Clinically significant HCMV reactivation after letermovir cessation is associated with both T-cell and “memory-like” NK-cell impairments.
HCMV manifestation severity is linked to the magnitude of impaired HCMV–specific T and “memory-like” NK-cell reconstitution post-letermovir.
Visual Abstract
Allogeneic hematopoietic stem cell transplantation (alloSCT) is the only cure for many hematologic malignancies. However, alloSCT recipients are susceptible to opportunistic pathogens, such as human cytomegalovirus (HCMV). Letermovir prophylaxis has revolutionized HCMV management, but the challenge of late HCMV reactivations has emerged. Immunological surrogates of clinically significant HCMV infection (csCMVi) after discontinuation of letermovir remain to be defined. Therefore, we studied natural killer (NK)-cell reconstitution along with the global and HCMV pp65-specific T-cell repertoire of 24 alloSCT recipients at 7 time points before (day +90) and after (days +120-270) cessation of letermovir prophylaxis. Patients who experienced csCMVi had lower counts of IFN-γ+ HCMV–specific CD4+ and CD8+ T cells than HCMV controllers. Furthermore, patients with csCMVi displayed late impairment of NK-cell reconstitution, especially suppression of “memory-like” CD159c+CD56dim NK-cell counts that preceded csCMVi events in most patients. Moreover, several surrogates of immune reconstitution were associated with the severity of HCMV manifestation, with patients suffering from HCMV end-organ disease and/or refractory HCMV infection harboring least HCMV–specific T cells and “memory-like” NK cells. Altogether, our findings establish an association of delayed or insufficient proliferation of both HCMV–specific T cells and “memory-like” NK cells with csCMVi and the severity of HCMV manifestations after discontinuation of letermovir prophylaxis.
Introduction
Allogeneic hematopoietic stem cell transplantation (alloSCT) is the sole curative treatment option for many patients with hematologic malignancies.1 However, alloSCT recipients are highly susceptible to opportunistic infections.1-3 The reactivation of latent human cytomegalovirus (HCMV) is the most relevant viral complication in these patients, resulting in considerable morbidity and mortality.4
The clinical management of HCMV in alloSCT recipients has undergone a notable transformation with the recent approval of letermovir for antiviral HCMV prophylaxis. This agent has a favorable toxicity profile and has demonstrated remarkable efficacy, resulting in its widespread use for primary prophylaxis in HCMV-seropositive alloSCT recipients for the first 100 days after transplantation.5,6 However, despite the benefits of letermovir prophylaxis in reducing early-onset HCMV reactivations, late-onset HCMV reactivations after the discontinuation of letermovir have emerged as a major cause of HCMV disease.4,7
Delayed T- and NK-cell reconstitution is a significant risk factor for prolonged HCMV viremia and the development of HCMV disease.4,8,9 In particular, HCMV-specific type 1–polarized T cells and a subset of natural killer (NK) cells known as “memory-like” NK cells were thought to play an important role in controlling clinically relevant HCMV events before the introduction of letermovir.4,8,9 Our recent research indicates that letermovir prophylaxis modifies the magnitude, kinetics, and balance of immune reconstitution.10 Specifically, alloSCT recipients with low levels of HCMV–specific T-helper (Th) cells and a high proportion of regulatory T cells (Tregs) during letermovir prophylaxis are at a high risk for future symptomatic and persistent HCMV reactivation.10 However, immune reconstitution was only analyzed until day 120 after transplantation, and therefore concluded before most HCMV reactivation events.10 Aiming to identify long-term differences in the kinetics of T- and NK-cell reconstitution between patients who successfully controlled HCMV infection and those with clinically significant HCMV infection (csCMVi), we performed follow-up immunological studies until day +270. Furthermore, we performed 3-group comparisons to study the association of the T- and NK-cell repertoire with HCMV end-organ disease (EOD) and refractory (Rf) HCMV infection.
Materials and methods
Institutional review board approval
This study was approved by the Ethics Committees of the University of Wuerzburg (Germany, protocol code 17/19-sc). All patients provided written informed consent.
Study population
This study included a total of 24 patients who were seropositive for HCMV who had undergone alloSCT (recipient [R]+donor[D]+, R+D−) at the University Hospital of Wuerzburg (Germany) between December 2019 and August 2022. All patients received letermovir prophylaxis at a dose of 480 mg once daily from day 1 until day 100 after transplantation, with a reduced dose (240 mg once daily) for those receiving cyclosporine A. HCMV DNAemia was quantified by real-time polymerase chain reaction once weekly until day 100 after transplantation and every other week after day +100. If HCMV DNA levels in the blood exceeded 1000 DNA copies/mL, letermovir prophylaxis was discontinued and preemptive systemic antiviral therapy with (val)ganciclovir was initiated. Clinical chart review was performed as previously described (Table 1; supplemental Table 1).10
Patient characteristics
| Variables . | HCMV controllers (n = 11) . | csCMVi (n = 13) . | P value∗ . |
|---|---|---|---|
| Age, median (range) | 57 (22-74) | 65 (33-77) | .17 |
| Sex, n (%) | |||
| Male | 7 (64) | 8 (62) | >.99 |
| Female | 4 (36) | 5 (38) | |
| Underlying disease, n (%) | |||
| Chronic leukemia | 0 (0) | 1 (8) | |
| Multiple myeloma | 0 (0) | 1 (8) | |
| Acute leukemia | 6 (55) | 10 (77) | - |
| Lymphoma | 1 (9) | 0 (0) | |
| Others | 4 (36) | 1 (8) | |
| HLA matching, n (%) | |||
| Matched related | 0 (0) | 5 (38) | |
| Matched unrelated | 9 (82) | 6 (46) | - |
| Haploidentical | 0 (0) | 0 (0) | |
| Mismatch | 2 (18) | 2 (15) | |
| Stem cell source, n (%) | |||
