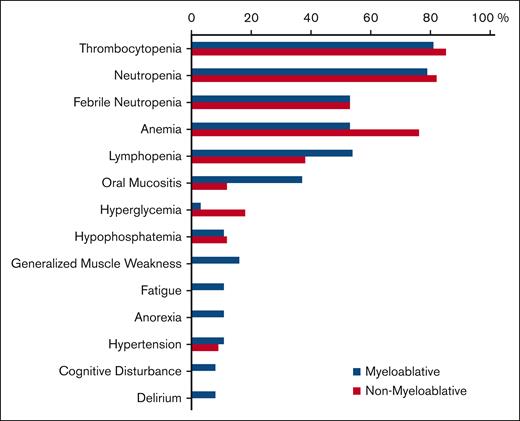
Notable grade ≥3 AEs during or after consolidation are presented by consolidation arm (Figure 2). Most patients experienced a grade ≥3 hematologic AE, with a comparable proportion between myeloablative and nonmyeloablative arms (92% vs 94%; P = .99). Grade ≥3 nonhematologic AEs were not significantly different between the 2 arms (79% in the myeloablative arm vs 68% in the nonmyeloablative arm; P = .30). All grade ≥3 AEs reported during induction and after the start of consolidation are provided in the supplemental Table 3.
Incidence of grade ≥3 AEs during consolidation. Comparison of myeloablative vs nonmyeloablative therapies is as follows: thrombocytopenia, 81% vs 85%; neutropenia, 79% vs 82%; febrile neutropenia, 53% vs 53%; anemia, 53% vs 76%; lymphopenia, 54% vs 38%; oral mucositis, 37% vs 12%; hyperglycemia, 3% vs 18%; hypophosphatemia, 11% vs 12%; generalized muscle weakness, 16% vs 0%; fatigue, 11% vs 0%; anorexia, 11% vs 0%; hypertension, 11% vs 9%; cognitive disturbance, 8% vs 0%; delirium, 8% vs 0%, respectively. Although the incidence of grade ≥3 cytopenias and febrile neutropenia were comparable between the consolidative arms, 2 patients on the myeloablative arm experienced grade 4 sepsis vs none in the nonmyeloablative arm. Furthermore, 3 patients in the myeloablative arm experienced grade 3 cognitive disturbances vs none in the nonmyeloablative arm; 1 of these occurred in a patient within 3 months after ending treatment, but the other 2 patients had these events occur 16 and 24 months after the end of treatment. Furthermore, among 108 patients who received induction therapy, the numbers of patients with dose modifications, omissions, or delays due to any of the 4 drugs administered were 84 (78%), 38 (35%), and 73 (68%), respectively. The numbers of patients with dose modifications during MTR induction followed by single administration of high-dose cytarabine induction were 81 (75%), 30 (28%), 1 (<1%), and 6 (6%) for each agent, respectively; the numbers of patients with dose omissions were 21 (19%), 24 (22%), 10 (9%), and 1 (<1%) for each agent, respectively; and the numbers of patients with dose delays were 57 (53%), 26 (24%), 18 (17%), and 6 (6%) for each agent, respectively.
Incidence of grade ≥3 AEs during consolidation. Comparison of myeloablative vs nonmyeloablative therapies is as follows: thrombocytopenia, 81% vs 85%; neutropenia, 79% vs 82%; febrile neutropenia, 53% vs 53%; anemia, 53% vs 76%; lymphopenia, 54% vs 38%; oral mucositis, 37% vs 12%; hyperglycemia, 3% vs 18%; hypophosphatemia, 11% vs 12%; generalized muscle weakness, 16% vs 0%; fatigue, 11% vs 0%; anorexia, 11% vs 0%; hypertension, 11% vs 9%; cognitive disturbance, 8% vs 0%; delirium, 8% vs 0%, respectively. Although the incidence of grade ≥3 cytopenias and febrile neutropenia were comparable between the consolidative arms, 2 patients on the myeloablative arm experienced grade 4 sepsis vs none in the nonmyeloablative arm. Furthermore, 3 patients in the myeloablative arm experienced grade 3 cognitive disturbances vs none in the nonmyeloablative arm; 1 of these occurred in a patient within 3 months after ending treatment, but the other 2 patients had these events occur 16 and 24 months after the end of treatment. Furthermore, among 108 patients who received induction therapy, the numbers of patients with dose modifications, omissions, or delays due to any of the 4 drugs administered were 84 (78%), 38 (35%), and 73 (68%), respectively. The numbers of patients with dose modifications during MTR induction followed by single administration of high-dose cytarabine induction were 81 (75%), 30 (28%), 1 (<1%), and 6 (6%) for each agent, respectively; the numbers of patients with dose omissions were 21 (19%), 24 (22%), 10 (9%), and 1 (<1%) for each agent, respectively; and the numbers of patients with dose delays were 57 (53%), 26 (24%), 18 (17%), and 6 (6%) for each agent, respectively.
