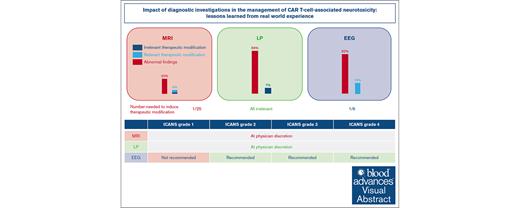
Data from a large cohort of CAR T-cell–treated patients question guidelines regarding diagnostic investigations in ICANS management.
Our results emphazise for the first time the role of EEG in the current guidelines but questions the need for systematic MRI and LP.
Visual Abstract
International guidelines regarding the management of immune effector cell–associated neurotoxicity syndrome (ICANS) recommend several diagnostic investigations, including magnetic resonance imaging (MRI), lumbar puncture (LP), and electroencephalogram (EEG) based on ICANS grade. However, the impact of these investigations has not yet been evaluated. Here, we aimed to describe the role of MRI, LP, and EEG in the management of ICANS in a cohort of real-life patients treated with chimeric antigen receptor (CAR) T cells at the University Hospital of Rennes, France. Between August 2018 and January 2023, a total of 190 consecutive patients were treated with CAR T cells. Among those, 91 (48%) developed ICANS. MRI was performed in 71 patients (78%) with ICANS, with a therapeutic impact in 4% of patients, despite frequent abnormal findings. LP was performed in 43 patients (47%), which led to preemptive antimicrobial agents in 7% of patients, although no infection was eventually detected. Systematic EEG was performed in 51 patients (56%), which led to therapeutic modifications in 16% of patients. Our study shows that EEG is the diagnostic investigation with the greatest therapeutic impact, whereas MRI and LP appear to have a limited therapeutic impact. Our results emphasize the role of EEG in the current guidelines but question the need for systematic MRI and LP, which might be left to the discretion of the treating physician.
Introduction
Chimeric antigen receptor (CAR) T cells have emerged during the last decade as a therapeutic breakthrough for various hematological malignancies. The 2 most common acute toxicities of CAR T cells include cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS). ICANS is commonly determined using the Amercian Society for Transplantation and Cellular Therapy (ASTCT) grading system by the most severe event (Immune effector Cell Encephalopathy (ICE) score, level of consciousness, convulsive or nonconvulsive seizure, motor findings, raised intracranial pressure, or cerebral edema).1 Some of these events could be defined by diagnostic investigations. These toxicities are common and may sometimes require management in an intensive care unit (ICU).2-7
National and international guidelines have emerged to guide physicians in the management of CRS and ICANS, whose treatment relies mostly on anticytokine therapy and steroids.6,8-10 These guidelines recommend 3 major diagnostic investigations that may be performed during ICANS: magnetic resonance imaging (MRI), lumbar puncture (LP), and electroencephalogram (EEG; supplemental Table 1). All these guidelines recommend performing MRI for ICANS grade 3+ but show discrepancies regarding ICANS grade 2. Some of them but not all recommend performing LP and even fewer encourage the use of EEG. Depending on ICANS grade, guidelines recommend initiating steroids (mainly for ICANS grade 2+) and/or tocilizumab (mainly for CRS grade 2+) and discussing additional therapies as siltuximab and/or anakinra for example.
These diagnostic investigations are performed either to detect specific ICANS-associated abnormalities and/or to rule out differential diagnoses. ICANS-related MRI abnormalities include T2 fluid-attenuated inversion recovery (T2-FLAIR) hypersignals, diffusion restriction, diffuse pachymeningitis, or cerebral edema.2-5 LP abnormalities during ICANS include hyperproteinorachy, reflecting the intensity of neuro-inflammation and hypercellularity, sometimes with CAR T cells found in the cerebral spinal fluid, whose exact roles in the occurrence and severity of ICANS are not fully understood.11 Moreover, LP is often used to rule out infectious causes of meningo-encephalitis, for which early diagnosis and treatment are associated with a better prognosis in septic meningitis.12 Finally, ICANS-related EEG abnormalities include diffuse or focal slowing, frontal intermittent rythmic delta activity, and even seizure activity, which could lead to convulsive or nonconvulsive status epilepticus.11,13,14 These findings may be used to manage the prophylaxis or curative anticonvulsive treatment.
However, none of the guidelines suggest treatment modification based on investigations’ results other than ICANS grade itself. Of note, EEG and MRI results may themselves modify the ICANS grade, for example, if they reveal epilepsy or cerebral edema. However, these patients would already have suggestive symptoms with low ICE score.
Yet, these investigations are invasive, time consuming, expensive in term of costs and human resources, and their clinical benefit remains unknown. Hence, guidelines suggesting the use of MRI, LP, and EEG rely on empirical practices and are only based on expert opinions with low scientific evidence.
The aim of this study is to evaluate the therapeutic impact of diagnostic investigations in a large cohort of CAR T-cell–treated patients experiencing neurotoxicity.
Material and methods
Patients and study design
Study period
All patients treated with CAR T cells at the tertiary University Hospital Centre of Rennes from 2018 to 2023 were included. Data regarding patient and disease characteristics, treatment courses, and clinical outcomes including toxicities were prospectively collected in our local registry.