| PBSC | 11 (100) | 13 (100) | 1 |
| Conditioning regimen, n (%) | |||
| Reduced intensity | 11 (100) | 13 (100) | 1 |
| Antithymocyte globulin, n (%) | |||
| No | 0 (0) | 4 (31) | .10 |
| Yes | 11 (100) | 9 (69) | |
| HCT-CI, n (%) | |||
| 0-2 | 7 (64) | 8 (62) | - |
| 3-4 | 2 (18) | 5 (38) | |
| ≥5 | 2 (18) | 0 (0) | |
| aGvHD, n (%) | |||
| 0-1 | 10 (91) | 7 (54) | .08 |
| 2-4 | 1 (9) | 6 (46) | |
| cGvHD, n (%) | |||
| No | 10 (91) | 10 (77) | .60 |
| Yes | 1 (9) | 3 (33) | |
| Serostatus (R/D), n (%) | |||
| +/+ | 10 (91) | 8 (62) | .17 |
| +/− | 1 (9) | 5 (38) | |
| 1-year mortality, n (%) | |||
| Alive | 11 (100) | 12 (92) | >.99 |
| Dead | 0 (0) | 1 (8) |
| Variables . | HCMV controllers (n = 11) . | csCMVi (n = 13) . | P value∗ . |
|---|---|---|---|
| Age, median (range) | 57 (22-74) | 65 (33-77) | .17 |
| Sex, n (%) | |||
| Male | 7 (64) | 8 (62) | >.99 |
| Female | 4 (36) | 5 (38) | |
| Underlying disease, n (%) | |||
| Chronic leukemia | 0 (0) | 1 (8) | |
| Multiple myeloma | 0 (0) | 1 (8) | |
| Acute leukemia | 6 (55) | 10 (77) | - |
| Lymphoma | 1 (9) | 0 (0) | |
| Others | 4 (36) | 1 (8) | |
| HLA matching, n (%) | |||
| Matched related | 0 (0) | 5 (38) | |
| Matched unrelated | 9 (82) | 6 (46) | - |
| Haploidentical | 0 (0) | 0 (0) | |
| Mismatch | 2 (18) | 2 (15) | |
| Stem cell source, n (%) | |||
| PBSC | 11 (100) | 13 (100) | 1 |
| Conditioning regimen, n (%) | |||
| Reduced intensity | 11 (100) | 13 (100) | 1 |
| Antithymocyte globulin, n (%) | |||
| No | 0 (0) | 4 (31) | .10 |
| Yes | 11 (100) | 9 (69) | |
| HCT-CI, n (%) | |||
| 0-2 | 7 (64) | 8 (62) | - |
| 3-4 | 2 (18) | 5 (38) | |
| ≥5 | 2 (18) | 0 (0) | |
| aGvHD, n (%) | |||
| 0-1 | 10 (91) | 7 (54) | .08 |
| 2-4 | 1 (9) | 6 (46) | |
| cGvHD, n (%) | |||
| No | 10 (91) | 10 (77) | .60 |
| Yes | 1 (9) | 3 (33) | |
| Serostatus (R/D), n (%) | |||
| +/+ | 10 (91) | 8 (62) | .17 |
| +/− | 1 (9) | 5 (38) | |
| 1-year mortality, n (%) | |||
| Alive | 11 (100) | 12 (92) | >.99 |
| Dead | 0 (0) | 1 (8) |
Characteristics of alloSCT patients who controlled HCMV (controllers) and patients with csCMVi.
aGvHD, acute GvHD; cGvHD, chronic GvHD; csCMVi, clinically significant HCMV infection; HCT-CI, hematopoietic cell transplantation comorbidity index; PBSC, peripheral blood stem cells.
Mann-Whitney U test or Fisher's exact test was used, as appropriate.
Definitions
Patients were divided into 2 groups, controllers and patients with csCMVi. Patients with csCMVi were defined as those who received antiviral therapy after HCMV DNA levels in the blood exceeded 1000 DNA copies/mL or were diagnosed with EOD defined in accordance with the criteria established by Ljungman et al.11 Controllers never exceeded the 1000-copy threshold and therefore, did not receive anti-HCMV therapy. For various analyses, patients with csCMVi were further subdivided into those with probable Rf HCMV infection as defined by Chemaly et al12 and/or EOD and those without Rf and/or EOD manifestations.
In some figures, numbers of various cell populations were compared before and after csCMVi. Pre-csCMVi measurements were obtained from peripheral blood mononuclear cells (PBMCs) isolated at the time point before the initiation of anti-HCMV therapy. Subsequent post-csCMVi assessments of cellular populations were conducted using PBMC samples measured at the earliest available time point after the discontinuation of anti-HCMV treatment.
Reanalysis of published data
As part of this study, we reanalyzed previously published results from days +90 and 120,10 albeit with revised patient grouping, as detailed above, and with a revised gating strategy for NK-cell analysis, as detailed below.
Immunoassays
EDTA-anticoagulated venous blood (36 mL) was obtained on days 90, 120, 150, 180, 210, 240, and 270 after transplantation. PBMCs were isolated and cryopreserved as described previously.10 After thawing, analysis of global T- and NK-cell phenotypes and quantification of HCMV pp65-specific T cells were performed using flow cytometry, following previously published protocols for resting, stimulation (HCMV–specific T cells only), and staining of PBMCs.10 Briefly, to detect HCMV–specific T cells, 5 × 105 PBMCs in 200 μL of RPMI Glutamax (Thermo Fisher, Gibco, Waltham, MA) + 10% fetal calf serum (Sigma-Aldrich, St. Louis, MO) + 50 μg/mL gentamycin (Gibco) were seeded into a 96-well round-bottom plate (Corning, Falcon, Glendale, AZ). The plate was incubated at 37°C, 5% CO2 for 2 hours. Thereafter, cells were stimulated with 0.1 μg/mL of a pp65 peptide mix (PepMix HCMVA pp65, >90% purity; JPT, Berlin, Germany), 0.1 μg/mL of an HIV peptide mix (PepMix HIV 1 NEF, Ultra; JPT) as background control, or phorbol myristate acetate (PMA, 0.5 μg/mL, Sigma-Aldrich) + ionomycin (1 μg/mL; Sigma-Aldrich) as positive control for 16 to 18 hours at 37°C and 5% CO2. After the first hour of stimulation, Brefeldin A (10 μg/mL; Sigma-Aldrich), GolgiStop (1.2 μL per well; Becton Dickinson, Franklin Lakes, NJ), and CD107a-APC (2.5 μL per well; Becton Dickinson) were added to all wells. The antibodies used for cell staining are summarized in reference.10 Cells were analyzed using a CytoFLEX cytometer (AS34240) and CytExpert v.2.4 software (Beckman Coulter, Brea, CA). Data were analyzed using Kaluza v.2.1 (Beckman Coulter) following our previously published gating strategy for T-cell quantification and phenotyping.10 The gating strategy for “memory-like” NK cells underwent minor modifications compared with that in our published protocol,10 as detailed in supplemental Figure 1. To estimate the abundance of T-cell and NK-cell subpopulations in peripheral blood, frequencies of cell populations identified using flow cytometry were multiplied by absolute lymphocyte counts per μL of whole blood, as determined by clinical hematology. Background-corrected HCMV–specific T-cell frequencies were calculated by subtracting HIV-induced unspecific background responses (all patients were HIV-negative) from HCMV-induced responses.