Among the 38 patients receiving myeloablative consolidation therapy, 2 (5%) had dose modifications of thiotepa, and 2 (5%) had dose delays; there were no omissions of drug. Two patients who began the myeloablative consolidation regimen did not complete it; 1 refused further treatment after stem cell mobilization, and another had unsuccessful stem cell collection. Among the 34 patients receiving nonmyeloablative consolidation therapy, 1 (3%) required a dose modification to cytarabine, and 2 (6%) had dose delays; there were no dose omissions. Overall, of 72 patients who received consolidation, only 2 did not complete consolidation therapy, and they were the 2 randomized to myeloablative therapy who discontinued treatment during stem cell collection, described above. Thus, the landmark analyses from the start of consolidation focus on the 70 patients who started and completed the core component of the consolidation therapy.
Among the 108 eligible patients who started induction therapy, 85 (79%) completed all 5 cycles. At the conclusion of induction therapy, 54 (50%; 95% CI, 40-60) achieved either a confirmed or unconfirmed CR, and 24 achieved a partial response for an overall radiographic response rate of 72% (95% CI, 63-80). By the end of induction, 17 (15.7%) had progressive disease (PD); specifically, 9% of those randomized to myeloablative vs 24% of those randomized to nonmyeloablative arms. As shown in Table 2, although all patients received the same induction regimen, more patients randomized to the myeloablative arm responded (CR/unconfirmed CR/partial response) to MTR than patients randomized to the nonmyeloablative arm (81% vs 63%; P = .026). Among those randomized to myeloablative consolidation therapy, 89% had a response or stable disease at the end of induction and were thus eligible to receive consolidation therapy, vs 70% of those randomized to nonmyeloablative consolidation therapy (P = .015).
Responses to induction
| . | All (N = 108) . | Myeloablative (n = 54) . | Nonmyeloablative (n = 54) . | P value . |
|---|---|---|---|---|
| Response, n (%) | ||||
| CR | 29 (27) | 12 (22) | 17 (31) | |
| CRu | 25 (23) | 18 (33) | 7 (13) | |
| PR | 24 (22) | 14 (26) | 10 (19) | .047 |
| SD | 8 (7) | 4 (7) | 4 (7) | |
| PD | 18 (17) | 5 (9) | 13 (24) | |
| Not Evaluated | 4 (4) | 1 (2) | 3 (6) | |
| CR [CR/CRu], n (%) | 54 (50) | 30 (56) | 24 (44) | .17 |
| Overall objective response [CR/CRu/PR], n (%) | 78 (72) | 44 (81) | 34 (63) | .026 |
| Clinical benefit rate [CR/CRu/PR/SD], n (%) | 86 (80) | 48 (89) | 38 (70) | .015 |
| . | All (N = 108) . | Myeloablative (n = 54) . | Nonmyeloablative (n = 54) . | P value . |
|---|---|---|---|---|
| Response, n (%) | ||||
| CR | 29 (27) | 12 (22) | 17 (31) | |
| CRu | 25 (23) | 18 (33) | 7 (13) | |
| PR | 24 (22) | 14 (26) | 10 (19) | .047 |
| SD | 8 (7) | 4 (7) | 4 (7) | |
| PD | 18 (17) | 5 (9) | 13 (24) | |
| Not Evaluated | 4 (4) | 1 (2) | 3 (6) | |
| CR [CR/CRu], n (%) | 54 (50) | 30 (56) | 24 (44) | .17 |
| Overall objective response [CR/CRu/PR], n (%) | 78 (72) | 44 (81) | 34 (63) | .026 |
| Clinical benefit rate [CR/CRu/PR/SD], n (%) | 86 (80) | 48 (89) | 38 (70) | .015 |
CRu, unconfirmed CR; PR, partial remission; SD, stable disease.