Regarding CAR T-cell–associated toxicities, every patient benefited from specific pretreatment clinical and diagnostic investigations, including an appointment with a trained neurologist. All patients had an MRI at baseline (ie, before CAR T-cell infusion), which allowed for comparison during ICANS to assess imputability of newly occurring brain abnormalities.
All studied patients were included in the French national DESCAR-T registry as requested by French health authorities for reimbursement. Thus, they received written information by local investigator on data collection.
ICANS management
ICANS management was left to the discretion to the treating physician, but adherence to international guidelines was strongly encouraged.6,8-10 We retrospectively analyzed MRI, LP, and EEG that had been performed during ICANS.
MRI at baseline and during ICANS were performed by a neuroradiologist in routine clinical practice. Results were categorized retrospectively as follows: normal (including no modification since baseline), ischemic stroke, cerebral edema, aspecific hypersignal, pachymeningitis, tumor flare, or disease progression.
Regarding LP, we depicted cellularity (defined as number of cells/mm3) and proteinorachy (defined as total amount of proteins in g/L) of cerebral spinal fluid. All samples were sent to a microbiological laboratory for direct microbiological examination and cultures. The use of multiplex or specific polymerase chain reaction to detect bacteria, parasites, fungi, or virus was left to the discretion of the treating physician.
For EEG, we focused on EEG requested due to ICE score worsening. Indeed, few EEG were performed due to abnormal movements. These EEGs were not included in this analysis, because they were not only justified by an isolated worsening of the ICANS after CAR T-cell infusion but also by the standard assessment of seizures. Hence, this approach led us to investigate the potential relevance of systematic EEG based on ICANS grade only.
Therapeutic modifications
We evaluated whether these diagnostic investigations led to a therapeutic modification or provided information on patient’s prognosis (eg, disease progression) affecting patient management (eg, switch to another line of treatment and change of status regarding ICU eligibility).
For MRI, we analyzed retrospectively whether MRI pathological results led to initiation, increase and/or discontinuation of central nervous system (CNS)-directed therapies (antiplatelet therapy [APT], anticoagulant drug [ACD], or steroids). In patients with central nervous system involvement, we determined whether MRI results led to change patient’s management (eg, ICU admission policy and switch to a new-line of treatment) in case of lymphoma progression.
For LP, we looked retrospectively whether LP abnormal results had a therapeutic impact and led to introduction of anti-infectious agents (antibiotics, antivirals, or antifungal therapy). We classified these treatment changes as “relevant” if there was a confirmed infection and “irrelevant” if not. We looked for potential side effects associated with anti-infectious agents.
For EEG, we sought retrospectively whether EEG pathological results led to initiation, increase, decrease, and/or discontinuation of antiepileptics. To focus on treatment modifications induced by EEG results, we chose to include only antiepileptics modification done after EEG results. We did not include antiepileptics modification done before EEG.
End points
The primary end point was to describe the therapeutic modifications induced by each diagnostic investigation. The secondary end points were to assess the specific abnormalities and differential diagnoses identified on MRI, LP, and EEG. We also performed subgroups for each ICANS grade.
Results
Patients’ characteristics
We analyzed 190 consecutive patients treated at the University Hospital of Rennes between August 2018 and January 2023. Patients’ baseline characteristics are presented in Table 1. Overall, 62% of patients were male, and the median age was 64 years (range, 15-81). Patients were mainly treated for a refractory/relapsed diffuse large B-cell lymphoma (73%) and received axicabtagene-ciloleucel (60%) after ≥2 prior therapies (90%). Overall, 165 patients (87%) developed a CRS, including 4% with grade ≥3. A total of 91 patients (48%) developed ICANS, including 25 with grade 1 (13%), 32 grade 2 (17%), 21 grade 3 (11%), and 12 grade 4 (6%). Overall, 46 patients (24%) required transfer in ICU either for CRS, ICANS, or both.