Statistical analysis
Statistical significance was determined using the Mann-Whitney U test, paired Wilcoxon test, Kruskal-Wallis test, or Fisher exact test, as appropriate. If applicable, Benjamini-Hochberg procedure was used to test for a false-positive discovery rate (FDR) of <0.2. Data were compiled, visualized, and analyzed using Microsoft Excel (Microsoft, Redmond, WA), Prism v.9.5 (GraphPad Software, Boston, MA), and R v.4.2.1 (R Core Team).
Results
Patient characteristics, HCMV reactivation, and viral load
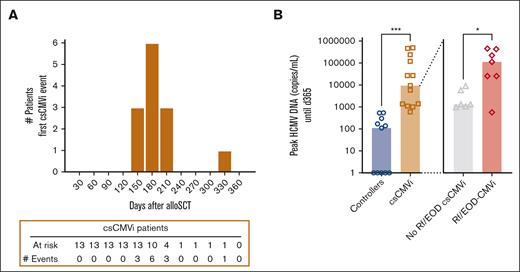
A total of 24 patients who underwent alloSCT receiving letermovir prophylaxis were included in the study, consisting of 11 controllers (46%) and 13 patients with csCMVi (54%). The csCMVi cohort included 6 patients without Rf/EOD-CMVi (47%) and 7 with Rf/EOD-CMVi (53%). For those with csCMVi, the median time of their first HCMV reactivation was on day +168. In all but 1 patient, the onset of csCMVi was observed within a specific timeframe, ranging from day 120 to day 210 after transplantation (Figure 1A). Controllers and patients with csCMVi did not show significant differences in demographics, underlying malignancy, donor type, donor HCMV status, stem cell source, conditioning regimen, comorbidity (hematopoietic cell transplantation comorbidity index) score, and chronic graft-versus-host disease (GvHD) incidence (Table 1). Although patients with csCMVi tended to have a higher occurrence of acute GvHD than HCMV controllers (P = .078), incidences of acute GvHD (P = .286) and chronic GvHD (P > .999) did not differ significantly between patients with no Rf/EOD-csCMVi and patients with Rf/EOD-CMVi. Furthermore, examination of the patterns of immunosuppressant and steroid administration for GvHD revealed no statistically significant differences between the controller and patient groups with csCMVi, either before or after the discontinuation of letermovir prophylaxis. However, a nonsignificant trend was observed, suggesting a higher dose of steroid treatment in the csCMVi group compared with the controllers after letermovir (P = .097, χ2 test) (supplemental Table 1).
Connection of HCMV load levels and clinical severity of HCMV manifestation. (A) The incidence of csCMVi onset among patients with csCMVi was monitored from day 0 to day +360. CsCMVi events were reported at the end of each month based on their diagnosis date. (B) Peak HCMV DNA copies per milliliter measured by polymerase chain reaction until day +365 in patients who underwent alloSCT, serving as control for HCMV (controllers, blue) or those with csCMVi (brown). Patients with csCMVi were further subdivided into patients without (gray) or with Rf/EOD (red). ∗P < .05, ∗∗∗P < .001 using Mann-Whitney U test.
Connection of HCMV load levels and clinical severity of HCMV manifestation. (A) The incidence of csCMVi onset among patients with csCMVi was monitored from day 0 to day +360. CsCMVi events were reported at the end of each month based on their diagnosis date. (B) Peak HCMV DNA copies per milliliter measured by polymerase chain reaction until day +365 in patients who underwent alloSCT, serving as control for HCMV (controllers, blue) or those with csCMVi (brown). Patients with csCMVi were further subdivided into patients without (gray) or with Rf/EOD (red). ∗P < .05, ∗∗∗P < .001 using Mann-Whitney U test.
HCMV controllers had significantly lower HCMV loads than patients with csCMVi (median, 110 vs 9400 HCMV copies/mL; P < .001). Among patients with csCMVi, those affected by Rf/EOD-CMVi had significantly higher HCMV loads than patients without Rf/EOD-CMVi (median, 120 000 vs 1400 HCMV copies/mL; P = .028; Figure 1B).
High numbers and frequencies of HCMV–specific CD4+ and CD8+ T cells are associated with HCMV control
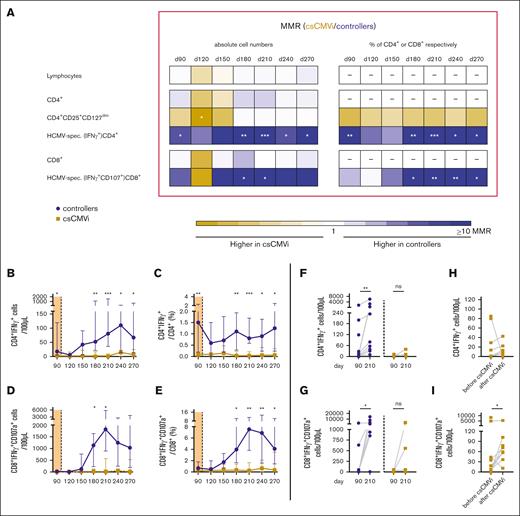
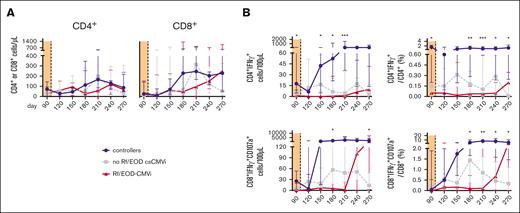
Lymphocyte counts, global CD4+ T-cell counts, and global CD8+ T-cell counts did not differ significantly between controllers and patients with csCMVi throughout the study period (day +90-270; Figure 2A). However, controllers had significantly higher levels of HCMV–specific CD4+ T cells than that of patients with csCMVi both before (median on day +90, 18 vs 3 CD4+IFN-γ+ cells/100 μL; P = .011) and after cessation of letermovir (P < .05 between days +180 and +270). The most marked differences in antigen-specific Th-cell counts between HCMV controllers and patients with csCMVi were found on day +210, with median counts of 80 vs <1 CD4+IFN-γ+ cells/100 μL (median-to-median ratio, 93; P < .001) (Figure 2A-B). Similarly, HCMV–specific CD8+ cytotoxic T-cell (CTL) counts were significantly higher in controllers than in patients with csCMVi from day +180 until day +210, with the strongest differences found on day +210 (median, 1817 vs 20 CD8+IFN-γ+CD107a+ CTLs/100 μL; P = .020) (Figure 2A-D). Correspondingly, relative frequencies of HCMV-specific cells among all CD4+ cells and CTLs were also significantly higher in controllers than in patients with csCMVi (Figure 2A,C,E).