Of the 108 patients who started induction therapy, 23 did not complete the induction treatment, and an additional 13 patients completed induction but opted not to continue to consolidation therapy. Among the 54 evaluable patients randomized to the myeloablative arm, 9 (17%) did not complete induction (3 due to PD, 2 for AEs, 1 death, and 3 for other reasons), and 7 completed induction but did not receive consolidation (2 due to PD, 3 treatment refusals, and 2 for other reasons, including insurance coverage). In the 54 evaluable patients randomized to the nonmyeloablative arm, 14 (26%) did not complete induction (7 due to PD, 4 for treatment refusal, and 3 deaths), and 6 completed induction but did not receive consolidation (5 due to PD and 1 for treatment refusal).
Collectively, there was an imbalance between the myeloablative and nonmyeloablative arms in the proportion of patients who went off treatment due to progression or death (11% vs 28%; P = .049) before the start of consolidation, despite patients receiving the same induction therapy.
The median PFS from the time of randomization was 6 years (3.9 to not reached) vs 2.4 years (0.6 to not reached) in the myeloablative and the nonmyeloablative arms (hazard ratio [HR], 0.51; 95% CI, 0.29-0.90; P =. 02, by log-rank and stratified log-rank tests), repsectively. As shown in Figure 3A, there is early separation in the PFS curves before consolidation, highlighting the differences introduced between arms during the common induction treatment phase. Specifically, 6-month PFS estimates were 89% (95% CI, 77-95) vs 71% (95% CI, 56-81) in the myeloablative and nonmyeloablative arms, respectively. By 2 years, the estimated PFS rates were 73% (95% CI, 58-83) vs 51% (95% CI, 36-63) in the myeloablative and nonmyeloablative arms, respectively.
Kaplan-Meier analysis of PFS and OS in 51101. (A) PFS of the modified intent-to-treat population according to consolidation group. (B) PFS from the time of consolidation according to consolidation group. (C) OS of the modified intent-to-treat according to consolidation group. (D) OS from the time of consolidation according to consolidation group.
Kaplan-Meier analysis of PFS and OS in 51101. (A) PFS of the modified intent-to-treat population according to consolidation group. (B) PFS from the time of consolidation according to consolidation group. (C) OS of the modified intent-to-treat according to consolidation group. (D) OS from the time of consolidation according to consolidation group.
Per protocol, a secondary analysis was performed for the subset of patients who completed consolidation therapy. This secondary analysis was important given the imbalance in the number of patients with significant events precluding the start of consolidation therapy. Among 72 patients who went on to consolidation therapy in this study, 70 were able to complete that therapy. Clinical characteristics of these 70 patients who went on to consolidation were balanced between arms (supplemental Table 1). Of these 70 patients (36 completing myeloablative consolidation and 34 completing nonmyeloablative consolidation), there was a nonsignificant trend for longer PFS in the myeloablative arm than the nonmyeloablative arm (HR, 0.58; 95% CI, 0.25-1.36; P = .21), with estimated 2-year PFS rates after the start of consolidation of 86% (95% CI, 69-94) vs 71% (95% CI, 52-83), respectively (Figure 3B). Across both treatment arms, the overall median PFS was 4.9 years (95% CI, 2.5 to not reached), and the estimated 2-year PFS rate was 62% (95% CI, 52-71).