Patient characteristics
| Characteristics . | All patients (n = 190) . |
|---|---|
| Age, median (range), y | 64 (15-81) |
| Men, n (%) | 118 (62%) |
| Hemopathy and hemopathy histological subtype, n (%) | |
| DLBCL | 138 (73%) |
| DLBCL NOS | 129 (68%) |
| PMBL | 4 (2%) |
| PCNSL | 5 (3%) |
| ALL | 17 (9%) |
| MCL | 13 (7%) |
| FL | 12 (6%) |
| MM | 10 (5%) |
| Type of CAR T cells, n (%) | |
| Axicabtagene ciloleucel | 115 (61%) |
| Tisagenlecleucel | 38 (20%) |
| Brexucabtagene autoleucel | 27 (14%) |
| Idecabtagene vicleucel | 10 (5%) |
| Number of prior lines | |
| Median (range) | 2 (1-7) |
| One prior line, n (%) | 19 (10%) |
| ≥2 prior lines, n (%) | 181 (90%) |
| Toxicities, n (%) | |
| CRS | 165/190 (87%) |
| CRS grade 3+ | 8/190 (4%) |
| ICANS | 91/190 (48%) |
| ICANS grade 3+ | 33/190 (17%) |
| ICU transfer | 46/190 (24%) |
| Characteristics . | All patients (n = 190) . |
|---|---|
| Age, median (range), y | 64 (15-81) |
| Men, n (%) | 118 (62%) |
| Hemopathy and hemopathy histological subtype, n (%) | |
| DLBCL | 138 (73%) |
| DLBCL NOS | 129 (68%) |
| PMBL | 4 (2%) |
| PCNSL | 5 (3%) |
| ALL | 17 (9%) |
| MCL | 13 (7%) |
| FL | 12 (6%) |
| MM | 10 (5%) |
| Type of CAR T cells, n (%) | |
| Axicabtagene ciloleucel | 115 (61%) |
| Tisagenlecleucel | 38 (20%) |
| Brexucabtagene autoleucel | 27 (14%) |
| Idecabtagene vicleucel | 10 (5%) |
| Number of prior lines | |
| Median (range) | 2 (1-7) |
| One prior line, n (%) | 19 (10%) |
| ≥2 prior lines, n (%) | 181 (90%) |
| Toxicities, n (%) | |
| CRS | 165/190 (87%) |
| CRS grade 3+ | 8/190 (4%) |
| ICANS | 91/190 (48%) |
| ICANS grade 3+ | 33/190 (17%) |
| ICU transfer | 46/190 (24%) |
ALL, Acute lymphoblastic leukemia; DLBCL, diffuse large B-cell lymphoma; FL, Follicular lymphoma; MCL, Mantel cell lymphoma; MM, Multiple myeloma; NOS, not otherwise specified; PMBL, Primary mediastinal B-cell lymphoma
Diagnostic investigation strategies
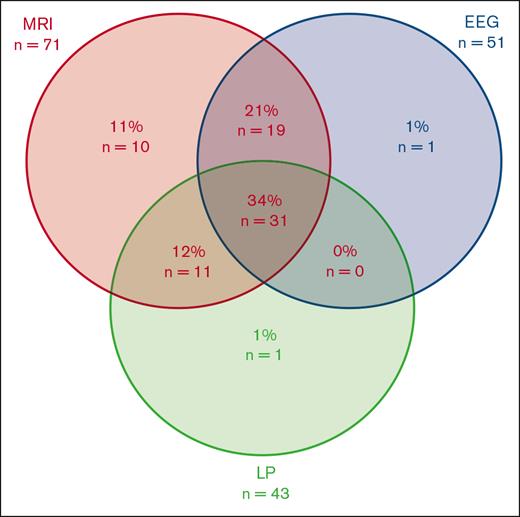
Most patients with ICANS (80%) underwent at least 1 investigation, and a third (34%) underwent MRI, LP, and EEG evaluation (Figure 1; supplemental Table 2). The most frequent diagnostic investigation was MRI (78%), followed by EEG (56%) and LP (47%).
Diagnostic investigation strategies (n = 91). EEG, Electroencephalogram; LP, Lumbar puncture; MRI, Magnetic resonance imaging.
Diagnostic investigation strategies (n = 91). EEG, Electroencephalogram; LP, Lumbar puncture; MRI, Magnetic resonance imaging.
The diagnostic investigation strategy varied over time (supplemental Table 3; supplemental Table 4). Indeed, at least 1 investigation was performed in 5 of 9 patients (56%) with ICANS grade 1 from 2018 to 2020, compared with 47% from 2021 to 2023, which may reflect the caution of physicians at the beginning of CAR T-cell therapy. When physicians chose to perform investigations in ICANS grade 1, MRI and LP were the first choice (33%) from 2018 to 2020, compared with MRI and EEG (29%) from 2021 to 2023.
Furthermore, the rate of patients who received MRI and EEG but not LP was 44% in ICANS grade 3 and 30% in ICANS grade 4 from 2021 to 2023, compared with both 0% from 2018 to 2020. This point may reflect that physicians in charge chose not perform LP in severe ICANS over time due to low benefit/risk ratio.
Therapeutic impact of MRI
A total of 71 MRI was performed in the 91 patients (78%) who developed an ICANS (Table 2; Figure 1), corresponding to 42% of patients with a grade 1, 88% of grade 2, and 100% of grades 3 and 4.
