Dynamic changes in neutrophil NETosis in patients with immune-mediated thrombotic thrombocytopenic purpura (iTTP) are assessed.
Similar to DNase I, recombinant ADAMTS13 or caplacizumab prevents the accumulation of neutrophil extracellular traps under flow in iTTP.
Visual Abstract
Neutrophil NETosis is a unique form of cell death, characterized by the release of decondensed chromatin and antimicrobial contents to the extracellular space, which is involved in inflammation and thrombosis. However, the role of NETosis in the pathogenesis of immune-mediated thrombotic thrombocytopenic purpura (iTTP) and how a targeted therapy affects the accumulation of neutrophil extracellular traps (NETs) under flow remain unknown. Flow cytometry demonstrated that the percentage of neutrophils undergoing NETosis in whole blood from patients with iTTP on admission was significantly increased, with a concurrent decrease in the capacity of inducible NETosis by shigatoxin. After therapy, the percentage of H3Cit+MPO+ neutrophils was significantly reduced, with an improvement in inducible NETosis in these patients. Additionally, little to no NET and thrombus formation was detected underflow in the whole blood from patients with iTTP when platelet counts were very low, but the NET and thrombus formation was dramatically increased following therapy when platelet counts rose to ≥50 × 109/L or were restored to normal with donor platelets. Similarly, there was no thrombus or NET accumulation under flow in the whole blood from vwf-/- mice, but NET accumulation was significantly higher in Adamts13-/- mice than in wild-type mice. Finally, recombinant ADAMTS13 or caplacizumab (or anfibatide) prevented NET and thrombus formation under flow in whole blood from patients with iTTP or from Adamts13-/- mice. These results indicate that neutrophil NETosis and NET formation depend on platelets and von Willebrand factor (VWF) in iTTP, and a targeted therapy such as recombinant ADAMTS13 or caplacizumab may prevent NET and thrombus formation under flow in iTTP.
Introduction
Immune-mediated thrombotic thrombocytopenic purpura (iTTP) is a rare but potentially fatal hematological disease. It is primarily caused by autoantibody-mediated inhibition of plasma ADAMTS13 activity1-3 and/or accelerated clearance of plasma ADAMTS13 antigen,4 resulting in a severe deficiency of ADAMTS13 activity. ADAMTS13, primarily synthesized in hepatic stellate cells5,6 and endothelial cells,7,8 is the key enzyme in circulation to cleave endothelium-derived ultra large (UL) von Willebrand factor (VWF) multimers.9 This proteolytic cleavage of ULVWF is crucial for maintaining normal hemostasis.10 Severe deficiency of plasma ADAMTS13 activity results in the accumulation of ULVWF multimers on vascular endothelial surfaces, in circulation, and at sites of vascular injury. This leads to excessive platelet aggregation and thrombus formation in small arterioles and capillaries, a characteristic pathological feature of iTTP.11 Patients with iTTP may present with a marked thrombocytopenia, microangiopathic hemolytic anemia, and various degrees of end organ damage.12-14 Therapeutic plasma exchange (TPE), caplacizumab, and immunosuppressants (eg, corticosteroids and rituximab), known as triple therapy, are the standard of care to date for the treatment of iTTP.15,16 Simple plasma infusion17,18 or recombinant ADAMTS1319 appears to be effective for the treatment of hereditary thrombotic thrombocytopenic purpura (hTTP), which results from mutations in ADAMTS13, leading to a severe deficiency in plasma ADAMTS13 activity.
Surprisingly, severe deficiency of plasma ADAMTS13 alone may not be sufficient to cause an acute episode of iTTP or hTTP, as demonstrated in human20 and mice.21 This suggests that additional environmental or genetic factors such as infections or acute systemic inflammation may be required to trigger acute TTP. For instance, Adamts13-/- mice do not develop spontaneous thrombocytopenia unless being challenged with a bacterial toxin such as shigatoxin-2 (Stx-2) or a large dose of unprocessed recombinant VWF.21-23 Stx-2 has been shown to activate endothelium to release ULVWF, resulting in endothelial injury21,22,24 and triggering neutrophils NETosis,25 all of which may lead to microvascular thrombosis when plasma ADAMTS13 activity is severely deficient. Adamts13-/- mice carrying a loss-functional heterozygous mutation (W1206R) in complement factor H (cfh) also developed spontaneous thrombotic microangiopathy (TMA), whereas a loss-of-function in either Adamts13 or cfh was not sufficient to cause the TMA disease.26
Neutrophil NETosis is part of the innate immune system for self-defense. It protects the host from invasion by external agents, such as bacteria, fungi, and viruses.27,28 NETosis may play a role in the pathogenesis of various inflammatory diseases, including sepsis,29,30 systemic lupus erythematosus,31 and thrombotic diseases (eg, deep vein thrombosis, heparin-induced thrombocytopenia,30,32 and acute iTTP.33-35 However, dynamic changes in NETosis and the ability to form neutrophil extracellular traps (NETs) under flow in patients with iTTP or Adamts13-/- mice have not been extensively studied. Additionally, the effect of a current targeted therapy for iTTP, such as recombinant ADAMTS13 or caplacizumab, on the NET accumulation under flow has not been investigated. This study aimed to determine the extent of neutrophil NETosis in vivo and ability to induce NETosis and NET formation by an external agonist in acute iTTP and during treatment, and to assess the role of platelets, VWF, and ADAMTS13, as well as a targeted therapeutic agent for iTTP on NET accumulation under arterial flow.
Methods
Patients
The Institutional Review Board (IRB) of the University of Kansas Medical Center approved the study protocol (#STUDY00145731). Consecutive patients at the University of Kansas Hospital between June 2022 and June 2023 with a suspected iTTP were prospectively enrolled in the study. The diagnosis of iTTP was made based on the following criteria: thrombocytopenia and microangiopathic hemolytic anemia with organ damage.36 All patients were subsequently confirmed to have a plasma ADAMTS13 activity level <10 IU/dL and positive anti-ADAMTS13 IgG (supplemental Table 1). One patient whose first sample was collected >48 hours after admission was excluded to avoid the impact of TPE on the assessment of plasma ADAMTS13 activity, inhibitor, and neutrophil NETosis. Samples from those without acute iTTP or other hematological diseases were obtained for the controls. All patients participated in the study were informed and consented.
Samples and clinical data
Venous blood samples were collected from non-TTP controls and patients admitted for acute iTTP before and during therapy. Whole blood was anticoagulated with EDTA for flow cytometric analysis or with 3.2% sodium citrate for the shear-based assay. Patient demographic information, clinical characteristics, and laboratory data were collected from electronic medical records. Laboratory findings included white blood cell count, neutrophil count, hemoglobin level, hematocrit, platelet count, lactate dehydrogenase (LDH) level, prothrombin time, activated partial thromboplastin time, creatinine level, ADAMTS13 activity, and anti-ADAMTS13 IgG levels.