Poor HCMV–specific T-cell reconstitution in csCMVi patients after discontinuation of letermovir. Samples from alloSCT recipients receiving letermovir prophylaxis until day +100 who controlled HCMV (controllers, blue) and those with csCMVi (brown) were compared using flow cytometry. (A) Heat map comparing the MMRs (csCMVi/controllers) of selected T-cell populations between the 2 cohorts. (B-E) Background-corrected median numbers (B) and frequencies (C) of HCMV–specific Th cells (CD4+IFN-γ+) as well as numbers (D) and frequencies (E) of cytotoxic T cells (CD8+IFN-γ+CD107a+). (A-E) Mann-Whitney U test and Benjamini-Hochberg procedure to test for an FDR of <0.2. (F,G) Background-corrected individual numbers of HCMV–specific (F) Th cells (CD4+IFN-γ+) and (G) cytotoxic T cells (CD8+IFN-γ+ CD107a+) on day +90 and day +210 are shown. (H-I) Background-corrected individual numbers of HCMV–specific (H) Th cells (CD4+IFN-γ+) and (I) cytotoxic T cells (CD8+IFN-γ+CD107a+) before and after csCMVi are displayed. (F-I) ∗P < .05, ∗∗P < .01, ∗∗∗P < .001 using paired Wilcoxon test. CD, cluster of differentiation; d, day; MMR, median-to-median ratio.
Poor HCMV–specific T-cell reconstitution in csCMVi patients after discontinuation of letermovir. Samples from alloSCT recipients receiving letermovir prophylaxis until day +100 who controlled HCMV (controllers, blue) and those with csCMVi (brown) were compared using flow cytometry. (A) Heat map comparing the MMRs (csCMVi/controllers) of selected T-cell populations between the 2 cohorts. (B-E) Background-corrected median numbers (B) and frequencies (C) of HCMV–specific Th cells (CD4+IFN-γ+) as well as numbers (D) and frequencies (E) of cytotoxic T cells (CD8+IFN-γ+CD107a+). (A-E) Mann-Whitney U test and Benjamini-Hochberg procedure to test for an FDR of <0.2. (F,G) Background-corrected individual numbers of HCMV–specific (F) Th cells (CD4+IFN-γ+) and (G) cytotoxic T cells (CD8+IFN-γ+ CD107a+) on day +90 and day +210 are shown. (H-I) Background-corrected individual numbers of HCMV–specific (H) Th cells (CD4+IFN-γ+) and (I) cytotoxic T cells (CD8+IFN-γ+CD107a+) before and after csCMVi are displayed. (F-I) ∗P < .05, ∗∗P < .01, ∗∗∗P < .001 using paired Wilcoxon test. CD, cluster of differentiation; d, day; MMR, median-to-median ratio.
Of note, controllers showed significant expansion of HCMV–specific CD4+ Th cells (median, 18-81 CD4+IFN-γ+ cells/100 μL; P = .004) and CD8+ CTLs (median, 26-1 817 CD8+IFN-γ+CD107a+ CTLs/100 μL; P = .012) between days +90 and +210, that is, after cessation of letermovir. In contrast, patients with csCMVi showed limited and statistically insignificant expansion of antigen-specific Th cells and CTLs by day +210 (Figure 2F-G), suggesting limited proliferation capacity of HCMV–specific T cells in patients with csCMVi.
Comparing HCMV–specific T-cell counts before and after csCMVi, it was noted that, although not significant, all but 1 patient either experienced an increase or maintained the same level of HCMV–specific CD4+ Th cells after the resolution of csCMVi (Figure 2H). In addition, there was a significant increase in HCMV–specific CD8+ CTL counts after csCMVi resolution (median, 7 vs 66 CD8+IFN-γ+CD107a+ CTLs/100 μL; P = .023; Figure 2I).
Moreover, median total Treg counts were significantly higher in patients with csCMVi than in HCMV controllers on day +120 (median, 4 vs 1 CD4+CD25+CD127dim cells/μL; P = .019). However, the difference in Treg counts between the 2 cohorts diminished between days +150 and +270 (Figure 2A).
Strong “memory-like” NK-cell reconstitution is associated with sustained HCMV control
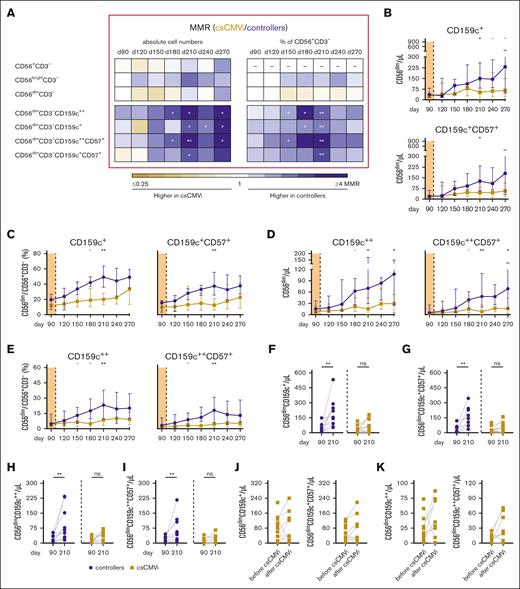
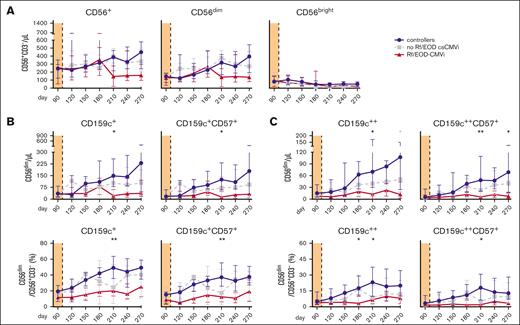
Total CD56+, CD56bright, and CD56dim NK-cell numbers and frequencies did not differ significantly between controllers and patients with csCMVi (Figure 3A). However, CD56dim NK cells include a subgroup of CD159c+ “memory-like” NK cells that are known to exhibit increased proliferation and enhanced anti-HCMV activity.13-20 Numbers of these cells were significantly higher in controllers than in patients with csCMVi between days +210 and +270 (Figure 3A-B), with the strongest difference found on day +270 (median, 231 vs 65 CD56dimCD159c+ cells/μL; P = .025). Likewise, proportions of CD56dimCD159c+ cells among NK cells were significantly higher in controllers than in patients with csCMVi on day +180 (P = .036) and day +210 (P = .002), with medians of 42% vs 19% and 49% vs 20% CD56dimCD159c+/CD56+CD3− cells, respectively (Figure 3A,C).