Diagnostic investigation results according to ICANS grade
| . | ICANS all grades (n = 91) . | Grade 1 (n = 26) . | Grade 2 (n = 32) . | Grade 3 (n = 21) . | Grade 4 (n = 12) . |
|---|---|---|---|---|---|
| MRI, n (%) | 71/91 (78%) | 11/26 (42%) | 27/32 (88%) | 21/21 (100%) | 12/12 (100%) |
| Normal | 57/71 (80%) | 10/11 (91%) | 25/27 (92%) | 17/21 (81%) | 5/12 (42%) |
| Edema | 5/71 (7%) | 1/11 (9%) | 1/27 (4%) | 0/21 (0%) | 3/12 (25%) |
| Stroke | 4/71 (6%) | 0/11 (0%) | 0/27 (0%) | 2/21 (10%) | 2/12 (17%) |
| Pachymeningitis∗ | 3/71 (4%) | 0/11 (0%) | 0/27 (0%) | 1/21 (5%) | 2/12 (17%) |
| Tumor flare† | 1/71 (1%) | 0/11 (0%) | 0/27 (0%) | 1/21 (5%) | 0/12 (0%) |
| Hemopathy progression‡ | 1/71 (1%) | 0/11 (0%) | 1/27 (4%) | 0/21 (0%) | 0/12 (0%) |
| Therapeutic impact§ | 3/71 (4%) | 1/26 (4%) | 0/32 (0%) | 2/21 (10%) | 0/12 (0%) |
| APT or ACD – no. (%) | 3/71 (4%) | 1/26 (4%) | 0/32 (0%) | 2/21 (10%) | 0/12 (0%) |
| LP, n (%) | 43 /91 (47%) | 5/26 (24%) | 16/32 (50%) | 12/21 (57%) | 10/12 (83%) |
| Normal LP‖, n (%) | 6/43 (14%) | 3/5 (60%) | 2/16 (13%) | 0/12 (0%) | 1/10 (10%) |
| Cells/mm3, median (IQR) | 3 (1-9) | 2 (1-24) | 5 (1-9) | 4.5 (1-9.25) | 2.5 (1-7.25) |
| Proteinorachy, median (IQR), g/L | 0.6 (0.44-1.36) | 0.35 (0.3-0.39) | 0.58 (0.45-0.88) | 0.75 (0.55-1.18) | 1.05 (0.6-1.80) |
| Microbial agent, n (%) | 0/43 (0%) | 0/5 (0%) | 0/16 (0%) | 0/12 (0%) | 0/10 (0%) |
| Therapeutic impact¶, n (%) | 3/43 (7%) | 1/5 (20%) | 1/16 (6%) | 0/12 (0%) | 1/10 (10%) |
| Preemptive antimicrobials exposure for unconfirmed infection, n (%) | 3/43 (7%) | 1/5 (20%) | 1/16 (6%) | 0/12 (0%) | 1/10 (10%) |
| Antiviral exposure, n (%) | 2/43 (5%) | 1/5 (20%) | 1/16 (6%) | 0/12 (0%) | 0/10 (0%) |
| Antifungal exposure, n (%) | 1/43 (2%) | 0/5 (0%) | 0/16 (0%) | 0/12 (0%) | 1/10 (10%) |
| EEG, n (%) | 51/ 91 (56%) | 4/26 (15%) | 18/32 (56%) | 17/21 (81%) | 12/12 (100%) |
| Normal | 9/51 (18%) | 2/4 (50%) | 5/18 (28%) | 1/17 (6%) | 1/12 (8%) |
| Abnormal EEG | 42/51 (82%) | 2/4 (50%) | 13/18 (72%) | 16/17 (94%) | 11/12 (92%) |
| Encephalopathy | 23/51 (45%) | 2/4 (50%) | 7/18 (39%) | 11/17 (65%) | 3/12 (25%) |
| Seizure or status epilepticus | 6/51 (12%) | 0/4 (0%) | 1/18 (6%) | 1/17 (6%) | 4/12 (33%) |
| Uncertain between encephalopathy or seizure | 3/51 (6%) | 0/4 (0%) | 0/18 (0%) | 2/17 (12%) | 1/12 (8%) |
| Background slowing | 8/51 (16%) | 0/4 (0%) | 4/18 (22%) | 2/17 (12%) | 2/12 (17%) |
| FIRDAS | 1/51 (2%) | 0/4 (0%) | 1/18 (6%) | 0/17 (0%) | 0/12 (0%) |
| Therapeutic impact# | 8/51 (16%) | 0/4 (0%) | 1/18 (6%) | 3/17 (18%) | 4/12 (33%) |
| AE changes because of EEG | 8/51 (16%) | 0/4 (0%) | 1/18 (6%) | 3/17 (18%) | 4/12 (33%) |
| Upgrading AE | 7/51 (14%) | 0/4 (0%) | 1/18 (6%) | 2/17 (12%) | 4/12 (33%) |
| Decreasing AE | 1/51 (2%) | 0/4 (0%) | 0/18 (0%) | 1/17 (6%) | 0/12 (0%) |
| . | ICANS all grades (n = 91) . | Grade 1 (n = 26) . | Grade 2 (n = 32) . | Grade 3 (n = 21) . | Grade 4 (n = 12) . |
|---|---|---|---|---|---|
| MRI, n (%) | 71/91 (78%) | 11/26 (42%) | 27/32 (88%) | 21/21 (100%) | 12/12 (100%) |
| Normal | 57/71 (80%) | 10/11 (91%) | 25/27 (92%) | 17/21 (81%) | 5/12 (42%) |
| Edema | 5/71 (7%) | 1/11 (9%) | 1/27 (4%) | 0/21 (0%) | 3/12 (25%) |