Murine model of TTP
All the animal experimental protocols were approved by the Institutional Animal Care and Use Committee (ACUP #2020-2574) of the University of Kansas Medical Center. Adamts13-/- mice of the CAST/Ei strain were used in these studies.21 Murine whole blood was collected using the retro-orbital bleeding technique after anesthesia. Approximately 70 μL of whole blood was collected and anticoagulated with the thrombin inhibitor D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK) (100 μmol/L). Whole blood was treated ex vivo with 100 ng/mL of Stx-2 for 60 min25 before perfusion through the microchannels coated with type 1 fibrillar collagen from equine tendons (Crono-log, Havertown, PA) under 100 dyne/cm2 for 120 seconds.37
Critical reagents
Recombinant ADAMTS13 was expressed in stably transfected HEK-293 cells using 10-layer cell factories (Thermo Fisher, Waltham, MA) in serum-free Dulbecco modified Eagle medium (DMEM)/F12 medium supplemented with 1% of insulin, transferrin, and selenium (ITS) cocktail (Millipore-Sigma, Burlington, MA).38,39 Approximately 2 L of conditioned medium was collected daily and loaded onto a Q-fast flow ion-exchange column (Thermo Fisher). Total protein was eluted with 1.0 M NaCl in 20 mM Tris-HCl (pH 8.0). All fractions containing proteins were pooled and loaded onto a 10 to 80 mL Ni-NTA affinity column (Thermo Fisher). After being washed with 20 mM and 40 mM of imidazole, the bound proteins were eluted with 250 mM of imidazole in 20 mM Tris-HCl (pH 8.0), and 400 mM NaCl. The peak fractions containing recombinant ADAMTS13 protein were pooled and concentrated with Centri-Prep30 (Millipore, Billerica, MA) and buffer-exchanged with 10 mM HEPES (pH 7.4) containing 150 mM NaCl and 5 mM CaCl2. This essentially removes imidazole and recharges rADAMTS13 with appropriate divalent metal ions. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) with Coomassie blue staining and the absorbance at 280 nm determined the purity and concentration of recombinant ADAMTS13.
Flow cytometry
Whole blood anticoagulated with EDTA was centrifuged at 500 × g for 10 minutes to obtain a buffy coat, which was washed with phosphate-buffered saline containing 2% bovine serum albumin.40 The cells were then incubated with 100 ng/mL of Stx-2 (Toxin Technology, Sarasota, FL) for 15 minutes to stimulate neutrophil NETosis.25 The cells were fixed with 2% paraformaldehyde for 30 minutes and neutrophils were identified using eFluor450 anti-CD15 (Invitrogen). Citrullinated histone H3 (H3Cit) was stained with anticitrullinated histone H3 (citrulline at R2, R8, and R17) IgG (Abcam, Waltham, MA), followed by goat Alexa488-conjugated anti-rabbit IgG (ThermoFisher). Myeloperoxidase (MPO) in neutrophils was stained with phycoerythrin anti-MPO antibody (Novus Biologicals, Centennial, CO) as previously described.29,30,32,41 The stained cells were analyzed using a BD LSR II flow cytometer.
Assay for plasma MPO levels
Plasma levels of MPO were determined using an ELISA kit34 (ThermoFisher) according to the manufacturer’s recommendations.
ADAMTS13 activity and antibody in patients with iTTP
Plasma ADAMTS13 activity and anti-ADAMTS13 IgG were determined using the Technozym ADAMTS13 activity and antibody kit (DiaPharma, West Chester, OH) as previously described.42 The lowest detection limit for plasma ADAMTS13 activity was 0.1%. Plasma level of anti-ADAMTS13 IgG >15 U/mL were interpreted as positive.
Microfluidic shear-based assay
Patient whole blood anticoagulated with sodium citrate was stained with rhodamine 6G (Sigma-Aldrich, St. Louis, MO) at 40 μg/mL for 15 minutes. Whole blood was then perfused at 100 dyne/cm2 over micro channels coated with type 1 fibrillar collagen (100 μg/mL) (Chrono-Log, Overland Park, KS) in 0.01M HCl and blocked with phosphate-buffered saline/0.5% bovine serum albumin.37 One of the channels was perfused with a whole blood aliquot preincubated with DNase I (or pulmozyme) (200 U/mL) (Genentech, San Francisco, CA), recombinant ADAMTS13 (6 μg/mL), or caplacizumab (3 μg/mL) (Sanofi, Chattanooga, TN) for 15 min. Additionally, Adamts13-/- mouse blood was anticoagulated using 100 μmol/L of PPACK (Sigma-Aldrich, St. Louis, MO) in the presence of 3 μmol/L of prostaglandin E1 (Sigma-Aldrich) was treated with 100 ng/mL of Stx-2 in a CO2 incubator at 37°C for 1 hour to trigger NETosis and diluted twofold with Tyrode’s buffer (10 mmol/L HEPES, pH 7.4, 134 mmol/L NaCl, 2.7 mmol/L KCl, 1.0 mmol/L MgCl2, 12 mmol/L NaHCO3, and 0.34 mmol/L Na2HPO4) before perfusion in the absence or presence of DNase I, recombinant ADAMTS13, or anfibatide (1.5 μg/mL). The rate of thrombus formation (or the rate of platelet and neutrophil accumulation) was recorded every 3 seconds for 120 seconds.37 The surface area coverage was determined using the Montage software. At the completion of the real-time experiment, the cells in the micro channels were fixed with 4% paraformaldehyde and stained with SytoxGreen (0.3 μmol/L) (ThermoFisher), nuclei with Hoechst 33342 (10 μg/mL) (Thermo Fisher), and platelets with anti-human CD41 (BD Biosciences, Lakes, NJ) or anti-mouse CD41 (5 μg/mL) conjugated with APC (Biolegend, San Diego, CA). Fluorescent images were obtained under a Nikon A1R confocal microscope using the NIS Elements Viewer 5.21 (Melville, NY).
Ex vivo restoration of thrombocytopenia in patients with iTTP with donor platelets
Platelet-rich plasma was prepared by centrifugation of donor blood anticoagulated with sodium citrate at 150 × g for 15 minutes. Tyrode buffer was added to platelet-rich plasma, followed by centrifugation at 900 × g for 10 minutes to obtain platelet pellets.37 These platelet pellets were resuspended in Tyrode buffer before use for the restoration of thrombocytopenia in the whole blood of patients with iTTP.
Cleavage of VWF by ADAMTS13 and DNase I under shear
Plasma-derived VWF (8.3 μg/mL) was incubated with recombinant ADAMTS13 (30 μg/mL) or DNase I (200 U/mL) in the absence or presence of EDTA for 30 min under constant vortexing at 2500 rpm in a polymerase chain reaction mixer. Sample buffer was added to the polymerase chain reaction tube and heated at 100°C for 5 min to terminate the reaction. Denatured proteins were separated using 1% agarose gel electrophoresis. VWF multimer distribution was determined by western blotting with anti-VWF IgG, followed by IRDye800 anti-rabbit IgG (LI-COR, Lincoln, Nebraska), as described previously.43
Statistical analysis
Mann-Whitney and Kruskal-Wallis ANOVA tests were used for nonparametric data of 2 groups and 3 groups, respectively. Paired t tests were used for changes in the same groups for normally distributed data, whereas Wilcoxon signed-rank tests were used for nonparametric data. Statistical significance was set at P < .05. Data analysis and graphing were performed using Prism 9 software (GraphPad, Boston, MA).
Results
Patient characteristics
All 6 patients enrolled in the study had clinical and laboratory characteristics consistent with the diagnosis of iTTP according to the 2020 International Society of Thrombosis and Haemostasis (ISTH) guidelines.36 The mean (± standard deviation [SD]) age was 53.8 (±13.8) years with an equal gender distribution. Hypertension and chronic renal disease were found in 2 of 6 (33.3%) cases. The mean (± SD) platelet count was 9.7 (± 4.8) × 109/L. All patients had plasma ADAMTS13 activity levels of <1.0 IU/dL (or 1% of normal) and were positive for anti-ADAMTS13 antibodies. Hemoglobin and hematocrit (mean ± SD) were 9.4 ± 3.9 g/dL and 27.0 ± 11.6%, respectively. Lactate dehydrogenase (LDH) and creatinine were 1444 ± 836 IU/L and 1.7 ± 1.1 mg/dL, respectively, with normal prothrombin time (PT) and activated thromboplastin time (APTT) (Table 1). All patients were treated with TPE daily, corticosteroids, and upfront rituximab. Five patients also received caplacizumab 3 to 4 days after admission. One patient case was complicated by intracranial bleeding and did not receive caplacizumab (supplemental Table 1).
