TO THE EDITOR:
Diffuse large B-cell lymphoma (DLBCL) is challenging to treat in the relapsed or refractory (R/R) setting.1-3 There is a need for therapies that can improve long-term outcomes, particularly for patients who are ineligible, unwilling, or unable to receive or who relapse after receiving hematopoietic stem cell transplantation or chimeric antigen receptor T-cell therapy.1,2
Loncastuximab tesirine (loncastuximab tesirine-lpyl [Lonca]), an antibody-drug conjugate comprising a humanized anti-CD19 antibody conjugated to a pyrrolobenzodiazepine dimer cytotoxin, received accelerated approval by the US Food and Drug Administration and has received conditional marketing authorization by the European Commission and the Medicines and Healthcare products Regulatory Agency in the United Kingdom to treat adult patients with R/R DLBCL after ≥2 lines of systemic therapy.4-6 The pivotal LOTIS-2 study demonstrated an overall response rate (ORR) of 48.3% with durable responses in a heavily pretreated, difficult-to-treat population.7 During treatment with Lonca, overall health-related quality of life (HRQoL) was either maintained or improved; patients reported improvement from baseline in symptoms such as pain, lumps/swelling, and weight loss; and treatment was tolerated as reported by a majority of patients. These measures are important for patients who struggle with the impact of the disease on their physical and mental health.8-10
Because the management of R/R DLBCL is challenging in older patients, who often have multiple comorbidities that make them ineligible for certain therapies, it is critical to better understand the impact of Lonca on efficacy, safety and tolerability, and HRQoL in this population.11,12 Side effect profile is especially important because these patients are vulnerable to toxicities from chemotherapy.11
This post hoc analysis evaluated the effects of Lonca treatment on efficacy, safety and tolerability, and patient-reported outcomes (PROs) related to HRQoL in older and younger patients from the LOTIS-2 trial (NCT03589469), a single-arm, open-label, phase 2 study in adults with R/R DLBCL, after ≥2 systemic treatments, who had measurable disease and Eastern Cooperative Oncology Group performance status score ranging from 0 to 2.7,8
This analysis was conducted using the data from study initiation (August 2018 to March 2021). Older patients were defined as those aged ≥70 years and younger patients as aged <70 years. Patients received Lonca as a 30-minute IV infusion on day 1 of each 3-week treatment cycle (0.15 mg/kg for 2 cycles; 0.075 mg/kg for subsequent cycles) for up to 1 year or until disease relapse or progression, unacceptable toxicity, death, or patient or investigator decision. This study was conducted in accordance with the International Council for Harmonisation Good Clinical Practice guidelines and ethical principles of the Declaration of Helsinki. Study protocol was approved according to the local regulations of the appropriate institutional review board/independent ethics committee. Informed consent was obtained from each patient and documented with a signed informed consent form before any study procedures.
ORR (primary efficacy end point) was defined as the proportion of patients with a best overall response of complete response or partial response, according to the 2014 Lugano classification.13 Safety and tolerability were measured by the frequency and severity of adverse events (AEs). PROs were measured using clinically validated instruments, including EuroQol instruments (EQ-5D-5L) and the Functional Assessment of Cancer Therapy–Lymphoma (FACT-Lym), at baseline (cycle 1, day 1, predose), on day 1 of each subsequent treatment cycle, and at the end-of-treatment visit (see supplemental Material 1A-B for more information on methods and outcomes).8
Safety and efficacy analyses were conducted for an all-treated population (patients who received ≥1 dose of Lonca; N = 145) when all responding patients had ≥6 months of follow-up after the initial documented response. PRO analyses were performed on the PRO population (N = 130) for patients who had a baseline score and ≥1 follow-up score. Comparative analyses between age groups were not performed with statistical testing; instead, numerical trends were noted across age groups on relevant measures.
A total of 145 patients with R/R DLBCL were treated and included as the all-treated population (age groups: <70 years, n = 95; ≥70 years, n = 50); among them, 130 patients were included in the PRO population (supplemental Figure 1A).8 Baseline characteristics are summarized in supplemental Table 1A. Patients <70 years received a median of 3 cycles (range, 1-17 cycles), and patients ≥70 years received a median of 4 cycles (range, 1-26 cycles) of Lonca as of the data cutoff (March 2021), with a median (minimum, maximum) of 43 (1, 358) and 69 (1, 569) days of treatment, respectively.
Patients in the younger (aged <70 years) and older (aged ≥70 years) groups had similar responses across nearly all efficacy measures (N = 145; Table 1; supplemental Figure 2A), including ORR (younger group, 46 of 95 [48.4%], 95% confidence interval [CI], 38.0%-58.9%; older group, 24 of 50 [48.0%], 95% CI, 33.7%-62.6%) and median time to complete response (younger group, 42 days, 95% CI, 37-247 days; older group, 41 days, 95% CI, 36-59 days).