Higher numbers and frequencies of NK cells and “memory-like” NK cells are associated with HCMV control in alloSCT recipients after discontinuation of letermovir. NK-cell reconstitution was compared in alloSCT recipients who controlled HCMV (controllers, blue) and those with csCMVi (brown). (A) Heat map comparing the MMRs (csCMVi/controllers) of selected NK-cell populations between the 2 cohorts. (B-E) Median absolute numbers (B) and frequencies (C) of CD56dimCD159c+(CD57+) cells as well as numbers (D) and frequencies (E) of CD56dimCD159c++ (CD57+) cells are shown. (A-E) Mann-Whitney U test and Benjamini-Hochberg procedure to test for a FDR of <0.2. Significant results with an FDR >0.2 are indicated by the gray stars. (F-I) Individual numbers of (F) CD56dimCD159c+, (G) CD56dimCD159c+CD57+, (H) CD56dimCD159c++, (I) CD56dimCD159c++CD57+ on day +90 and day +210 are shown. (J,K) Individual absolute numbers of (J) CD56dimCD159c+ and CD56dimCD159c+CD57+ as well as (K) CD56dimCD159c++ and CD56dimCD159c++CD57+ before and after csCMVi are displayed. (F-K) ∗P < .05, ∗∗P < .01 using paired Wilcoxon test. CD, cluster of differentiation; d, day; MMR, median-to-median ratio.
Higher numbers and frequencies of NK cells and “memory-like” NK cells are associated with HCMV control in alloSCT recipients after discontinuation of letermovir. NK-cell reconstitution was compared in alloSCT recipients who controlled HCMV (controllers, blue) and those with csCMVi (brown). (A) Heat map comparing the MMRs (csCMVi/controllers) of selected NK-cell populations between the 2 cohorts. (B-E) Median absolute numbers (B) and frequencies (C) of CD56dimCD159c+(CD57+) cells as well as numbers (D) and frequencies (E) of CD56dimCD159c++ (CD57+) cells are shown. (A-E) Mann-Whitney U test and Benjamini-Hochberg procedure to test for a FDR of <0.2. Significant results with an FDR >0.2 are indicated by the gray stars. (F-I) Individual numbers of (F) CD56dimCD159c+, (G) CD56dimCD159c+CD57+, (H) CD56dimCD159c++, (I) CD56dimCD159c++CD57+ on day +90 and day +210 are shown. (J,K) Individual absolute numbers of (J) CD56dimCD159c+ and CD56dimCD159c+CD57+ as well as (K) CD56dimCD159c++ and CD56dimCD159c++CD57+ before and after csCMVi are displayed. (F-K) ∗P < .05, ∗∗P < .01 using paired Wilcoxon test. CD, cluster of differentiation; d, day; MMR, median-to-median ratio.
“Memory-like” NK cells can additionally express CD57, indicating a mature state of NK cells associated with enhanced cytotoxicity.21,22 Consistent with overall CD56dimCD159c+ NK-cell kinetics, CD56dimCD159c+CD57+ cell numbers and frequencies were significantly higher in controllers than in patients with csCMVi, with the most marked differences observed on day +210 (median, 145 vs 44 CD56dimCD159c+CD57+ cells/μL [P = .013]; 38% vs 13% CD56dimCD159c+CD57+/ CD56+CD3− cells [P = .004]; Figure 3A-C).
Of note, some previous studies specifically defined only NK cells with strong CD159c expression (CD159c++) as “memory-like” NK cells.23,24 Therefore, we additionally quantified this population. Indeed, (CD57+) CD56dimCD159c++ NK-cell numbers and frequencies were significantly higher in controllers than in patients with csCMVi, and their kinetics were comparable to the total CD56dimCD159c+ population (Figure 3A,D,E).
Similar to HCMV–specific T cells, “memory-like” NK cells significantly expanded in controllers between days +90 and +210, that is, after letermovir cessation. Specifically, 7- to 10-fold increases in median counts of CD56dimCD159c+ cells (36 vs 149, P = .002), CD56dimCD159c+CD57+ cells (17 vs 125, P = .002), CD56dimCD159c++ cells (16 vs 70, P = .002), and CD56dimCD159c++CD57+ cells (5 vs 49, P = .002) were seen in controllers between days +90 and +210 (Figure 3F-I). In contrast, none of “memory-like” NK-cell populations significantly expanded in patients with csCMVi (Figure 3F-I).
A vast majority of patients with csCMVi had higher “memory-like” NK-cell counts after the resolution of csCMVi compared with the counts quantified before csCMVi. Although not statistically significant, the most notable increase was observed in the count of CD56dimCD159c++CD57+ cells (7 vs 22 /μL, P = .055) (Figure 3J,K).
Low HCMV–specific T-cell and “memory-like” NK-cell numbers and frequencies are associated with severe HCMV infections
Next, we sought to test whether HCMV–specific T-cell numbers and frequencies were associated with the severity of HCMV infection. Therefore, patients were subdivided into 3 groups: controllers, patients with csCMVi without Rf/EOD manifestations, and patients with Rf/EOD HCMV disease. Although global T-cell reconstitution did not differ significantly between the 3 groups (Figure 4A), significant differences were found in the numbers and frequencies of HCMV–specific T cells (Figure 4B-C). In particular, HCMV–specific CD4+ T-cell counts differed significantly between the 3 groups on day +90 and between days +150 and +210, with the most marked differences found on day +210 (median, 81 vs 5 vs 1 CD4+IFN-γ+ cells/100 μL in controllers, patients with no Rf/EOD-csCMVi, and patients with Rf/EOD, respectively; P < .001; Figure 4B). Similarly, HCMV–specific CTL counts differed significantly between the 3 groups on day +180 (P = .033) and day +270 (P = .046) (median, day +180, 1 141 vs 57 vs 5; day +270, 1039 vs 14 vs 343 CD8+IFN-γ+CD107a+ cells/100 μL in controllers, patients with no Rf/EOD-csCMVi, and patients with Rf/EOD, respectively; Figure 4C). These differences in absolute cell counts were mirrored by similar intergroup differences in the kinetics of HCMV–specific Th-cell and CTL frequencies (Figure 4B-C).