Nine patients received nonprotocol therapy before a PFS event (5 in the absence of a PFS event) and are included as events for EFS. In the subset of patients who completed consolidation, EFS estimates at 2 years after the start of consolidation were higher in the myeloablative arm vs the nonmyeloablative arm (86% [95% CI, 69%- 94%] vs 68% [95% CI, 49%-81%]), but there was not a significant difference between the EFS curves (HR, 0.61; 95% CI, 0.27-1.37; P = .22).
With a median follow-up of 4.1 years, there have been 26 deaths in the 108 evaluable patients. Median OS was not reached in either arm, and there was no difference in OS in the myeloablative vs nonmyeloablative arms (HR, 0.60; 95% CI, 0.27-1.31; P = .19; Figure 3C). OS estimates at 2 years were 87% (95% CI, 74-93) and 78% (95% CI, 64-87) in those randomized to the myeloablative and nonmyeloablative arms, respectively. In the subset of patients who completed consolidation, there were only 8 deaths (5 in the myeloablative arm and 3 in the nonmyeloablative arm). Few deaths occurred in the first 2 years after the start of consolidation, with 2-year OS estimates of 97% (95% CI, 81-100) and 91% (95% CI, 75-97) in the myeloablative and the nonmyeloablative arms, respectively (Figure 3D). Additional planned correlative secondary end points per protocol will be presented in a future publication.
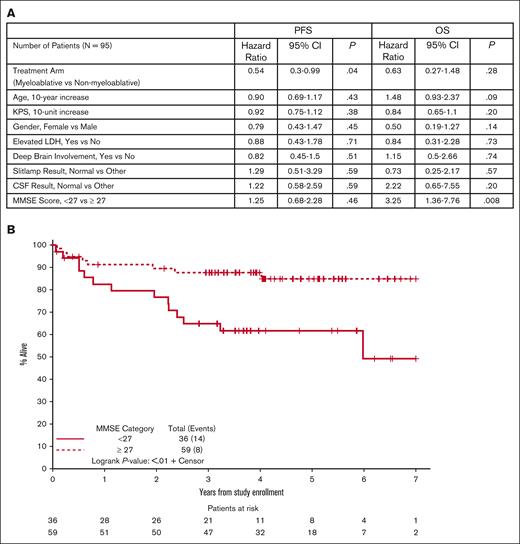
To identify individual clinical prognostic variables, we first evaluated candidates from the International Extranodal Lymphoma Study Group and Memorial Sloan-Kettering prognostic scoring systems for PCNSL.15,16 Outcome was not correlated with age, KPS, lactate dehydrogenase, deep brain involvement, or CSF involvement. We also considered baseline MMSE scores, available in 99 patients, of which 95 were evaluable for the primary end point. Unlike other clinical variables considered, baseline MMSE score was an independent prognostic variable for OS (but not PFS). Using data of van der Meulen et al,17 plus independent recursive partitioning analysis, we identified a cut point for the baseline MMSE score of 27 as a categorical variable, in which scores <27 correlated with inferior OS (HR, 3.25; 95% CI, 1.36-7.76; P = .008, Figure 4A). Results were retained even after adjusting for treatment arm and age in the model (HR, 3.36; 95% CI, 1.39-8.12; P = .006).