| Stroke | 4/71 (6%) | 0/11 (0%) | 0/27 (0%) | 2/21 (10%) | 2/12 (17%) |
| Pachymeningitis∗ | 3/71 (4%) | 0/11 (0%) | 0/27 (0%) | 1/21 (5%) | 2/12 (17%) |
| Tumor flare† | 1/71 (1%) | 0/11 (0%) | 0/27 (0%) | 1/21 (5%) | 0/12 (0%) |
| Hemopathy progression‡ | 1/71 (1%) | 0/11 (0%) | 1/27 (4%) | 0/21 (0%) | 0/12 (0%) |
| Therapeutic impact§ | 3/71 (4%) | 1/26 (4%) | 0/32 (0%) | 2/21 (10%) | 0/12 (0%) |
| APT or ACD – no. (%) | 3/71 (4%) | 1/26 (4%) | 0/32 (0%) | 2/21 (10%) | 0/12 (0%) |
| LP, n (%) | 43 /91 (47%) | 5/26 (24%) | 16/32 (50%) | 12/21 (57%) | 10/12 (83%) |
| Normal LP‖, n (%) | 6/43 (14%) | 3/5 (60%) | 2/16 (13%) | 0/12 (0%) | 1/10 (10%) |
| Cells/mm3, median (IQR) | 3 (1-9) | 2 (1-24) | 5 (1-9) | 4.5 (1-9.25) | 2.5 (1-7.25) |
| Proteinorachy, median (IQR), g/L | 0.6 (0.44-1.36) | 0.35 (0.3-0.39) | 0.58 (0.45-0.88) | 0.75 (0.55-1.18) | 1.05 (0.6-1.80) |
| Microbial agent, n (%) | 0/43 (0%) | 0/5 (0%) | 0/16 (0%) | 0/12 (0%) | 0/10 (0%) |
| Therapeutic impact¶, n (%) | 3/43 (7%) | 1/5 (20%) | 1/16 (6%) | 0/12 (0%) | 1/10 (10%) |
| Preemptive antimicrobials exposure for unconfirmed infection, n (%) | 3/43 (7%) | 1/5 (20%) | 1/16 (6%) | 0/12 (0%) | 1/10 (10%) |
| Antiviral exposure, n (%) | 2/43 (5%) | 1/5 (20%) | 1/16 (6%) | 0/12 (0%) | 0/10 (0%) |
| Antifungal exposure, n (%) | 1/43 (2%) | 0/5 (0%) | 0/16 (0%) | 0/12 (0%) | 1/10 (10%) |
| EEG, n (%) | 51/ 91 (56%) | 4/26 (15%) | 18/32 (56%) | 17/21 (81%) | 12/12 (100%) |
| Normal | 9/51 (18%) | 2/4 (50%) | 5/18 (28%) | 1/17 (6%) | 1/12 (8%) |
| Abnormal EEG | 42/51 (82%) | 2/4 (50%) | 13/18 (72%) | 16/17 (94%) | 11/12 (92%) |
| Encephalopathy | 23/51 (45%) | 2/4 (50%) | 7/18 (39%) | 11/17 (65%) | 3/12 (25%) |
| Seizure or status epilepticus | 6/51 (12%) | 0/4 (0%) | 1/18 (6%) | 1/17 (6%) | 4/12 (33%) |
| Uncertain between encephalopathy or seizure | 3/51 (6%) | 0/4 (0%) | 0/18 (0%) | 2/17 (12%) | 1/12 (8%) |
| Background slowing | 8/51 (16%) | 0/4 (0%) | 4/18 (22%) | 2/17 (12%) | 2/12 (17%) |
| FIRDAS | 1/51 (2%) | 0/4 (0%) | 1/18 (6%) | 0/17 (0%) | 0/12 (0%) |
| Therapeutic impact# | 8/51 (16%) | 0/4 (0%) | 1/18 (6%) | 3/17 (18%) | 4/12 (33%) |
| AE changes because of EEG | 8/51 (16%) | 0/4 (0%) | 1/18 (6%) | 3/17 (18%) | 4/12 (33%) |
| Upgrading AE | 7/51 (14%) | 0/4 (0%) | 1/18 (6%) | 2/17 (12%) | 4/12 (33%) |
| Decreasing AE | 1/51 (2%) | 0/4 (0%) | 0/18 (0%) | 1/17 (6%) | 0/12 (0%) |
AE, antiepileptics; FIRDAS, Frontal Intermittent Rythmic Delta Activity Legend.
Most important findings in table are in bold.
Defined as fibrosing and inflammatory process causing thickening of dura matter, seen on MRI.
Increased PCNSL lesion but later decreasing on MRI, suggesting a pseudoprogression.
Increased PCNSL lesion confirmed on later MRI during follow-up, suggestive of hemopathy progression.
Treatment introduction or modification regarding to MRI results.
Normal lumbar punction is defined as cells below 5 per mm3 and proteinorachy below 0.40 g/L.
Treatment introduction or modification regarding to LP results.
Therapeutic modification done after EEG asked without clinical signs of epilepsy. All EEG asked after abnormal movements or other signs of epilepsy were excluded.
