In this issue of Blood Advances, Lauruschkat et al move us forward in our understanding of the immune signature of individuals who do vs do not control cytomegalovirus (CMV) after cessation of letermovir.1 Letermovir for prevention of CMV reactivation after allogeneic stem cell transplantation (allo-HCT) has resulted in a significant reduction in the incidence of clinically significant CMV infection (csCMVi) and CMV–related nonrelapse mortality. CMV reactivation after discontinuation of letermovir occurs in 12% to 45% of at-risk allo-HCT recipients.2,3 Risk factors for the development of csCMVi after letermovir discontinuation have been identified,4 but disappointingly prolonging the duration of prophylaxis reduces the risk of reactivation in some5 but not all high-risk populations.6 In addition, there is evidence that prophylaxis may delay CMV–specific T cell reconstitution and late events can frequently occur even after the use of letermovir for prolonged durations beyond 100 days.7 Taken together, this landscape highlights the critical need to identify immune milestones that can predict control of CMV in recipients at risk of csCMVi.
In the absence of letermovir prophylaxis, some milestones of immune reconstitution critical for the control of CMV reactivation have been identified: investigators have identified (using mass cytometry) an early immune signature with predominance of innate cells (monocytes and natural killer [NK] cells) and a later adaptive signature characterized by memory–exposed activated effector memory T cells and effector memory T cells reexpressing CD45RA CD8+ T cells.8 Other groups have evaluated the use of functional assays, some of which are commercially available.9,10 However, these studies have not been performed in recipients of letermovir-based prophylaxis who are at risk for late csCMVi.
Thus, there is a critical need to define the immune reconstitution milestones required to control CMV in the letermovir era. This article identifies the immune signature of individuals who do vs do not control CMV after cessation of letermovir. Although the cohort is small, 3 groups are evaluated: those who do not have csCMVi after discontinuation, those who have csCMVi but not refractory viremia or end-organ disease, and those who have refractory viremia or end-organ disease. These authors previously identified alterations in NK and T cell populations, including low numbers (and proportions) of CMV–specific CD4+ (Th1) cells and a high proportion of regulatory T cells, as risk factors for csCMVi in allo-HCT recipients on letermovir prophylaxis. They now extend that analysis to evaluate both innate and adoptive immunity between day +90 and day +270 in individuals discontinuing letermovir on day +100, covering a prolonged period of risk after discontinuation of letermovir.
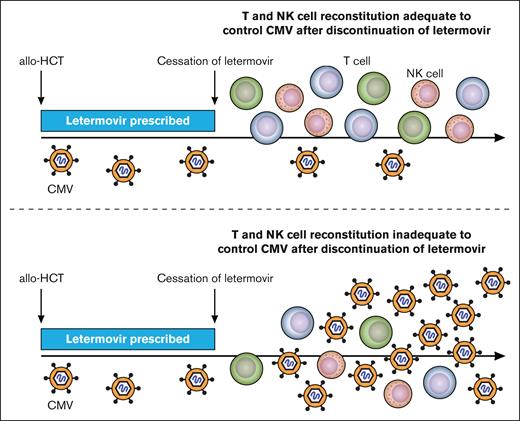
One of the strengths of the analysis performed is the evaluation of both innate and adaptive populations with the authors finding that those who experienced csCMVi had lower numbers of subsets of T and NK cells rather than more global defects in immune reconstitution. Specifically, they identified that individuals who developed csCMVi had fewer IFN-γ+ CMV-pp65–specific CD4+ and CD8+ T cells than CMV controllers and a deficit in the reconstitution of “memory-like” CD159c+CD56dim NK cells (see figure). Importantly, the later defect appeared as a new event before csCMVi in some patients and underscores the need for serial and event-driven monitoring of immune reconstitution. These immune milestones were also associated with the severity of csCMVi in those developing refractory and/or end-organ disease with lower numbers of CMV–specific T cells and memory-like NK cells. After stopping letermovir, patients who never had csCMVi demonstrated continued expansion of CMV–specific T cells, whereas noncontrollers did not. The absolute and relative number and expansion of memory-like NK cells were also higher in controllers than in noncontrollers, even at late time points.
Immune milestones associated with control of clinically significant CMV reactivation. Over time (x-axis) allo-HSCT recipients before day +100 had control of clinically significant CMV infection while on letermovir (blue bar). After discontinuation of letermovir prophylaxis on day +100 those with clinically significant CMV infection (virus level depicted as above the level of detection) had lower CD4+ (purple) and CD8+ (green) CMV–specific T cells and lower memory-like NK cells (pink).
Immune milestones associated with control of clinically significant CMV reactivation. Over time (x-axis) allo-HSCT recipients before day +100 had control of clinically significant CMV infection while on letermovir (blue bar). After discontinuation of letermovir prophylaxis on day +100 those with clinically significant CMV infection (virus level depicted as above the level of detection) had lower CD4+ (purple) and CD8+ (green) CMV–specific T cells and lower memory-like NK cells (pink).
Although this study improves our understanding of the immunologic control of CMV reactivation after transplantation, it is limited by the relatively small cohort with a very high incidence of late CMV reactivation and csCMVi. Additionally, the size of the cohort made it impossible to formally assess the role of immune suppression for prophylaxis and/or treatment of graft-versus-host disease (GVHD). All patients in this cohort received reduced-intensity conditioning and the majority had partial in vivo T cell depletion with antithymocyte globulin. It will be important to validate these findings in a large cohort of allo-HCT recipients undergoing transplantation with a range of approaches to GVHD prophylaxis. This will be especially important to assess given the expanding use of haploidentical donor transplants with in vivo T cell depletion with posttransplant cyclophosphamide as well as ex vivo T cell depletion.
There are several critical questions that remain unanswered in defining milestones of effective CMV-specific immunity. The present study did not assess the potential role of immune dominance of specific HLA alleles, or to what extent the diversity of the CMV–specific T cell repertoire is important and evaluated only responses to pp65 CMV epitopes. In addition, defining the specifics of how letermovir prophylaxis delays CMV–specific T cell reconstitution will require a large cohort and characterization of CMV events occurring before day 100, including low-level viremia in relation to immune profiling of controllers and noncontrollers.
Conflict-of-interest disclosure: S.P. receives support for the conduct of clinical trials through Boston Children’s Hospital from AlloVir, Atara Biotherapeutics, and Jasper; is inventor of the intellectual property related to the development of third-party viral-specific T cells program, of which all rights are assigned to Memorial Sloan Kettering Cancer Center; received honoraria from Pierre Fabre and Regeneron; and reports consultancy for Cellevolve and Century Therapeutics. J.S.L. declares no competing financial interests.