Characteristics and laboratory findings on admission of 6 patients with iTTP used in microfluidic assays
| Parameter . | Reference‡ . | iTTP (n = 6) . |
|---|---|---|
| Age (years) | — | 53.8 ± 13.8∗ |
| Gender, male, n (%) | — | 3 (50.0) |
| Comorbidities | ||
| Hypertension, n (%) | — | 2 (33.3) |
| Diabetes, n (%) | — | 0 (0) |
| Chronic kidney disease, n (%) | — | 2 (33.3) |
| History of malignancy, n (%) | — | 2 (33.3) |
| White Blood Cell (× 109/L) | 4.5-11.0 | 10.7 ± 3.0∗ |
| Neutrophil (× 109/L) | 4.1-7.7 | 8.5 ± 2.3∗ |
| Hemoglobin (g/dL) | 12.0-16.5 | 9.4 ± 3.9∗ |
| Hematocrit (%) | 36-50 | 27.0 ± 11.6∗ |
| Platelet count (× 109/L) | 150-400 | 9.7 ± 4.8∗ |
| ADAMTS13 activity (IU/dL) | 40-133 | |
| Anti-ADAMTS13 IgG (U/mL) | <15 | 0 (0-0.7)† |
| Positive | ||
| LDH (IU/L) | 100-200 | 1444 ± 836∗ |
| Creatinine (mg/dL) | 0.46-1.09 | 1.7 ± 1.1∗ |
| INR | 0.85-1.15 | 1.2 ± 0.2∗ |
| APTT (sec) | 20-40 | 29.0 ± 2.1∗ |
| Parameter . | Reference‡ . | iTTP (n = 6) . |
|---|---|---|
| Age (years) | — | 53.8 ± 13.8∗ |
| Gender, male, n (%) | — | 3 (50.0) |
| Comorbidities | ||
| Hypertension, n (%) | — | 2 (33.3) |
| Diabetes, n (%) | — | 0 (0) |
| Chronic kidney disease, n (%) | — | 2 (33.3) |
| History of malignancy, n (%) | — | 2 (33.3) |
| White Blood Cell (× 109/L) | 4.5-11.0 | 10.7 ± 3.0∗ |
| Neutrophil (× 109/L) | 4.1-7.7 | 8.5 ± 2.3∗ |
| Hemoglobin (g/dL) | 12.0-16.5 | 9.4 ± 3.9∗ |
| Hematocrit (%) | 36-50 | 27.0 ± 11.6∗ |
| Platelet count (× 109/L) | 150-400 | 9.7 ± 4.8∗ |
| ADAMTS13 activity (IU/dL) | 40-133 | |
| Anti-ADAMTS13 IgG (U/mL) | <15 | 0 (0-0.7)† |
| Positive | ||
| LDH (IU/L) | 100-200 | 1444 ± 836∗ |
| Creatinine (mg/dL) | 0.46-1.09 | 1.7 ± 1.1∗ |
| INR | 0.85-1.15 | 1.2 ± 0.2∗ |
| APTT (sec) | 20-40 | 29.0 ± 2.1∗ |
LDH, lactate dehydrogenase; INR, international normalized ratio; APTT, activated thromboplastin time.
Data are shown as the mean ± standard deviation (SD).
median (range).
Laboratory reference ranges are provided.
NETosis and the potential of inducible NETosis in patients with acute iTTP
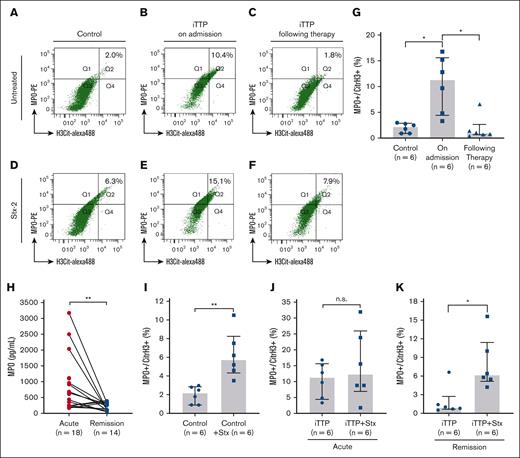
Using flow cytometry, we were able to detect a small percentage (median, 2.1%) of neutrophils undergoing NETosis, marked by H3Cit+MPO+, in whole blood from non-TTP controls (Figure 1A). The median percentage of H3Cit+ and MPO+ neutrophils in patients with acute iTTP was 11.3% (Figure 1B), which was significantly higher than that in non-TTP controls. Following a standard of care therapy, the median percentage of H3Cit+ and MPO+ neutrophil was significantly reduced (to 0.9%) (Figure 1C). The difference in the percentage of H3Cit+ and MPO+ neutrophils was statistically significant between the control and patients with iTTP on admission (P < .05) or between patients with iTTP on admission and those during clinical remission (P < .05) (Figure 1G). The plasma levels of myeloperoxidase (MPO) in patients with iTTP on admission were significantly higher than those during clinical response/remission (P < .05) (Figure 1H). These results indicate that neutrophil NETosis is significantly elevated in acute iTTP, which is markedly reduced following a standard of care therapy or during clinical remission.
Flow cytometric detection of NETosis in whole blood from patients with iTTP and non-TTP controls. (A-C) The gating profiles and percentage of H3Cit+MPO+ neutrophils (Q2) in unstimulated samples from non-TTP controls, patients with acute iTTP on admission, and those following therapy, respectively. (D-F) The gating profile and percentage of H3Cit+MPO+ neutrophils (Q2) in Stx-2-stimulated blood samples from non-TTP controls, patients with iTTP on admission, and patients with iTTP following therapy, respectively. (G) Quantitation and statistical analysis of H3Cit+MPO+ neutrophils in unstimulated neutrophil collected from control, iTTP on admission, and iTTP after therapy. (H) Individual plasma MPO level in patients with acute iTTP and during remission. (I-K) (paired t test). The effects of Stx-2 on the percentage of H3Cit+MPO+ neutrophils in non-TTP controls, patients with iTTP on admission, and those following therapy, respectively. Kruskal-Willis test was performed in panel A and Mann-Whitney test in panels I-K. The data shown are individual values (dots), median (bar), and interquartile range (IQR) (G-K). n.s., ∗, and ∗∗ indicates P > 0.05, P < 0.05, and P < 0.01, respectively.
Flow cytometric detection of NETosis in whole blood from patients with iTTP and non-TTP controls. (A-C) The gating profiles and percentage of H3Cit+MPO+ neutrophils (Q2) in unstimulated samples from non-TTP controls, patients with acute iTTP on admission, and those following therapy, respectively. (D-F) The gating profile and percentage of H3Cit+MPO+ neutrophils (Q2) in Stx-2-stimulated blood samples from non-TTP controls, patients with iTTP on admission, and patients with iTTP following therapy, respectively. (G) Quantitation and statistical analysis of H3Cit+MPO+ neutrophils in unstimulated neutrophil collected from control, iTTP on admission, and iTTP after therapy. (H) Individual plasma MPO level in patients with acute iTTP and during remission. (I-K) (paired t test). The effects of Stx-2 on the percentage of H3Cit+MPO+ neutrophils in non-TTP controls, patients with iTTP on admission, and those following therapy, respectively. Kruskal-Willis test was performed in panel A and Mann-Whitney test in panels I-K. The data shown are individual values (dots), median (bar), and interquartile range (IQR) (G-K). n.s., ∗, and ∗∗ indicates P > 0.05, P < 0.05, and P < 0.01, respectively.