Prognostic variables including MMSE in CALGB 51101. (A) Analysis of baseline clinical characteristics, adjusting for treatment arm, demonstrated that baseline MMSE was the only variable that correlated with OS. Mean baseline MMSE score for the myeloablative group was 25.7 (SD, 5.92). Mean baseline MMSE score for the nonmyeloablative group was 25.8 (SD, 6.31). Median baseline MMSE scores were 28 in each group. MMSE scores were summarized as a continuous measure as well as categorically, both as any neurocognitive impairment (MMSE, <27 vs not) as well as level of neurocognitive impairment (severe, 0-9; vs moderate, 10-20; vs mild, 21-26; vs normal, 27-30). (B) Kaplan-Meier analysis using a MMSE score of 27 as a categorical variable suggests a significant correlation between baseline neurocognitive impairment on survival in PCNSL in CALGB 51101 (P < .01). The MMSE score of 27 as a categorical variable significantly correlated with OS in patients who received myeloablative therapy (P < .02) and nonmyeloablative therapy (P < .07). We found that the negative impact of impaired neurocognition is heavily influenced by the significant impact of severe cognitive impairment (MMSE score, 0-9) on OS in relation to those with normal cognition at baseline (HR, 19.8; 95% CI, 4.94-79.0; P < .001), even after adjusting for age and treatment arm. In this same model, we found that the mild (MMSE, 21-26) and moderate (MMSE, 10-20) cognitive impairment corresponded to a tendency toward worse OS outcomes (mild HR, 2.85; 95% CI, 1.06-7.66; P = .03; moderate HR, 2.42; 95% CI, 0.62-9.43; P = .20). Furthermore, although an MMSE <27 (vs not) at baseline was not associated with worse PFS, we found that severe cognitive impairment at baseline was significantly associated with worse PFS (HR, 7.32; 95% CI, 2.09-25.6; P = .001) in relation to those with normal baseline cognition, even adjusting for age and treatment arm. Caveats with these findings are based on the fact that there were more limited numbers of patients with moderate (n = 9) and severe (n = 3) cognitive impairment at baseline. Furthermore, those with MMSE scores at baseline of at least 27 (ie, no cognitive impairment) tended to have better KPS than those with any cognitive impairment (median, 80 vs 70; P < .001); based on the nonsignificant influence of KPS on OS (P = .27), this does not appear to have any confounding effects on the influence of baseline MMSE on OS. No other baseline characteristics were significantly associated with baseline MMSE status. Baseline MMSE continuous scores as well as categorical status (eg, <27 vs ≥27) were also not significantly different between the treatment arms. (Figure 4A; supplemental Table 2). SD, standard deviation.
Prognostic variables including MMSE in CALGB 51101. (A) Analysis of baseline clinical characteristics, adjusting for treatment arm, demonstrated that baseline MMSE was the only variable that correlated with OS. Mean baseline MMSE score for the myeloablative group was 25.7 (SD, 5.92). Mean baseline MMSE score for the nonmyeloablative group was 25.8 (SD, 6.31). Median baseline MMSE scores were 28 in each group. MMSE scores were summarized as a continuous measure as well as categorically, both as any neurocognitive impairment (MMSE, <27 vs not) as well as level of neurocognitive impairment (severe, 0-9; vs moderate, 10-20; vs mild, 21-26; vs normal, 27-30). (B) Kaplan-Meier analysis using a MMSE score of 27 as a categorical variable suggests a significant correlation between baseline neurocognitive impairment on survival in PCNSL in CALGB 51101 (P < .01). The MMSE score of 27 as a categorical variable significantly correlated with OS in patients who received myeloablative therapy (P < .02) and nonmyeloablative therapy (P < .07). We found that the negative impact of impaired neurocognition is heavily influenced by the significant impact of severe cognitive impairment (MMSE score, 0-9) on OS in relation to those with normal cognition at baseline (HR, 19.8; 95% CI, 4.94-79.0; P < .001), even after adjusting for age and treatment arm. In this same model, we found that the mild (MMSE, 21-26) and moderate (MMSE, 10-20) cognitive impairment corresponded to a tendency toward worse OS outcomes (mild HR, 2.85; 95% CI, 1.06-7.66; P = .03; moderate HR, 2.42; 95% CI, 0.62-9.43; P = .20). Furthermore, although an MMSE <27 (vs not) at baseline was not associated with worse PFS, we found that severe cognitive impairment at baseline was significantly associated with worse PFS (HR, 7.32; 95% CI, 2.09-25.6; P = .001) in relation to those with normal baseline cognition, even adjusting for age and treatment arm. Caveats with these findings are based on the fact that there were more limited numbers of patients with moderate (n = 9) and severe (n = 3) cognitive impairment at baseline. Furthermore, those with MMSE scores at baseline of at least 27 (ie, no cognitive impairment) tended to have better KPS than those with any cognitive impairment (median, 80 vs 70; P < .001); based on the nonsignificant influence of KPS on OS (P = .27), this does not appear to have any confounding effects on the influence of baseline MMSE on OS. No other baseline characteristics were significantly associated with baseline MMSE status. Baseline MMSE continuous scores as well as categorical status (eg, <27 vs ≥27) were also not significantly different between the treatment arms. (Figure 4A; supplemental Table 2). SD, standard deviation.