Severity of HCMV infection is linked to numbers and frequencies of HCMV–specific T cells. T-cell reconstitution was compared in alloSCT recipients who controlled HCMV (controllers, blue), those who experienced csCMVi without Rf/EOD (gray), and those who had csCMVi with Rf/EOD (red). (A) Absolute numbers of total Th cells (CD4+) and cytotoxic T cells (CD8+). (B-C) Background-corrected numbers and percentages of HCMV–specific Th cells (CD4+IFN-γ+, B) and cytotoxic T cells (CD8+IFN-γ+CD107a+, C) after 16 to 18 h of stimulation with pp65 HCMV-peptide mix. (A-C) Symbols/lines and error bars indicate medians and interquartile ranges, respectively. Kruskal-Wallis test and Benjamini-Hochberg procedure to test for a FDR of <0.2. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. CD, cluster of differentiation.
Severity of HCMV infection is linked to numbers and frequencies of HCMV–specific T cells. T-cell reconstitution was compared in alloSCT recipients who controlled HCMV (controllers, blue), those who experienced csCMVi without Rf/EOD (gray), and those who had csCMVi with Rf/EOD (red). (A) Absolute numbers of total Th cells (CD4+) and cytotoxic T cells (CD8+). (B-C) Background-corrected numbers and percentages of HCMV–specific Th cells (CD4+IFN-γ+, B) and cytotoxic T cells (CD8+IFN-γ+CD107a+, C) after 16 to 18 h of stimulation with pp65 HCMV-peptide mix. (A-C) Symbols/lines and error bars indicate medians and interquartile ranges, respectively. Kruskal-Wallis test and Benjamini-Hochberg procedure to test for a FDR of <0.2. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. CD, cluster of differentiation.
Although global NK-cell counts did not differ significantly between the 3 groups (Figure 5A), “memory-like” NK-cell counts and frequencies, especially the CD56dimCD159c++ population, showed significant associations with HCMV control and severity of HCMV disease. Specifically, counts of CD56dimCD159c++(CD57+) cells steadily decreased from controllers to patients with no Rf/EOD-csCMVi to those with Rf/EOD, with medians of 70 vs 40 vs 13 CD56dimCD159c++ cells/μL (P = .019) and 49 vs 23 vs 6 CD56dimCD159c++CD57+ cells/μL (P = .004), respectively, on day +210 (Figure 5C). These intergroup differences further increased until day +270, with medians of 107 vs 51 vs 13 CD56dimCD159c++ cells/μL (P = .032) and 69 vs 40 vs 8 CD56dimCD159c++CD57+ cells/μL (P = .012) in controllers, patients with no Rf/EOD-csCMVi, and those with Rf/EOD, respectively (Figure 5C). Relative frequencies of CD56dimCD159c++ CD57+ cells showed similar kinetics (Figure 5C). Similar trends were observed for the kinetics of CD56dimCD159c++ (CD57+) and CD56dimCD159c+CD57+ counts and frequencies (Figure 5B-C).
Numbers and frequencies of “memory-like” NK cells are associated with the severity of HCMV infection. NK-cell reconstitution was compared in alloSCT recipients who controlled HCMV (controllers, blue), those who experienced csCMVi without Rf/EOD (gray), and those who faced csCMVi with Rf/EOD (red). (A) CD56+CD3−, CD56brightCD3− and CD56dimCD3− NK-cell numbers. (B-C) Absolute cell numbers and frequencies of (B) CD56dimCD159c+(CD57+) and (C) CD56dimCD159c++(CD57+) “memory-like” NK cells. (A-C) Symbols/lines and error bars indicate medians and interquartile ranges, respectively. Kruskal-Wallis test and Benjamini-Hochberg procedure to test for a FDR of <0.2. The significant result with an FDR >0.2 is indicated by a gray star. ∗P < .05, ∗∗P < .01. CD, cluster of differentiation.
Numbers and frequencies of “memory-like” NK cells are associated with the severity of HCMV infection. NK-cell reconstitution was compared in alloSCT recipients who controlled HCMV (controllers, blue), those who experienced csCMVi without Rf/EOD (gray), and those who faced csCMVi with Rf/EOD (red). (A) CD56+CD3−, CD56brightCD3− and CD56dimCD3− NK-cell numbers. (B-C) Absolute cell numbers and frequencies of (B) CD56dimCD159c+(CD57+) and (C) CD56dimCD159c++(CD57+) “memory-like” NK cells. (A-C) Symbols/lines and error bars indicate medians and interquartile ranges, respectively. Kruskal-Wallis test and Benjamini-Hochberg procedure to test for a FDR of <0.2. The significant result with an FDR >0.2 is indicated by a gray star. ∗P < .05, ∗∗P < .01. CD, cluster of differentiation.
Taken together, patients with Rf/EOD facing the most severe HCMV-associated illnesses harbored the lowest counts and frequencies of HCMV–specific CD4+ and CD8+ T cells and “memory-like” NK cells. Those with milder HCMV manifestations, that is, patients with csCMVi but without Rf/EOD, had intermediate counts and frequencies of these cell populations, whereas HCMV controllers had the highest numbers and frequencies of HCMV–specific CD4+ and CD8+ T cells and “memory-like” NK cells.
Low HCMV–specific CD4+ T-cell counts, often coupled with impaired reconstitution of “memory-like” NK cells, might be immunological predictors of Rf/EOD-CMVi events.
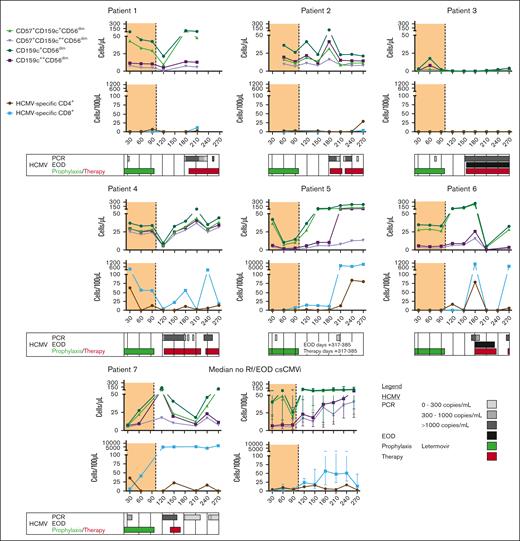
Although our sample size is limited, individual analysis of longitudinal kinetics revealed that all but 1 patient with Rf/EOD-CMVi had persistently low counts (<25/100 μL) of HCMV–specific Th1 cells throughout at least day +210. In contrast, “memory-like” NK-cell numbers in Rf/EOD-CMVi showed greater fluctuations over time. Notably, either persistent lack of reconstitution or a precipitous drop in NK-cell numbers preceded the HCMV Rf/EOD event in most patients (patients 1-5 in Figure 6), mostly in the context of newly prescribed immunosuppressive therapy to treat GvHD. However, in some patients (patients 6 and 7, and a second drop in patient 2 in Figure 6), a drop in “memory-like” NK-cell counts was found in the aftermath of HCMV reactivation, especially in the context of HCMV EOD.