To assess the NETosis reserve (or the potential of inducible NETosis), Stx-2, which was shown to induce thrombotic microangiopathy (TMA) in humans44 and mice,21,45,46 was used to trigger neutrophil NETosis. A whole blood sample was incubated with Stx-2 (100 ng/mL) at 37°C for 15 min before fixation, staining, and flow cytometric analysis. The results showed that following Stx-2 stimulation, the percentage of neutrophils with H3Cit+MPO+ staining increased by 2.6-fold (from 2.2%-5.7%) (P < .01) (Figure 1D), but much less so in iTTP on admission (from 11.3%-12.2%, (P>.05) (Figure 1E). The percentage significantly increased again in iTTP during clinical response/remission (from 0.9%-6.1%; 6.8-fold increase) (P < .05) (Figure 1F). Quantitative data and statistical analysis of the percentage of NETosis before and after the Stx-2 challenge are shown (Figure 1I-K). These results indicate that the neutrophil NETosis reserve may be significantly reduced in acute iTTP, which recovers following intensive therapy.
Thrombus and NET accumulation on a collagen surface under flow in wild-type (WT), vwf-/-, and Adamts13-/- mice
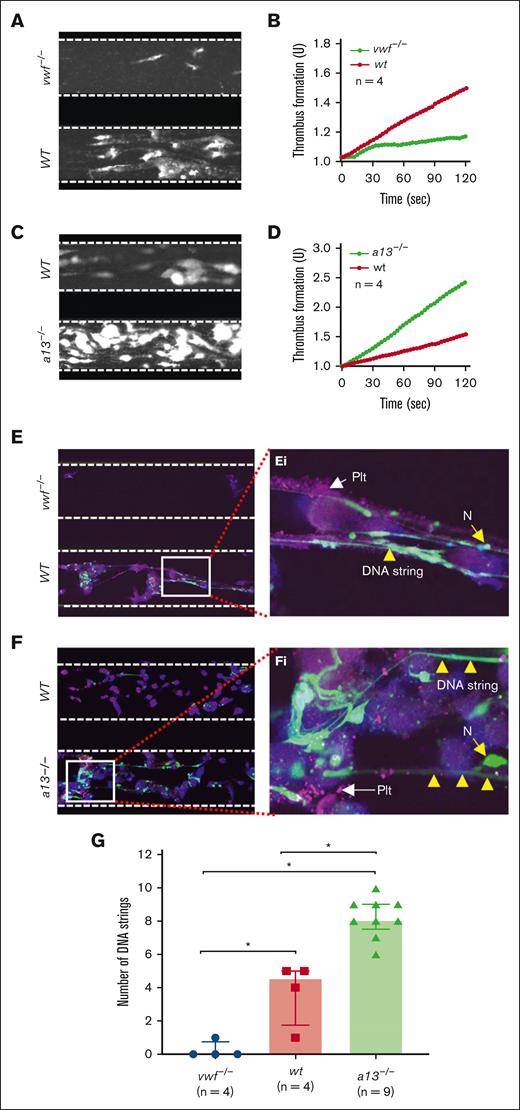
To determine whether VWF or ADAMTS13 regulates the shear-dependent accumulation of NETs, we collected thrombin inhibitor (PPACK)-anticoagulated whole blood samples from WT, vwf-/-, and Adamts13-/- mice (CAST/Ei strain) and challenged with Stx-2 (100 ng/mL) at 37°C for 1 hour to induce neutrophil NETosis. Without Stx-2 stimulation, no NET was detected, despite many platelets and neutrophils adhering to the collagen surface under flow (supplemental Figure 2). After perfusion of Stx-2-stimulated whole blood, the rate of thrombus formation under flow was significantly lower in vwf-/- mice than in WT controls (Figure 2A-B; supplemental Video 1) as expected. Conversely, the rate of thrombus formation following perfusion of stimulated blood from Adamts13-/- mice was much higher than that in WT controls (Figure 2C-D; supplemental Video 2). Interestingly, NET accumulation was also significantly reduced following perfusion of whole blood from vwf-/- mice (Figure 2E/Ei) but increased in Adamts13-/- mice (Figure 2F/Fi) when compared with that in WT mice. The number of elongated DNA strings under a fluorescent microscope following perfusion of whole blood from WT, vwf-/-, and Adamts13-/- mice was significantly different (Figure 2G). Together, these results indicate that, similar to thrombus formation, neutrophil NETosis and NET accumulation under flow depend on plasma VWF, which is regulated by ADAMTS13.
Microfluidic assay determines ex vivo thrombus and NETs formation under arterial flow in vwf-/- and Adamts13-/- mice. (A-B) The final mean surface coverage of platelets and leukocytes on the collagen surface and the mean rate of thrombus formation over time, respectively, following perfusion of whole blood prestimulated with Stx-2 under flow from vwf-/- (top) and WT (bottom) mice, as indicated. (C-D) The final surface coverage of platelets and leukocytes and the rate of thrombus formation over time, respectively, following perfusion of whole blood prestimulated with Stx-2 from WT (top) and Adamts13-/- (bottom) mice, as indicated. (E-F) Representative confocal microscopic images demonstrate the finally adhered platelets (purple), neutrophils (green), and extracellular DNA strings (elongated green) following perfusion of whole blood samples from vwf-/- vs WT, and WT vs Adamts13-/- mice, respectively. (Ei-Fi) are enlarged (5×) areas from the bottom panels of E and F, respectively. Here, a13-/- is Adamts13-/-; vwf-/- is vwf null; n, number of mice; N, neutrophil; PLT, platelets. (G) Quantitation of the number of DNA strings under fluorescent microscope in the blood samples from vwf-/-, WT, and Adamts13-/- mice. The data shown in G are individual values (dots), median (bar), and interquartile range (IQR). Kruskal-Wallis test determined the statistical significance among the 3 groups. ∗ indicates P < .05.
Microfluidic assay determines ex vivo thrombus and NETs formation under arterial flow in vwf-/- and Adamts13-/- mice. (A-B) The final mean surface coverage of platelets and leukocytes on the collagen surface and the mean rate of thrombus formation over time, respectively, following perfusion of whole blood prestimulated with Stx-2 under flow from vwf-/- (top) and WT (bottom) mice, as indicated. (C-D) The final surface coverage of platelets and leukocytes and the rate of thrombus formation over time, respectively, following perfusion of whole blood prestimulated with Stx-2 from WT (top) and Adamts13-/- (bottom) mice, as indicated. (E-F) Representative confocal microscopic images demonstrate the finally adhered platelets (purple), neutrophils (green), and extracellular DNA strings (elongated green) following perfusion of whole blood samples from vwf-/- vs WT, and WT vs Adamts13-/- mice, respectively. (Ei-Fi) are enlarged (5×) areas from the bottom panels of E and F, respectively. Here, a13-/- is Adamts13-/-; vwf-/- is vwf null; n, number of mice; N, neutrophil; PLT, platelets. (G) Quantitation of the number of DNA strings under fluorescent microscope in the blood samples from vwf-/-, WT, and Adamts13-/- mice. The data shown in G are individual values (dots), median (bar), and interquartile range (IQR). Kruskal-Wallis test determined the statistical significance among the 3 groups. ∗ indicates P < .05.
Recombinant ADAMTS13 or anfibatide inhibits thrombus formation and NET accumulation on a collagen surface under flow in Adamts13-/- mice
To determine whether a targeted therapy, recombinant ADAMTS13, which cleaves VWF or anfibatide, which inhibits VWF-platelet interaction, inhibits NET accumulation under flow, we perfused a PPACK-anticoagulated whole blood from Adamts13-/- mice stimulated with Stx-2 over a type I fibrillar collagen coated surface under 100 dyne/cm2 in the absence or presence of recombinant human ADAMTS13 or anfibatide. The results showed that the rate of thrombus formation was significantly reduced in the channels in which DNase I (Figure 3A,D; supplemental Video 3), recombinant ADAMTS13 (Figure 3B/3E; supplemental Video 4), or anfibatide (Figure 3C,F; supplemental Video 5) was present, compared with that in the channel in which DNase I, recombinant ADAMTS13, or anfibatide was absent. More interestingly, the characteristic, elongated, and extracellularly stained DNA strings (NETs) were found only in the channels in the absence of DNase I, or recombinant ADAMTS13, or anfibatide present (Figure 3G/Gi, Figure H/Hi, Figure I/Ii). The number of DNA strings observed under a microscope with or without DNase I was statistically significantly different (Figure 3J-L). These results indicate that neutrophil NETosis induced by shigatoxin in whole blood from Adamts13-/- mice was significantly enhanced and could be removed by DNase I or prevented by recombinant human ADAMTS13 or anfibatide.