Discussion
The results of CALGB 51101, which to our knowledge is the first randomized trial for PCNSL to be initiated in which neither arm involved WBRT, strongly support the feasibility, safety, and efficacy of 2 dose-intensive chemotherapy-based consolidation strategies, with both myeloablative and nonmyeloablative arms achieving excellent PFS and OS without radiotherapy. Although comparisons between the 2 arms are limited by relatively small sample size and confounded by significant differences in the frequency of disease progression and death as well as by response proportions between the 2 arms during identical induction therapy, the estimated 2-year PFS rates for the myeloablative arm of 73% is encouraging and consistent with the outcomes in other phase 2 randomized trials that evaluated ASCT in newly diagnosed PCNSL.11,18 Notably, Kaplan-Meier analysis demonstrates that the nonmyeloablative arm showed evidence for the emergence of a stable plateau in the PFS curve starting at 4 years, similar to previous studies using EA consolidation.4
Notably, Illerhaus et al presented results of the IELSG43 trial in PCNSL that compared a BCNU/thiotepa-based transplant-based consolidation with a distinct nonmyeloablative consolidation program, the DeVic regimen, based on ifosfamide, etoposide plus carboplatin. In this phase 3 study, PFS and OS results, currently available in abstract form (2022 annual meeting of the American Society of Hematology), significantly favored ASCT compared with the nonmyeloablative regimen.
It is important to note that CALGB 51101 also supports the feasibility of autologous stem cell transplant as consolidation in older patients with PCNSL, consistent with the results of the MARITA trial, which demonstrated the safety and efficacy of myeloablative consolidation in 14 older patients with PCNSL, aged 69 to 79 years.19
Notably, in CALGB 51101, the incidence of disease progression during the first year after consolidation was markedly higher with nonmyeloablative, EA-based consolidation. However, 1 year after consolidation, the frequency of CNSL progression was higher among patients who received the myeloablative therapy, likely reflecting the differential impact of dose intensity in chemotherapy-based consolidation on the timing of early vs delayed progression in PCNSL.
Both regimens were well tolerated in multicenter execution, and there was limited severe clinical neurotoxicity; however, detailed formal neurocognitive testing has not yet been completed. Importantly, there was no treatment-related mortality associated with either consolidative arm in this study. Notably, this is distinct from other prospective studies of similar size, in which ASCT, using a different consolidation regimen, thiotepa, busulfan, and cyclophosphamide, has been reproducibly associated with treatment-related mortality rates of ∼11%.11,20 Our results therefore support the safety and efficacy of the carmustine/thiotepa combination as a transplant conditioning regimen in newly diagnosed PCNSL, in patients aged 18 to 75 years.
Importantly, CALGB 51101 also highlights the limitations of current methotrexate-based induction approaches in PCNSL. Although the MTR induction followed by single administration of high-dose cytarabine combination was well tolerated and associated with an overall 50% rate of CR, 19.4% of patients with newly diagnosed PCNSL in this study exhibited disease progression or died during induction therapy, without receipt of consolidation, similar to the experience in previous studies.4,6,10,11 Identification of molecular biomarkers that identify the subpopulation of patients with PCNSL destined to experience early disease progression during methotrexate-based induction therapy is a research priority, as is the incorporation of targeted agents with greater antilymphoma efficacy within induction strategies. Based on the CSF penetration and activity of lenalidomide in relapsed CNSLs,21,22 as well as evidence for activity of checkpoint blockade in this setting,23 a successor trial, Alliance A051901 (NCT04609046), has been developed to address the need for more effective induction strategies in PCNSL.
Our study confirms that baseline MMSE score in PCNSL has independent prognostic significance.18 In our data set inclusive of patients aged ≤75 years, it was the most significant individual clinical prognostic variable. We conclude that baseline MMSE score needs to be considered a prognostic factor in PCNSL, used in patient counseling in practice, and in risk stratification within design of future trials.