Efficacy measures in patients aged <70 and ≥70 years
| . | <70 y (n = 95) . | ≥70 y (n = 50) . | Total (N = 145) . |
|---|---|---|---|
| BOR, n (%) | |||
| CR | 21 (22.1) | 15 (30.0) | 36 (24.8) |
| PR | 25 (26.3) | 9 (18.0) | 34 (23.4) |
| Stable disease | 16 (16.8) | 6 (12.0) | 22 (15.2) |
| PD | 18 (18.9) | 12 (24.0) | 30 (20.7) |
| Not evaluable | 15 (15.8) | 8 (16.0) | 23 (15.9) |
| ORR (CR + PR) | 46 (48.4) | 24 (48.0) | 70 (48.3) |
| 95% CI for ORR | 38.0-58.9 | 33.7-62.6 | 39.9-56.7 |
| 95% CI for CR | 14.2-31.8 | 17.9-44.6 | 18.0-32.7 |
| . | <70 y (n = 95) . | ≥70 y (n = 50) . | Total (N = 145) . |
|---|---|---|---|
| BOR, n (%) | |||
| CR | 21 (22.1) | 15 (30.0) | 36 (24.8) |
| PR | 25 (26.3) | 9 (18.0) | 34 (23.4) |
| Stable disease | 16 (16.8) | 6 (12.0) | 22 (15.2) |
| PD | 18 (18.9) | 12 (24.0) | 30 (20.7) |
| Not evaluable | 15 (15.8) | 8 (16.0) | 23 (15.9) |
| ORR (CR + PR) | 46 (48.4) | 24 (48.0) | 70 (48.3) |
| 95% CI for ORR | 38.0-58.9 | 33.7-62.6 | 39.9-56.7 |
| 95% CI for CR | 14.2-31.8 | 17.9-44.6 | 18.0-32.7 |
| . | <70 y (n = 46) . | ≥70 y (n = 24) . | Total (N = 70) . |
|---|---|---|---|
| Time to CR/PR, d, median (min, max) | 41.5 (35, 247) | 41.0 (36, 142) | 41.0 (35, 247) |
| . | <70 y (n = 46) . | ≥70 y (n = 24) . | Total (N = 70) . |
|---|---|---|---|
| Time to CR/PR, d, median (min, max) | 41.5 (35, 247) | 41.0 (36, 142) | 41.0 (35, 247) |
| . | <70 y (n = 21) . | ≥70 y (n = 15) . | Total (N = 36) . |
|---|---|---|---|
| Time to CR, d, median (min, max) | 42.0 (37, 247) | 41.0 (36, 59) | 42 (36, 247) |
| . | <70 y (n = 21) . | ≥70 y (n = 15) . | Total (N = 36) . |
|---|---|---|---|
| Time to CR, d, median (min, max) | 42.0 (37, 247) | 41.0 (36, 59) | 42 (36, 247) |
| . | <70 y (n = 49) . | ≥70 y (n = 24) . | Total (N = 73) . |
|---|---|---|---|
| Median PFS, mo (95% CI) | 3.81 (2.69-8.08) | 7.36 (2.99-NA) | 4.93 (2.89-8.31) |
| . | <70 y (n = 49) . | ≥70 y (n = 24) . | Total (N = 73) . |
|---|---|---|---|
| Median PFS, mo (95% CI) | 3.81 (2.69-8.08) | 7.36 (2.99-NA) | 4.93 (2.89-8.31) |
| . | <70 y (n = 17) . | ≥70 y (n = 6) . | Total (N = 23) . |
|---|---|---|---|
| Median DOR, mo (95% CI) | 9.26 (4.63-NA) | NR | 13.37 (6.87-NA) |
| . | <70 y (n = 17) . | ≥70 y (n = 6) . | Total (N = 23) . |
|---|---|---|---|
| Median DOR, mo (95% CI) | 9.26 (4.63-NA) | NR | 13.37 (6.87-NA) |
| . | <70 y (n = 63) . | ≥70 y (n = 33) . | Total (N = 96) . |
|---|---|---|---|
| Median OS, mo (95% CI) | 9.89 (6.14-12.09) | 8.90 (6.74-12.42) | 9.53 (6.93-11.47) |
| . | <70 y (n = 63) . | ≥70 y (n = 33) . | Total (N = 96) . |
|---|---|---|---|
| Median OS, mo (95% CI) | 9.89 (6.14-12.09) | 8.90 (6.74-12.42) | 9.53 (6.93-11.47) |
BOR, best overall response; CR, complete response; DOR, duration of response; max, maximum; min, minimum; NA, not available; NR, not reached; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response.