Revised ICANS management guideline
| . | ICANS grade 1 . | ICANS grade 2 . | ICANS grade 3 . | ICANS grade 4 . |
|---|---|---|---|---|
| MRI | At physician discretion | |||
| LP | At physician discretion | |||
| EEG | Not recommended | Recommended | Recommended | Recommended |
| . | ICANS grade 1 . | ICANS grade 2 . | ICANS grade 3 . | ICANS grade 4 . |
|---|---|---|---|---|
| MRI | At physician discretion | |||
| LP | At physician discretion | |||
| EEG | Not recommended | Recommended | Recommended | Recommended |
The most common result was a normal MRI, corresponding to 80 % of MRI. The proportion of normal MRI ranged from 91% to 81% in ICANS grades 1 to 3, and only 42% of MRI were normal in ICANS grade 4.
One of the most frequent abnormal results was aspecific hypersignal, which occurred in 4 patients (6%) with ICANS, including 1 patient with grade 2 (1/27 [4%]) and 3 patients with grade 4 (3/12 [25%]).
The second most frequent abnormal result was stroke, which occurred in 4 patients, all with ICANS grade 3 to 4. All strokes were minimal ischemic stroke, from which description and relevance were previously reported.15 Moreover, there were 2 tumor flares and 1 disease progression in patients treated for primary central nervous system lymphoma (PCNSL), who had increased PCNSL lesions compared with baseline, confirmed on later MRI during follow-up.
Notably, there was no diffuse edema depicted on MRI, even in the most severe ICANS grade 4. Five MRIs (7%) depicted minimal lesions described as edema, 1 in ICANS grade 1 (9%), another in ICANS grade 2 (4%), and 3 (25%) in ICANS grade 4.
Overall, 3 MRIs (4% of all MRI) generated therapeutic modification (Figure 2). Two stroke descriptions on MRI in patients with ICANS grade 3 led to initiation or increase in APT therapy. Notably, the 2 other strokes in patients with ICANS grade 4 did not lead to treatment modification because these 2 patients were already treated for atrial fibrillation with ACD. One patient with ICANS grade 1 received APT because MRI described a stroke event, which was a T2 FLAIR aspecific hypersignal, already described on baseline MRI. The MRI results were reclassified normal a posteriori. Nevertheless, the physician decided to initiate APT without knowing this discrepancy.
Therapeutic modifications due to investigations. EEG, Electroencephalogram; LP, Lumbar puncture; MRI, Magnetic resonance imaging.
Therapeutic modifications due to investigations. EEG, Electroencephalogram; LP, Lumbar puncture; MRI, Magnetic resonance imaging.
Thus, the number-needed-to-induce 1 therapeutic modification for MRI was 50 for ICANS grades 1 to 2 and 11 for ICANS grades 3 to 4.
There was no change of anticancer treatment based on MRI results in the patient with disease progression.
There was no bleeding in patients who were newly prescribed APT during their stay (results not shown). No MRI findings changed a grade of ICANS.
Therapeutic impact of LP
A total of 43 LPs were performed among the 91 patients (47%) who developed an ICANS (Table 2; Figure 1). LP was performed in 24% of patients with grade 1, compared with 50%, 57%, and 83% of patients with ICANS grade 2, 3, and 4, respectively. The median proteinorachy and the number of cells were 0.62 g/L and 3 per mm3, respectively. No LP found septic meningitis. All systematic bacteriological examinations and specific or multiplex polymerase chain reaction were negative.
There were 3 preemptive therapeutic modifications for unconfirmed infection (Figure 2). Two LPs led to preemptive antivirals introduction (aciclovir) in patients with ICANS grades 1 and 2 because of lymphocytic meningitis. One LP led to preemptive antifungal introduction (voriconazole) in a patient with ICANS grade 3 for a suspected Aspergillus species meningitis, which was not confirmed by infectious disease physician’s expertise. All antiviral and antifungal treatments were stopped when infections were excluded. No antivirals induced significant adverse events. Exposure to antifungal treatment (voriconazole) was followed by hepatic cytolysis a few days after initiation.
Therapeutic impact of EEG
Overall, 51 EEGs were performed in the 91 patients (56%) who developed an ICANS (Table 2; Figure 1). In ICANS grade 1, up to 16% of patients underwent an EEG, compared with 56%, 81%, and 100% of patients with ICANS grades 2, 3, and 4, respectively.
Only 18% of EEGs were normal in the cohort, ranging from 50% in ICANS grade 1 to 6% in ICANS grade 4.
The most common finding was encephalopathy in 45% of patients. Notably, 6 EEGs (12%) reported seizure or status epilepticus. There were 2 patients with ICANS grades 2 and 3 (6% of EEG), respectively, and 4 patients (33%) with ICANS grade 4 who developed seizure or status epilepticus on their EEGs, despite the absence of clinical symptoms of epilepsy. Moreover, in the entire cohort, 3 EEGs were described as “uncertain” between encephalopathy or seizure.
Finally, 8 EEGs (16%) led to therapeutic modification in the entire cohort (Figure 2), of which 7 were in the severe and life-threatening ICANS (grade 3+) group (24%). All EEGs that found seizure or status epilepticus led to an increase in antiepileptics prophylaxis by levetiracetam or introduction of a new antiepileptics (mainly phenytoin). In patients with ICANS grade 3, 1 EEG reported as “uncertain” between seizure or encephalopathy led to an increase in antiepileptics prophylaxis, whereas another EEG that found a toxic encephalopathy attributed to levetiracetam prophylaxis led to discontinuation of antiepileptics prophylaxis.