Acknowledging the limitations of a randomized phase 2 trial, Alliance 51101 demonstrates, to our knowledge, for the first time that each dose-intensive consolidation strategy, myeloablative and nonmyeloablative, provides excellent disease control after MTR induction therapy in PCNSL, with acceptable toxicity. Although there is a nonsignificant trend toward improved PFS, but not OS, among patients treated in the myeloablative arm, we envision that further insights into PCNSL biology will identify the subset of patients who require ASCT for optimal outcome as well as the subset who can achieve long-term survival and potentially cure with nonmyeloablative therapy alone.
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA180820 and UG1CA233196 (to the Eastern Cooperative Oncology Group), U10CA180888 and UG1CA233230 (to the Southwest Oncology Group), UG1CA233180, UG1CA233331, UG1CA233339, and R01CA139-83-01A1 (J.L.R.); and the Leukemia and Lymphoma Society (J.L.R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. https://acknowledgments.alliancefound.org.
The authors are grateful to the patients and their families for participation in this clinical study. The authors also acknowledge the essential contributions of the physicians, nurses, and study coordinators from the 27 participating sites.
Authorship
Contribution: T.T.B. wrote the first draft of the manuscript with input from S.G., A.S.R., S.M.G., S.E.S., N.M., L.J.S., J.W.F., B.S.K., N.L.B., E.D.H., B.D.C., N.W.-J., L.N., J.P.L., and J.L.R.; S.G., A.S.R., and S.M.G. performed the statistical analysis; S.G. and S.M.G. directly accessed and verified the underlying data reported in this manuscript; and all authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict-of-interest disclosure: T.T.B. reports funding from ONO Pharmaceuticals. A.S.R. reports funding from Eli Lilly and Telios. N.M. reports funding from Biogen. L.J.S. reports funding from AbbVie. B.S.K reports funding from Genentech/Roche. N.L.B. reports funding from ADC Therapeutics, Affimed, Autolus, BMS/Celgene, Forty Seven, Gilead/Kite Pharma, Immune Design, Janssen, Merck, Millennium, Pfizer, Pharmacyclics, Roche/Genentech, and Seattle Genetics. E.D.H. reports funding from Eli Lilly, AbbVie, Virtuoso Therapeutics, Novartis, Astellas, and CytomX. N.W.-.J. reports funding from Karyopharm, Seagen, Grunenthal, Epizyme, ERSA, Cuno Science, and Dava Oncology. L.N. reports consulting fees from Ono, Genmab, Brave Bio, Miltenyi, and Curis; advisory board fees from Ono and Kite/Gilead; royalty from UpToDate (Wolters Kluwer); and grant from Leukemia and Lymphoma Society. J.P.L. reports funding from Regeneron, MEI Pharma, Genmab, Karyopharm, Roche/Genentech, Epizyme, Celgene/BMS, Incyte, AbbVie, Sutro, AstraZeneca, Bayer, Janssen, Eisai, Mustang Bio, Second Genome, Merck, Constellation, Grail, Pfizer, Caribou Biosciences, Astellas, Lilly, Beigene, Kite/Gilead, Novartis, Seagen, and Lymphoma Research Foundation. J.L.R. reports funding from Incyte and NURIX. The remaing authors declare no competing financial interests.
Correspondence: Tracy Batchelor, Department of Neurology, Hale Building for Transformative Medicine, 4th Floor, Brigham and Women’s Hospital, 60 Fenwood Rd, Boston, MA 02115; email: tbatchelor@bwh.harvard.edu.
References
Author notes
Presented in Oral Abstract Form at the 2020 and 2021 Annual Meetings of the American Society of Clinical Oncology.
Deidentified patient data may be requested from Alliance for Clinical Trials in Oncology via concepts@alliancenctn.org if data are not publicly available. A formal review process includes verifying the availability of data, conducting a review of any existing agreements that may have implications for the project, and ensuring that any transfer is in compliance with the institutional review board. The investigator will be required to sign a data release form before transfer.
The full-text version of this article contains a data supplement.