DNase I, recombinant ADAMTS13, and anfibatide inhibit thrombus and prevent NET accumulation under flow in whole blood of Adamts13-/- mice. (A-C) The final surface coverage of platelets and leukocytes on a collagen surface following perfusion of Adamts13-/- murine blood samples stimulated with Stx-2 in the presence (top) or absence (bottom) of DNase I (200 IU/mL) or recombinant ADAMTS13 (+rA13) (12 μg/mL), or anfibatide (+ANF) (1.5 μg/mL), respectively, under 100 dyne/cm2. (D-F) The rate of thrombus formation over time (mean ± standard error of the mean [SEM]) following perfusion of Adams13-/- murine blood samples stimulated with Stx-2 in the presence (red) or absence (blue) of DNase I or rA13, ANF, under flow. (G-I) Representative confocal microscopic images demonstrate adhered platelets (purple), neutrophils (green), and extracellular DNA strings (elongated green) at the end of perfusion of whole blood samples from adamts13-/- mice in the presence (top) or absence (bottom) of DNase I, rA13, or ANF, following fixation and additional staining. (Gi-Ii) Enlarged (5×) areas from the bottom of panels G, H, and I, respectively, indicate the extracellular DNA strings, platelets, and neutrophils. (J-L) Number of elongated DNA strings formed at the end of perfusion of blood samples from Adamts13-/- mice in the presence or absence of DNase I, rA13, and ANF (n = 3), respectively. Data are shown as the median (bar) and IQR. Mann-Whitney test was used for the differences between the 2 groups. rA13, recombinant ADAMTS13; ANF, anfibatide; N, neutrophil; PLT, platelet. ∗∗ indicates P < .01.
DNase I, recombinant ADAMTS13, and anfibatide inhibit thrombus and prevent NET accumulation under flow in whole blood of Adamts13-/- mice. (A-C) The final surface coverage of platelets and leukocytes on a collagen surface following perfusion of Adamts13-/- murine blood samples stimulated with Stx-2 in the presence (top) or absence (bottom) of DNase I (200 IU/mL) or recombinant ADAMTS13 (+rA13) (12 μg/mL), or anfibatide (+ANF) (1.5 μg/mL), respectively, under 100 dyne/cm2. (D-F) The rate of thrombus formation over time (mean ± standard error of the mean [SEM]) following perfusion of Adams13-/- murine blood samples stimulated with Stx-2 in the presence (red) or absence (blue) of DNase I or rA13, ANF, under flow. (G-I) Representative confocal microscopic images demonstrate adhered platelets (purple), neutrophils (green), and extracellular DNA strings (elongated green) at the end of perfusion of whole blood samples from adamts13-/- mice in the presence (top) or absence (bottom) of DNase I, rA13, or ANF, following fixation and additional staining. (Gi-Ii) Enlarged (5×) areas from the bottom of panels G, H, and I, respectively, indicate the extracellular DNA strings, platelets, and neutrophils. (J-L) Number of elongated DNA strings formed at the end of perfusion of blood samples from Adamts13-/- mice in the presence or absence of DNase I, rA13, and ANF (n = 3), respectively. Data are shown as the median (bar) and IQR. Mann-Whitney test was used for the differences between the 2 groups. rA13, recombinant ADAMTS13; ANF, anfibatide; N, neutrophil; PLT, platelet. ∗∗ indicates P < .01.
Recombinant ADAMTS13 and caplacizumab inhibit thrombus formation and NET accumulation on collagen surface under flow in iTTP
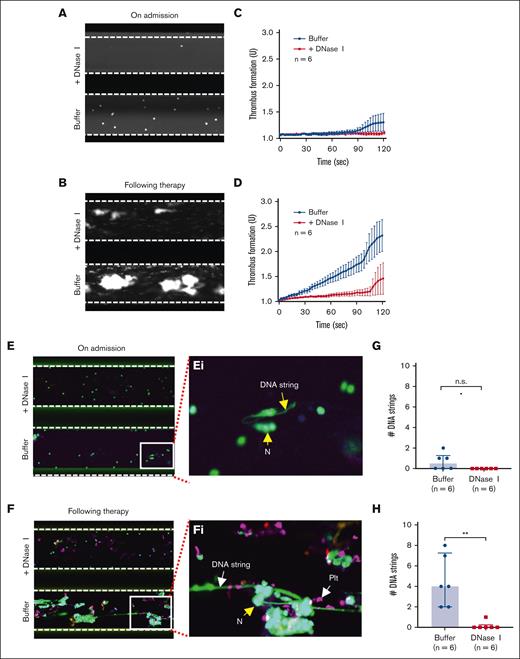
To assess the dynamic changes in thrombus formation and NET accumulation under flow and the therapeutic effects of DNase I, recombinant ADAMTS13, and caplacizumab on eliminating or preventing thrombus formation and NET accumulation, we collected citrated-anticoagulated whole blood samples from 6 patients with acute iTTP on admission when the platelet count was very low (<10 × 109/L), during clinical responses/remission (5 cases), or when platelet counts rose to >30 × 109/L (1 case) (supplemental Table 1). The anticoagulated whole blood was then perfused over a type I fibrillar collagen-coated surface at 100 dyne/cm2 in the absence or presence of DNase I, recombinant ADAMTS13, or caplacizumab. As expected, no platelet or neutrophil adhered to the collagen surface when whole blood (very low platelet count) from patients with acute iTTP on admission was perfused (Figure 4A,C; supplemental Video 6). At the same time, little or no NET was observed on the collagen surface following SytoxGreen staining (Figure 4E/Ei). However, when donor platelets were added to these patient samples with initial iTTP to normalize platelet counts, both thrombus formation and NET accumulation on the collagen surface dramatically increased under the same flow conditions, which was abrogated by caplacizumab (supplemental Figure 3; supplemental Video 7). Moreover, thrombus formation (Figure 4B,D; supplemental Video 8) and NET accumulation (Figure 4F/Fi) were also significantly increased in patients with iTTP samples following the standard of care therapy when platelet counts were normalized or substantially increased despite treated with TPE and/or caplacizumab. Quantitation of the number of elongated DNA strings in the absence or presence of DNase I demonstrated the significant difference in patient samples collected after therapy (Figure 4F,H). These results demonstrated that platelets may play a role in NET accumulation on the collagen surface under flow in patients with acute iTTP, which can be completely removed by DNase I.
Platelets are required for the formation of NETs under flow in whole-blood samples of acute iTTP. (A-B) Representative images illustrate adhered neutrophils and platelets on a collagen-coated surface following perfusion of a whole blood sample from patients with iTTP on admission and after therapy, respectively, with or without DNase I (200 IU/mL) as indicated. (C-D) The rate of thrombus formation as a function of time (mean ± SEM, n = 6) following perfusion of iTTP blood samples collected on admission and after therapy, respectively, with or without DNase I (200 IU/mL) as indicated. (E-F) Final platelets, leukocytes, and DNA strings on a collagen surface following perfusion, fixation, and restained with anti-CD41 APC and SytoxGreen with or without DNase I as indicated. (Ei-Fi) Enlarged (5×) areas of the bottom images in E and F, respectively, indicate the extracellular DNA strings, platelets (PLT), and neutrophils (N). (G-H) The number of DNA strings formed on a collagen surface following perfusion of blood samples collected from patients with iTTP on admission and during remission, respectively, with or without DNase I. Data are shown as median (bar) and IQR. Mann-Whitney test was used to determine differences. N, neutrophil; PLT, platelet; n.s. and ∗∗ indicate P > .05 and P < .01, respectively.