Thus, the number-needed-to-induce 1 therapeutic modification for EEG was 20 for ICANS grades 1 to 2 and 4 for ICANS grades 3 to 4.
One seizure finding in ICANS grade 2 should have led to reconsider ICANS grading to ICANS grade 3, which would have led to increased steroids as recommended by the guidelines. However, given a reassuming clinical assessment, steroid dose was not upgraded, and only the dose of antiepileptics was upgraded.
Discussion
Current guidelines on ICANS management are mainly based on experts’ opinion rather than evidence-based medicine, and their clinical impact in daily practice has not been specifically evaluated.5,7-9 These guidelines recommend the use of diagnostic investigations to rule out differential diagnoses and identify specific CAR T-cell–associated manifestations.
Our findings highlight some divergences between guidelines and daily practice regarding diagnostic investigations. Physicians chose to explore 42%, 24%, and 15% of grade 1 ICANS with MRI, LP, and EEG, respectively, which is supported by any guidelines. These discrepancies between international guidelines and daily practice may reflect the clinical polymorphism of such patients at bedside that may prompt physicians to eliminate differential diagnoses even in patients with mild neurologic toxicity. Moreover, a significant proportion of patients with ICANS grade 3+ did not undergo LP or EEG (12% and 33%, respectively), which is not in line with the guidelines. This discrepancy could be interpreted in several ways. Physicians may have chosen not to perform LP because of an unfavorable risk-benefit ratio regarding an invasive and potentially harmful investigations, with few clinical arguments for a septic meningitis.16 Furthermore, the relatively low number of EEG rates raises questions about its accessibility in daily routine, even in severe and life-threatening ICANS treated in ICU.
A high rate of LPs and EEGs were abnormal in our cohort (86% and 82%, respectively), most of them in grade 2+ ICANS (82% and 85%, respectively) rather than in grade 1 ICANS (40% and 50%, respectively). Thus, physician should be aware that there is a high probability of abnormal LP and EEG when these investigations are performed in patients with ICANS, which increases with ICANS grade. Contrary to LP and EEG, MRI results are often normal (80%), except in patients with grade 4, in whom only 42% of MRIs are depicted as normal.
Our study shows that diagnostic investigations induced therapeutic modifications in 4% and 16% of patients after MRIs and EEGs, respectively, and exposed 7% of patients to preemptive antimicrobial agent exposure for unconfirmed infections after LPs. No investigation showed a differential diagnosis or a treatable specific lesion (except for a few strokes of fortuitous discovery and not responsible for symptoms). Regarding MRI, the most frequent abnormal result was aspecific flair hypersignal, which occurred mainly in ICANS grade 4 (25% of ICANS grade 4) and whose clinical significance is unknown. Another frequent finding was stroke, which occurred in 10% and 17% of ICANS grades 3 and 4, respectively. Moreover, tumor flare occurring in PCNSL was 2 times more frequent than disease progression (as confirmed by repeated MRIs). Regarding LP, several studies reported hypercellularity and hyperproteinorachy during ICANS, but no data suggested a link with ICANS severity or patient’s prognosis.11 Moreover, current guidelines recommend performing LPs in severe ICANS to formally rule out infectious causes of meningitis in patients who are immunocompromised with neurological symptoms. However, LP is not a risk-free procedure in patients with thrombocytopenia, often requires platelet transfusion in this context, and could lead to bleeding and neurological adverse events.16 In our cohort, no infection was found in LP, even in the most severe ICANS subgroup. For EEG, the main abnormal finding was aspecific encephalopathy, without knowing whether encephalopathy was due to ICANS itself or another differential diagnose. EEG seizures and status epilepticus without clinical signs of epilepsy were common in our cohort because it was reported in 12% in ICANS, ranging from 0% in ICANS grade 1 to 33% in ICANS grade 4. Considering that EEG is part of the classical management of epilepsy, regardless of ICANS grade, we excluded 3 EEGs that were requested after abnormal movements in our analysis. Thus, it allowed for us to finely examine the added value of systematic EEG during ICANS. We chose to exclude 3 additional situations in which antiepileptics treatment was increased before performing EEG, because these treatment modifications were not justified by EEG results.