Impaired reconstitution of “memory-like” NK cells and HCMV–specific T cells in patients with Rf/EOD CMVi. HCMV–specific CD4+ and CD8+ T cells as well as “memory-like” NK cells were quantified in patients with Rf/EOD-CMVi. Individual kinetics of all 7 patients with Rf/EOD-CMVi are shown. CD, cluster of differentiation; PCR, polymerase chain reaction.
Impaired reconstitution of “memory-like” NK cells and HCMV–specific T cells in patients with Rf/EOD CMVi. HCMV–specific CD4+ and CD8+ T cells as well as “memory-like” NK cells were quantified in patients with Rf/EOD-CMVi. Individual kinetics of all 7 patients with Rf/EOD-CMVi are shown. CD, cluster of differentiation; PCR, polymerase chain reaction.
In summary, persistently low HCMV–specific CD4+ T-cell counts, often combined with suppressed reconstitution of “memory-like” NK cells, appear to be common immunological denominators and surrogates of Rf/EOD-CMVi events.
Discussion
Letermovir prophylaxis is highly effective in mitigating clinically significant HCMV reactivation but does not eliminate the risk of late-onset reactivations.4 Despite the use of letermovir, quantitative and qualitative immune reconstitution remains critical for HCMV control, especially after discontinuation of prophylaxis. However, long-term studies of immune reconstitution after cessation of letermovir and detailed information regarding protective immune characteristics have been lacking. Our findings in this study underscore the importance of HCMV–specific T cells to protect against late-onset HCMV reactivation and reveal a previously understudied association between insufficient reconstitution of “memory-like” NK cells and csCMVi.
Studying antigen-specific T-cell immunity, we found that IFN-γ+ HCMV–specific type-1 Th and CTL (Th1 and Tc1) counts and frequencies were consistently low in patients with csCMVi before their first HCMV reactivation, especially those with Rf/EOD-CMVi. This suggests an association between the number of HCMV–specific T cells and the severity of the HCMV manifestation. Interestingly, even though individual kinetics of T-cell and NK-cell reconstitution of the patients with Rf/EOD-CMVi were heterogeneous, low numbers of HCMV–specific Th1 cells until day +210 appeared to be a common denominator of HCMV risk and severity. These long-term trends underscore the association of poor and delayed HCMV–specific T-cell reconstitution with severe and persistent HCMV reactivation, corroborating our previous report that low HCMV–specific Th1 counts before letermovir cessation are a critical predictor of future prolonged and symptomatic HCMV reactivation in alloSCT recipients.10 Moreover, our findings align with numerous reports from the pre-letermovir era establishing a protective role of HCMV–specific T cells in HCMV control in alloSCT recipients.4,25-27
The crucial role of HCMV–specific IFN-γ+ Th1 and Tc1 cells in sustained viral control after letermovir prophylaxis reaffirms the need to systematically evaluate functional immunoassays, including cytokine release assays, as a tool to inform HCMV management during and after letermovir prophylaxis. These assays, which have already demonstrated their predictive capacity for future significant HCMV reactivations in patients receiving preemptive therapy,28-31 could provide essential guidance to individually tailor the duration of letermovir prophylaxis or the use of adjunctive immunotherapeutic interventions, such as third-party donor T cells. There might be several approaches to further improve the capacity of functional immunoassays in HCMV management. Firstly, the striking and statistically significant differences in antigen-specific T-cell reconstitution between controllers, patients with no Rf/EOD-csCMVi, and those with Rf/EOD-CMVi on and after day +150 suggest that extended surveillance of anti-HCMV T-cell immunity after the discontinuation of letermovir, combined with consideration of clinical variables (eg, GvHD therapy), might improve HCMV risk stratification. Although immunodominant HCMV antigens, such as immediate-early protein (IE) 1 and phosphoprotein (pp)65, have been comprehensively studied, emerging evidence suggests that the T-cell response specific to HCMV relies on a broader spectrum of HCMV antigens.32-34 Thus, screening of a broad array of HCMV antigens in the context of letermovir prophylaxis might help to further improve HCMV risk stratification. This approach could also allow us to elucidate whether the magnitude of T-cell responses to specific immunodominant antigens or rather the diversity of HCMV-specific responses is most predictive of HCMV control.
Inclusion of additional readouts might present another avenue for refinement of functional immune monitoring. In our previous publication, we observed significantly higher quantities and frequencies of regulatory T cells (Treg) in patients receiving letermovir prophylaxis with prolonged and symptomatic HCMV reactivation between day +30 and day +120 compared with patients with no or less severe HCMV reactivations.10 Although this study revealed significant differences in regulatory T-cell counts between HCMV controllers and patients with csCMVi on day +120, these differences diminished until day +240. In fact, this pattern might be a consequence of the HCMV reactivation events observed in all our patients with csCMVi between days +150 and +240, because Treg counts and their inhibitory functionality often decline at the onset of acute infections.35 Importantly, such late and possibly reactivation-induced effects would not compromise the use of Treg markers (eg, IL10) at the conclusion of letermovir prophylaxis, which might improve HCMV risk stratification.
Beyond the known role of T-cell reconstitution and polarization in HCMV control, to our knowledge, this is the first study showing that late-onset HCMV reactivation after discontinuation of letermovir prophylaxis could be associated with impaired NK-cell reconstitution. Counts of CD56dim NK cells, especially “memory-like” NK cells, were higher in controllers than in patients with csCMVi. Consistent with our observations for HCMV–specific T cells, patients with Rf/EOD-CMVi had the fewest “memory-like” NK cells, pointing to an association of “memory-like” NK-cell counts and the severity of the HCMV manifestation. However, our results suggest that differences in “memory-like” NK cells emerge later than the disparate kinetics of HCMV–specific T cells described previously10 and above. Although differences in HCMV–specific T cells were found as early as day +90, significant differences in “memory-like” NK-cell counts first emerged between days +180 and +210. Hence, it would be conceivable that impaired NK-cell reconstitution is a consequence rather than a predictor of clinically significant HCMV events. Consistent with this hypothesis, our previous study10 did not reveal early disparities in “memory-like” NK-cell reconstitution as a significant predictor of future prolonged and symptomatic HCMV reactivation events in patients receiving letermovir prophylaxis. Furthermore, in the pre-letermovir era, significant numbers of “memory-like” NK cells were commonly detected only ∼6 months after transplantation, expanded until 2 years after transplantation, and were associated with improved disease-free survival.15,19,36 In contrast, herein and in our previously published study,10 we found measurable initial reconstitution of these cells before day +150, especially in HCMV controllers. Additionally, we previously reported faster reconstitution of these cells in patients receiving letermovir than in those managed with preemptive therapy,10 further suggesting that HCMV reactivation could initially suppress “memory-like” NK-cell reconstitution, as seen in some patients in this study.