Platelets are required for the formation of NETs under flow in whole-blood samples of acute iTTP. (A-B) Representative images illustrate adhered neutrophils and platelets on a collagen-coated surface following perfusion of a whole blood sample from patients with iTTP on admission and after therapy, respectively, with or without DNase I (200 IU/mL) as indicated. (C-D) The rate of thrombus formation as a function of time (mean ± SEM, n = 6) following perfusion of iTTP blood samples collected on admission and after therapy, respectively, with or without DNase I (200 IU/mL) as indicated. (E-F) Final platelets, leukocytes, and DNA strings on a collagen surface following perfusion, fixation, and restained with anti-CD41 APC and SytoxGreen with or without DNase I as indicated. (Ei-Fi) Enlarged (5×) areas of the bottom images in E and F, respectively, indicate the extracellular DNA strings, platelets (PLT), and neutrophils (N). (G-H) The number of DNA strings formed on a collagen surface following perfusion of blood samples collected from patients with iTTP on admission and during remission, respectively, with or without DNase I. Data are shown as median (bar) and IQR. Mann-Whitney test was used to determine differences. N, neutrophil; PLT, platelet; n.s. and ∗∗ indicate P > .05 and P < .01, respectively.
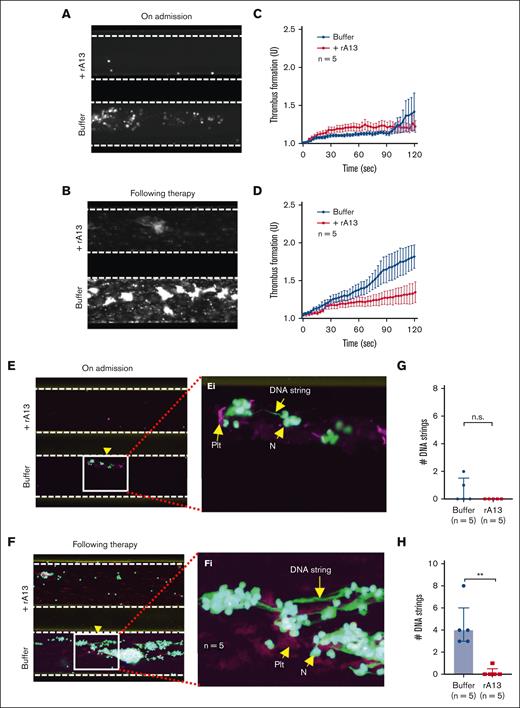
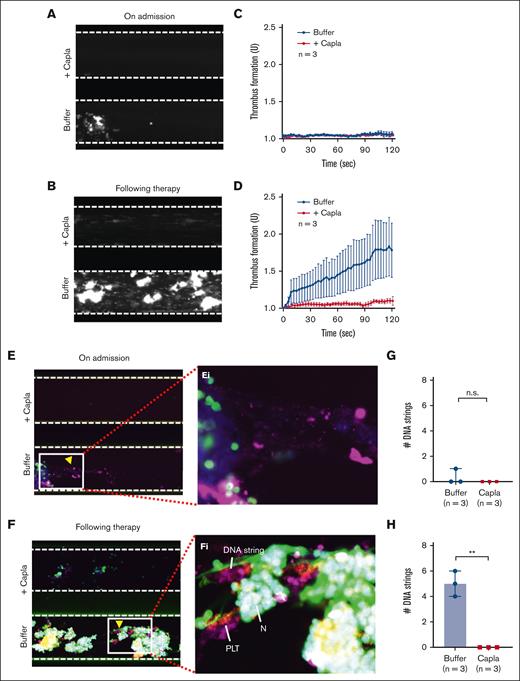
To further determine whether recombinant ADAMTS13 is efficacious in preventing NET accumulation on the collagen surface under flow, we perfused anticoagulated whole blood from patients with iTTP in the presence or absence of recombinant human ADAMTS13 (6 μg/mL) or caplacizumab (3 μg/mL) over the collagen-coated surface. Consistently, the rate of thrombus formation on the collagen surface was very low in blood sample of patients with iTTP collected on admission when platelet counts were very low, despite severe ADAMTS13 deficiency (Figure 5A,C; Figure 6A,C). Thrombus formation was dramatically increased in samples obtained from patients with iTTP after therapy when platelet counts were normalized or substantially increased but plasma ADAMTS13 activity remained quite low (Figure 5B,D; Figure 6 and 6D). Similarly, the NETs formed on the collagen surface were also extremely low in the patient admission blood samples (Figure 5E/Ei,G; Figure 6E/Ei,G) but dramatically increased in the samples during clinical remission when platelet counts were normalized or substantially increased (Figure 5F/Fi,H; Figure 6F/Fi,H). Interestingly, addition of either recombinant ADAMTS13 (Figure 5; supplemental Video 9) or caplacizumab (3 μg/mL) (Figure 6; supplemental Video 10) further reduced thrombus formation and NET accumulation on the collagen surface in all blood samples. Together, our results demonstrate that thrombus formation and NET accumulation under flow in whole blood from patients depends on platelet counts and VWF function. Similar to DNase I, recombinant ADAMTS13 and caplacizumab may prevent thrombus formation and NET accumulation under flow in iTTP (Figure 7).
Recombinant ADAMTS13 prevents NET formation under flow in iTTP. (A-B) Representative images illustrating the final coverage of neutrophils and platelets on a collagen-coated surface following perfusion of whole blood samples from iTTP on admission and after therapy, respectively, with or without recombinant ADAMTS13 (6 μg/mL) under flow. (C-D) The rate of thrombus formation (mean ± SEM) as a function of time after perfusion of whole blood samples of iTTP on admission and following treatment, respectively, with or without rADAMTS13 under flow. (E-F) Representative confocal fluorescent images show platelets (purple), neutrophils (green), and extracellular DNA strings (elongated green) following perfusion of whole blood samples from iTTP on admission and following therapy, respectively, with or without rADAMTS13, following fixation and restaining. (Ei-Fi) Enlarged areas (5×) from E and F, respectively, indicate extracellular DNA strings, platelets, and neutrophils, respectively. (G-H) The number of elongated DNA strings formed at the end of perfusion of whole blood samples from iTTP on admission and following therapy, respectively, with or without rADAMTS13 (n = 5). The data shown are median (bar) and IQR. Mann-Whitney test was used for the differences. n.s. and ∗∗ indicate P > .05 and P < .01, respectively. rA13, recombinant ADAMTS13; N, neutrophil; PLT, platelet; SEM, standard error of the mean.
Recombinant ADAMTS13 prevents NET formation under flow in iTTP. (A-B) Representative images illustrating the final coverage of neutrophils and platelets on a collagen-coated surface following perfusion of whole blood samples from iTTP on admission and after therapy, respectively, with or without recombinant ADAMTS13 (6 μg/mL) under flow. (C-D) The rate of thrombus formation (mean ± SEM) as a function of time after perfusion of whole blood samples of iTTP on admission and following treatment, respectively, with or without rADAMTS13 under flow. (E-F) Representative confocal fluorescent images show platelets (purple), neutrophils (green), and extracellular DNA strings (elongated green) following perfusion of whole blood samples from iTTP on admission and following therapy, respectively, with or without rADAMTS13, following fixation and restaining. (Ei-Fi) Enlarged areas (5×) from E and F, respectively, indicate extracellular DNA strings, platelets, and neutrophils, respectively. (G-H) The number of elongated DNA strings formed at the end of perfusion of whole blood samples from iTTP on admission and following therapy, respectively, with or without rADAMTS13 (n = 5). The data shown are median (bar) and IQR. Mann-Whitney test was used for the differences. n.s. and ∗∗ indicate P > .05 and P < .01, respectively. rA13, recombinant ADAMTS13; N, neutrophil; PLT, platelet; SEM, standard error of the mean.