Importantly, the therapeutic impact varied between diagnostic investigations. On 1 hand, systematic EEG based on ICANS grade only was often followed by therapeutic modifications (16% of cases). Therapeutic impact of EEG was more important in severe ICANS; 18% and 33% of EEG performed for grades 3 and 4 ICANS, respectively, resulted in a change of antiepileptics treatment. Notably, these systematic EEGs have unmasked seizure without clinical signs or have diagnosed encephalopathy due to antiepileptics prophylaxis and thus allowed physicians in charge either to increase or decrease antiepileptics prophylaxis as required. Thus, EEG provided relevant information and enabled adapted therapeutic modifications of antiepileptics treatment, which supports a broad use of EEG. On the other hand, systematic LP was never associated with relevant therapeutic modification, even in case of severe ICANS. Moreover, this broad LP policy resulted in initiation of antimicrobial agents for unconfirmed infections in 3 patients (7% of all LPs) and induced unnecessary liver toxicity in 1 of them. Nevertheless, because of the limited number of LPs in our cohort, our results should be interpreted with caution, and we cannot draw definitive conclusions regarding the real need for LP in these patients. However, because of its low therapeutic impact and its potential complications, systematic LP may not be recommended for all patients but rather be discussed on a case-by-case basis, according to clinical assessment of the treating physician. The need for systematic MRI assessment based on ICANS grade only (without clinical symptoms of seizure) is also questionable. Overall, only 4% of MRIs lead to a therapeutic modification. Strikingly, there was no diffuse edema in our cohort, even in ICANS grade 4, which is 1 of the main concerns of treating physicians managing severe ICANS. Of note, all international guidelines recommend the use of steroids based on ICANS grade, regardless of MRI results. Surprisingly, the only steroid upgrading in our cohort was done in a patient with ICANS grade 3 because of neurological worsening during steroids tapering, without performing MRI. Notably, our study included 5 patients with PCNSL. Three patients (60%) experienced either disease progression or tumor flare, however, these findings did not lead to treatment modification, probably because of the difficulty in distinguishing 1 from the other initially. Because of the limited number of patients in this specific subgroup, these results should be interpreted with caution. However, current guidelines did not exclude this subgroup and still recommend performing systematic investigations based on ICANS grade. Finally, systematic MRIs have unmasked 4 silent strokes, but only half of stroke findings had a therapeutic impact (ACD or APT initiation). This could be explained by the fact that these events occurred in patients with preexisting cardiac diseases who were already treated with optimal treatment. One patient with ICANS grade 2 received APT because of a hypersignal suggestive of stroke depicted on MRI, although this hypersignal was already present on baseline MRI. The treating physician who was unaware of this prior image chose to introduce APT. Clinical significance of these immune cell–associated acute stokes and their management remains unknown. One could argue that associated strokes are just an epiphenomenon in post–CAR T-cell infusion, but CAR T-cell–mediated inflammatory state might play a role in the occurrence of such events.15
Considering these findings, we may suggest revised guidelines (Table 3) based on diagnostic investigations with optimal therapeutic impact on patients experiencing ICANS. LP might be left at the physician discretion, because systematic LPs did not lead to differential diagnosis and may expose patients to complications and/or to unnecessary preemptive treatments. Similarly, MRI might be left at the physician’s discretion, because systematic MRI led to abnormal findings without therapeutic impact. Because of its significant therapeutic impact, EEG should still be performed based on ICANS grade as supported by the guidelines.
Our study has several strengths. First, our cohort is 2 to 4 times larger than previous studies focusing on ICANS10-13,16,17 and its management.10,12,16 Second, it is, to our knowledge, the first real-life study to evaluate the impact of diagnostic investigations recommended by international guidelines for ICANS management. Moreover, all patients benefited from preinfusion, baseline MRI, allowing for comparison with postinfusion MRI. Last, our subgroup analysis allowed for us to depict finely the proportion of abnormal results in each ICANS grade and describe varying investigation strategies through the study period.
Nevertheless, our study has some limitations due to its retrospective nature. First, MRI and EEG assessments were subject to a lack of standardization in the study. Around half of EEGs were classified as encephalopathy without knowing the clinical significance of such EEG abnormality. Six percent of EEGs could not distinguish between encephalopathy and seizure, which led to underestimating the proportion of seizure in the cohort. Future studies should probably include standardized EEG analysis. Second, the limited number of patients in our cohort might not be sufficient to detect rare events.
Our study shows that diagnostic investigations recommended by international guidelines for ICANS management rarely result in therapeutic changes for MRI (4%) and LP (7%, all irrelevant), questioning the need for systematic assessment. On the contrary, EEG should still be performed in patients with ICANS grade 2+, considering its significant therapeutic impact. Our results highlight the need for novel ICANS management guidelines, which will limit the use of investigations to situations with a significant therapeutic impact and with an optimal risk-benefit ratio.
Authorship
Contribution: G.M. and R.H. designed the research; M.M. performed the research; M.M. and G.M. wrote the manuscript; S.L., Q.Q., F.L., S.D.G. and R.H. reviewed the manuscript.
Conflict-of-interest disclosure: R.H. reports honoraria from Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda, and Roche; and consultancy at Kite/Gilead, Novartis, Bristol Myers Squibb/Celgene, ADC Therapeutics, Incyte, and Miltenyi. G.M. reports honoraria from Bristol Myers Squibb/Celgene, Gilead-Kite, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Guillaume Manson, Department of Hematology, CHU Rennes, 2 Rue Henri Le Guilloux, Rennes 35033 Cedex 9, France; email: guillaume.manson@chu-rennes.fr.
References
Author notes
Presented in abstract form at the 65th American Society of Hematology Annual Meeting in December 2023.
Data are available upon reasonable request from the corresponding author, Guillaume Manson (guillaume.manson@chu-rennes.fr).
The full-text version of this article contains a data supplement.