However, review of individual NK-cell kinetics in our limited number of patients with Rf/EOD-CMVi suggested that lack of reconstitution and/or precipitous drops in “memory-like” NK-cell counts preceded HCMV Rf/EOD events in most patients. Later reconstitution of “memory-like” NK cells compared with antigen-specific T cells might to be due, at least in part, to their dependence on HCMV neutralizing antibodies produced by HCMV–specific B cells, which are initially low in numbers during the early posttransplantation period and gradually expand after transplantation.19 Consistent with this hypothesis, plasma or serum from individuals HCMV-seropositive but not from HCMV seronegative donors enhanced the expansion and functionality of adaptive NK cells when cocultured with autologous monocytes or macrophages in vitro, likely due to enhanced CD16 signaling.17,19,23,37,38 Therefore, pharmacological suppression of adaptive immune reconstitution and B-cell functionality might compound the impact of iatrogenic immunosuppression on NK-cell reconstitution. Such indirect links could have contributed to the sharp drop in “memory-like” NK cells seen in several of our patients with Rf/EOD-CMVi in the context of GvHD therapy, preceding persistent and/or severe HCMV reactivation events. Importantly, our observation that NK-cell impairment mostly preceded Rf/EOD events suggests that extended monitoring of NK-cell counts and phenotypes might contribute to a more nuanced risk assessment regarding late HCMV events. Of note, based on research in kidney transplant recipient, “memory-like” NK cells are thought to predominantly contribute to curbing progression of HCMV infection rather than controlling early viral replication,39 further supporting an association between low “memory-like” NK-cell counts and Rf/EOD-csCMVi risk.
Our study has certain limitations. We studied a relatively small patient cohort, which prevented us from performing definitive assessment of the prospective value of extended NK-cell monitoring (eg, receiver operating characteristics analysis). Therefore, detailed long-term studies in larger cohorts are needed to corroborate the prognostic significance of functional NK-cell assays for future csCMVi events. In the future, well-powered studies should also elucidate the role of NK-cell impairment in refractory HCMV reactivations after the initial reactivation event and determine whether these late changes to the NK-cell repertoire might predispose patients with csCMVi to other infections. Furthermore, larger cohorts are needed to further validate the association between HCMV–specific Th1-cell reconstitution and the severity of HCMV reactivations and to elucidate more definitively the role of regulatory T cells in shaping anti-HCMV immunity. Additionally, despite well-matched patient cohorts, other variables such as donor and recipient HCMV serotypes as well as immunosuppression could potentially affect immune reconstitution and HCMV-reactivation timelines after alloSCT.4,40,41 Hence, these parameters would need to be considered in larger studies using multivariate analysis. Moreover, owing to the limited size of our patient cohort, we were unable to robustly assess the impact of additional factors, such as low-level HCMV reactivations and HLA restrictions on immune reconstitution and HCMV control. This analysis requires a larger patient sample to achieve the necessary statistical power. Future studies with expanded cohorts are essential to effectively evaluate these factors. Furthermore, our study used cryopreserved PBMCs, which is a routine methodology but cryopreserved cells may be susceptible to alterations of T-cell phenotypes and antigen-specific responses compared with freshly isolated cells.42-45 Lastly, our calculation of absolute counts of leukocyte subsets relied on a combination of flow cytometry and lymphocyte counts from clinical hematology; hence, there might be some variation compared with direct whole blood–based flow cytometric assessment because of cell isolation and in vitro stimulation.10
Despite these limitations, our study underscores the vital role of HCMV–specific T cells in HCMV control and reveals a thus far underappreciated association between impaired reconstitution of “memory-like” NK cells and late, clinically significant HCMV reactivations after discontinuation of letermovir. Expansion and clinical translation of this knowledge could result in more nuanced functional immunoassay probing both the T- and NK-cell repertoire to guide HCMV management. Additionally, future in-depth analyses using advanced molecular techniques, such as single-cell RNA sequencing, could illuminate the distinct mechanisms underpinning impaired Th1-, Tc1-, and (“memory-like”) NK-cell reconstitution and its impact on other infectious complications and long-term outcomes. Ultimately, such studies might unveil novel avenues for targeted immunotherapeutic interventions to prevent severe HCMV disease.
Acknowledgments
The authors thank all the patients for their blood donation. The authors also acknowledge the assistance of Lubov Darst, Selina Grafelmann and Anna Groß in obtaining patient samples, and Oana Butto for her help with PBMC isolation.
This work was supported by a Deutsche Forschungsgemeinschaft grant within the Research Unit FOR 2830 (KR 5761/1-2, project number 398367752, to S.K.; EI 269/10-2, project number 398367752, to H.E.; DO 1275/7-2, to L.D.; ER 927/1-2, to F.E.; FA 483/2-2, to C.S.F.). S.K. was further supported by a fellowship (project number ZZ-28) of the Interdisciplinary Center for Clinical Research (IZKF).
Author contributions
Contribution: C.D.L., G.U.G., H.E., S.W., and S.K. conceived the study; D.G. and S.K. performed patient enrollment and clinical documentation; C.D.L., A.F.R., C.K., and A.N. planned and performed experiments; C.D.L., I.M., F.E., S.W., and S.K. analyzed the data; C.D.L. performed data visualization; C.D.L., F.E., L.D., H.E., S.W., and S.K. led project administration and supervision; F.E., L.D., H.E., and S.K. acquired funding; C.D.L., S.W., and S.K. wrote the original draft; and all authors reviewed, edited, and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sabrina Kraus, Department of Internal Medicine II, University Hospital of Würzburg, Oberdürrbacherstraße 6, 97080 Würzburg, Germany; email: Kraus_S3@ukw.de; and Chris David Lauruschkat, Department of Internal Medicine II, University Hospital of Würzburg, Josef-Schneider Str. 2, C11, 97080 Würzburg, Germany; email: Lauruschka_C@ukw.de.
References
Author notes
Requests for materials should be addressed to the corresponding author, Sabrina Kraus (Kraus_S3@ukw.de).
The data sets generated and analyzed during this study are available on reasonable request from the corresponding author, Sabrina Kraus (Kraus_S3@ukw.de). Individual participant data will not be shared.
The full-text version of this article contains a data supplement.