Caplacizumab also prevents the formation of NETs under flow in iTTP. (A-B) Representative images illustrating the final coverage of neutrophils and platelets on a collagen-coated surface following perfusion of whole blood samples from iTTP on admission and after therapy, with or without caplacizumab (3 μg/mL). (C-D) The rate of thrombus formation (mean ± SEM) (n = 3) as a function of time in the blood samples of iTTP on admission and after treatment, respectively, under flow with or without caplacizumab. (E-F) Representative microscopic images show the staining of platelets (purple), neutrophils (green), and extracellular DNA strings (elongated green) following perfusion of whole blood samples from iTTP on admission and following therapy, respectively, with or without caplacizumab, after fixation and restaining with anti-CD41 and SytoxGreen. (Ei-Fi) Enlarged areas (5×) from E and F, respectively, indicate extracellular DNA strings, platelets, and neutrophils. (G-H) The number of elongated DNA strings (mean ± SEM) (n = 3) formed at the end of perfusion of whole blood samples from patients with iTTP on admission and after treatment with or without caplacizumab. The data shown are median (bar) and IQR. Mann-Whitney test was used for the differences between the 2 groups. Capla, caplacizumab; N, neutrophil; PLT, platelet; n.s. and ∗∗ indicate P > .05 and P < .01, respectively.
Caplacizumab also prevents the formation of NETs under flow in iTTP. (A-B) Representative images illustrating the final coverage of neutrophils and platelets on a collagen-coated surface following perfusion of whole blood samples from iTTP on admission and after therapy, with or without caplacizumab (3 μg/mL). (C-D) The rate of thrombus formation (mean ± SEM) (n = 3) as a function of time in the blood samples of iTTP on admission and after treatment, respectively, under flow with or without caplacizumab. (E-F) Representative microscopic images show the staining of platelets (purple), neutrophils (green), and extracellular DNA strings (elongated green) following perfusion of whole blood samples from iTTP on admission and following therapy, respectively, with or without caplacizumab, after fixation and restaining with anti-CD41 and SytoxGreen. (Ei-Fi) Enlarged areas (5×) from E and F, respectively, indicate extracellular DNA strings, platelets, and neutrophils. (G-H) The number of elongated DNA strings (mean ± SEM) (n = 3) formed at the end of perfusion of whole blood samples from patients with iTTP on admission and after treatment with or without caplacizumab. The data shown are median (bar) and IQR. Mann-Whitney test was used for the differences between the 2 groups. Capla, caplacizumab; N, neutrophil; PLT, platelet; n.s. and ∗∗ indicate P > .05 and P < .01, respectively.
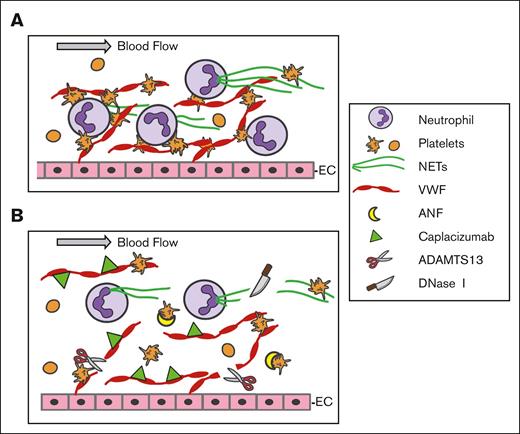
A proposed model of action for DNase I, recombinant ADAMTS13, and caplacizumab to inhibit NET and thrombus formation under flow. (A) Under flow, VWF is released from endothelial cells (EC) upon stimulation and remains anchored on the endothelial surface, which captures flowing platelets and neutrophils. Neutrophils undergo NETosis and release histone-DNA and histone-MPO complexes (NETs) that bind to VWF and activate platelets to enhance thrombus formation. (B) Whether extracellular DNA strings are degraded by DNase I, VWF strings are removed by rA13, or platelet-VWF interactions are inhibited by Capla or ANF, the result is to destabilize the thrombus structure, dampening inflammation, and thrombosis under flow. ANF, anfibatide; Capla, caplacizumab; EC, endothelial cell; N, neutrophil; PLT, platelet; rA13, recombinant ADAMTS13.
A proposed model of action for DNase I, recombinant ADAMTS13, and caplacizumab to inhibit NET and thrombus formation under flow. (A) Under flow, VWF is released from endothelial cells (EC) upon stimulation and remains anchored on the endothelial surface, which captures flowing platelets and neutrophils. Neutrophils undergo NETosis and release histone-DNA and histone-MPO complexes (NETs) that bind to VWF and activate platelets to enhance thrombus formation. (B) Whether extracellular DNA strings are degraded by DNase I, VWF strings are removed by rA13, or platelet-VWF interactions are inhibited by Capla or ANF, the result is to destabilize the thrombus structure, dampening inflammation, and thrombosis under flow. ANF, anfibatide; Capla, caplacizumab; EC, endothelial cell; N, neutrophil; PLT, platelet; rA13, recombinant ADAMTS13.
DNase I does not cleave VWF under flow
To determine whether DNase I cleaved VWF, we incubated purified plasma VWF with a high concentration of DNase I (200 U/mL) or recombinant ADAMTS13 (30 μg/mL) for 30 minutes under constant shear. Based on agarose gel electrophoresis and western blotting analysis of VWF multimer distribution, we found that DNase I at the concentration used in the microfluidic assays did not cleave VWF multimers directly, whereas recombinant ADAMTS13 did, as expected. The cleavage of VWF by ADAMTS13 was completely inhibited by the addition of EDTA (20 mM), suggesting that the cleavage of VWF is specific to the metalloprotease ADAMTS13 (supplemental Figure 1).
Discussion
To our knowledge, this is the first demonstration of dynamic changes in neutrophil NETosis, NETosis reserve, thrombus formation, and NET accumulation in acute iTTP. More importantly, we demonstrated that, similar to DNase I, recombinant ADAMTS13 or caplacizumab (or anfibatide) prevents NET accumulation in iTTP under flow through proteolytic cleavage of VWF or inhibition of VWF-platelet interaction, providing novel insight into the mechanism by which recombinant ADAMTS13 or anti-VWF nanobody may function as an antithrombotic and anti-inflammatory agent.
iTTP is primarily caused by autoantibodies against ADAMTS13, resulting in a severe deficiency in plasma ADAMTS13 activity.1-3 However, plasma ADAMTS13 deficiency alone may not be sufficient to cause an acute episode of TTP, suggesting that a second trigger may be necessary. Pregnancy, infection, and inflammation are the most common physiological and environmental factors that trigger acute iTTP.47-49 This is because pregnancy may activate the complement system and induce a hypercoagulable state, including increased expression of VWF and other coagulation factors but reduced activity of anticoagulants, such as ADAMTS13, protein C, protein S, tissue plasminogen inhibitors (PAI-1), and so on.50,51 During pregnancy, the balance between proinflammatory Th2 cytokines (eg, interferon-γ and tumor necrosis factor-α) and anti-inflammatory Th2 cytokines (eg, interleukin-10) is fundamental for successful gestation.52-54
Inflammation may trigger neutrophils to undergo NETosis, resulting in the release of NETs.55 NETosis is an innate immune response that helps localize infection and serves as a scaffold for the development of platelet-rich thrombi.56-58 The formation of NETs requires VWF and platelets, which together serve as a platform for neutrophil rolling interaction and adherence (Figure 7).59-61 NETs are rapidly degraded by DNase I, the activity of which is significantly decreased in patients with acute iTTP62 and other pathological conditions, such as COVID-1963,64 and lupus.65,66 Excessive NETs may result in vascular endothelial injury and subsequent organ damage.67,68
Elevated plasma levels of soluble NETs fragments (ie, histone-DNA or histone-MPO complexes) or cell-free DNA in patients with acute iTTP have been reported.33,34 Increased levels of NETs are associated with disease severity and mortality rate of iTTP.33 Several previous studies have used flow cytometry to assess NETosis in patients with sepsis, systemic lupus erythematous, deep vein thrombosis, and heparin-induced thrombocytopenia.29-32,69 Phorbol myristate acetate, bacteria, and viruses are known to stimulate NET formation,70-72 and changes following stimulation with phorbol myristate acetate are much less pronounced in patients with sepsis than in healthy controls, suggesting a reduced NETosis reserve in these patients.30 In our study, we showed that Stx-2, implicated in the development of HUS, does not further stimulate NETosis in the admission blood from acute iTTP, but does increase NETosis in the blood samples from patients after therapy. These results indicate that the number of neutrophils capable of undergoing NETosis (or NETosis reserve) in acute iTTP may be significantly reduced because massive NETosis and a NETosis reserve can be restored in iTTP following a standard of care therapy.
The therapeutic efficacy of DNase I, which eliminates NETs, has been previously tested in a murine model of acute respiratory distress syndrome73 and in patients with acute COVID-19.64 DNase I does not directly cleave VWF multimers under shear but digests the extracellular DNA strings (Figure 7), thus reducing inflammation and thrombosis.
To assess whether NET formation or accumulation under flow can be prevented by cleaving VWF or inhibiting VWF-platelet interaction, we performed microfluidic assays. Our results demonstrate that there are no thrombi or NET accumulation on the collagen surface under flow in iTTP on admission when the platelet count is extremely low (<10 × 109/L). Both thrombus formation and NET accumulation under flow increase dramatically following the reconstitution of platelet counts using donor platelets or after platelet counts rise following therapy. This supports that both thrombus formation and NET accumulation are dependent on platelets, which are required for neutrophils to adhere to the VWF-collagen surface via CD62.74 This hypothesis is further supported by demonstrating that the disruption of platelet-VWF interaction with recombinant ADAMTS13 cleaves VWF or caplacizumab, which binds the A1 domain of VWF75,76 or anfibatide that binds platelet GPIb,46,77 inhibits thrombus formation and NET accumulation under flow (Figure 7) in both patients with iTTP and Adamts13-/- murine whole blood perfused over a collagen-coated surface under flow.
There are some limitations in this study. Although flow cytometry is widely used for assessing neutrophil NETosis in vivo, the stability and structural accessibility of CitrH3 and MPO in activated neutrophils may create variability in quantitation. Therapeutic interventions, such as plasma exchange and caplacizumab may affect the levels of soluble NETs in the plasma. Finally, the number of acute iTTP cases studied for NETosis in vivo and NET accumulation under flow remains small. Nevertheless, these limitations did not affect our interpretation or conclusions of the study.
We conclude that neutrophil NETosis is significantly elevated and its NETosis reserve is significantly reduced in acute iTTP. Both returned to normal following a standard of care therapy. Little or no thrombus formation and NET accumulation under flow were observed in iTTP on admission when the platelet count was very low but they were significantly increased following therapy when platelet counts were substantially increased or normalized. Most importantly, similar to DNase I, recombinant ADAMTS13 or caplacizumab (or anfibatide) prevent the accumulation of NETs on the collagen surface under flow, likely via inhibition of VWF function or disruption of VWF-platelet-neutrophil interaction. Together, our findings support the potential contribution of NETosis and NET formation to the pathogenesis of iTTP and reveal an additional mechanism underlying the anti-inflammatory and antithrombotic effects of recombinant ADAMTS13 or caplacizumab (or anfibatide) as a targeted therapy for iTTP and perhaps other inflammatory and thrombotic disorders.
Acknowledgments
The study was supported in part by grants from the National Heart, Lung, and Blood Institute (NHLBI) (HL144552, HL157975-01A1, and HL164016-01A1) and Answering TTP Foundation in Canada (X.L.Z.). The authors express their gratitude to Lucy Zheng at Stowers Institute for Medical Research, Kansas City, KS, for her assistance in designing the graphic abstract and subtitle labeling using iMovie.
Authorship
Contribution: N.Y., Q.Z., and X.L.Z. designed the study, analyzed the results, and wrote the manuscript; N.Y., A.B., and S.H.G. performed experiments and data analysis; L.Z. helped with the microfluidic assay and flow cytometry experiments; D.S., K.H., and Z.Y. helped recruit patients with iTTP; and all authors approved the final version of the manuscript for submission.
Conflict-of-interest disclosure: X.L.Z. is a consultant for Alexion, Apollo, GC Biopharma, Sanofi, Stago, and Takeda, and a cofounder of Clotsolution. The remaining authors declare no competing financial interests.
Correspondence: X. Long Zheng, Department of Pathology and Laboratory Medicine, The University of Kansas Medical Center, 5016 Delp, Mail Stop 3045 3901 Rainbow Blvd, Kansas City, KS 66160; email: xzheng2@kumc.edu.
References
Author notes
All raw data will be shared upon request through the corresponding author, X. Long Zheng (xzheng2@kumc.edu).
The full-text version of this article contains a data supplement.


![DNase I, recombinant ADAMTS13, and anfibatide inhibit thrombus and prevent NET accumulation under flow in whole blood of Adamts13-/- mice. (A-C) The final surface coverage of platelets and leukocytes on a collagen surface following perfusion of Adamts13-/- murine blood samples stimulated with Stx-2 in the presence (top) or absence (bottom) of DNase I (200 IU/mL) or recombinant ADAMTS13 (+rA13) (12 μg/mL), or anfibatide (+ANF) (1.5 μg/mL), respectively, under 100 dyne/cm2. (D-F) The rate of thrombus formation over time (mean ± standard error of the mean [SEM]) following perfusion of Adams13-/- murine blood samples stimulated with Stx-2 in the presence (red) or absence (blue) of DNase I or rA13, ANF, under flow. (G-I) Representative confocal microscopic images demonstrate adhered platelets (purple), neutrophils (green), and extracellular DNA strings (elongated green) at the end of perfusion of whole blood samples from adamts13-/- mice in the presence (top) or absence (bottom) of DNase I, rA13, or ANF, following fixation and additional staining. (Gi-Ii) Enlarged (5×) areas from the bottom of panels G, H, and I, respectively, indicate the extracellular DNA strings, platelets, and neutrophils. (J-L) Number of elongated DNA strings formed at the end of perfusion of blood samples from Adamts13-/- mice in the presence or absence of DNase I, rA13, and ANF (n = 3), respectively. Data are shown as the median (bar) and IQR. Mann-Whitney test was used for the differences between the 2 groups. rA13, recombinant ADAMTS13; ANF, anfibatide; N, neutrophil; PLT, platelet. ∗∗ indicates P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/10/10.1182_bloodadvances.2023011617/2/m_blooda_adv-2023-011617-gr3.jpeg?Expires=1765917148&Signature=CQNAAiCXO9FB8NVH6kjn9v-N~Xy3~l2vQ3xf0t3-BHon9vr-6IV3Hjv4prtMDkbGxbTS3rlqioTwt7vh5sg6o8QTqPDibFng34RtRBYn6vlPwwq89AOVJ8O34xEqHkBKCjt3anT8Dv6kik4LYoH4xxm9i3SqwlhzWOx2Suz4mDlipVonc7jecB1tBYYo2OHkBV0yvlIK3icoIa9mWhaOdOZLArqc55atHoZCtAxFlnEP-Ioca3fI~MXoOHj3kCHgOJSpC9z6RaObdXtSECkRZGqAuUsorbr-1YarlqQHoytBjq6Wykv4wHdgkzw1Afr-KeZVQaMIcNzJQwPobU6VNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)