Overall treatment-emergent adverse events (TEAEs) were similar across age groups (N = 145; supplemental Table 2A). Percentages of patients with any TEAE leading to dose delay or reduction (<70 years, 51.6%; ≥70 years, 52%) or any TEAE leading to withdrawal (<70 years, 24.2%; ≥70 years, 26%) were also similar. Grade ≥3 AEs occurring in ≥10% of patients revealed similar rates of hematologic TEAEs; however, there was a lower percentage of increased gamma-glutamyltransferase in older patients (<70 years, 23.2%; ≥70 years, 6%).
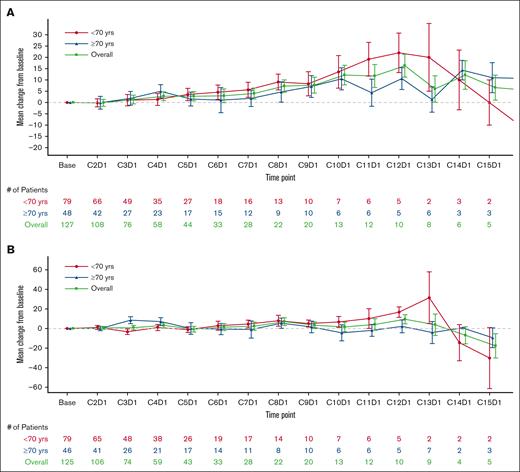
Change from baseline in EQ-VAS and FACT-Lym total scores were stable or improved over the treatment period in both age groups (N = 130; Figure 1A-B). A majority of patients in both groups responded “a little bit” or “not at all” to the GP5 (FACT-Lym) question on tolerability; however, a higher percentage of patients in the older age group responded “not at all” than in the younger age group (Figure 1C).
Change from baseline scores for EQ-VAS and FACT-Lym and pecentages of responses to GP5. Change from baseline scores based on the visit for (A) EQ-VAS; (B) FACT-Lym total score; and (C) percentages of responses to GP5 (“I am bothered by side effects of treatment”) by visit of patients aged <70 and ≥70 years.
Change from baseline scores for EQ-VAS and FACT-Lym and pecentages of responses to GP5. Change from baseline scores based on the visit for (A) EQ-VAS; (B) FACT-Lym total score; and (C) percentages of responses to GP5 (“I am bothered by side effects of treatment”) by visit of patients aged <70 and ≥70 years.
An analysis was also conducted in patients aged <65, 65 to <75, and ≥75 years, with similar efficacy, safety, and PROs reported across these age groups (supplemental Material 1B).
Consistent with the overall results from the LOTIS-2 study, Lonca had an acceptable safety and tolerability profile and produced quick and durable responses in both younger and older patients with R/R DLBCL in this subgroup analysis.7 In addition, PROs (as measured by the EQ-VAS and FACT-Lym total scores) were stable or improved, and a majority of patients responded “a little bit” or “not at all” to the GP5 (FACT-Lym) question on tolerability in both groups. Therefore, these outcome data, combined with a favorable ORR, durable response, and manageable safety and tolerability profile, suggest that Lonca is suitable for and has the potential to improve HRQoL for both older and younger patients.
This analysis was conducted on data from the single-arm, open-label LOTIS-2 study, which included a broad patient population, comprising patients who were heavily pretreated, older (>70 years), and had difficult-to-treat disease, including high-grade B-cell lymphoma with MYC and BCL2 or BCL6 rearrangements (double-hit) or with MYC, BCL2, and BCL6 rearrangements (triple-hit); patients with transformed disease; and patients with primary refractory DLBCL. Of the 184 patients screened for LOTIS-2, 61 were aged ≥70 years, and of the 145 patients eligible for the study, 50 were aged ≥70 years. However, results may not be comparable with those of other studies. Although we used validated PRO scales, patients might have been biased in reporting positive results.14 A comparative statistical analysis was not performed across the age groups; only descriptive statistics within and not between the groups were reported. In addition, because patients received treatment for up to 1 year with a median of 3 or 4 cycles, sample sizes in later cycles of treatment were small and enriched with responders.
Although older patients are more challenging to treat, these data suggest that older age does not have a negative impact on response or safety and tolerability of Lonca treatment.11,12 Of note, the findings are similar to those seen with chimeric antigen receptor T-cell therapy, as demonstrated in the ZUMA-7 clinical trial of axicabtagene ciloleucel, in which older patients (≥65 years) had clinical outcomes similar to the overall patient population.15 In addition, results of a real-world, retrospective analysis in Germany demonstrated that being age >65 years was not a risk factor for clinical outcomes after axicabtagene ciloleucel or tisagenlecleucel treatment.16 The age breakdowns used in the subanalysis of LOTIS-2 reported herein are of particular relevance, considering the later age of disease onset and diagnosis of DLBCL.17
Taken together, data from this subgroup analysis and previously reported findings suggest that Lonca is an effective R/R DLBCL treatment, with a durable response and favorable safety and tolerability profile, which improves or maintains PROs in younger and older patients alike.
Acknowledgments: The authors thank their colleague Eric Yu for his contribution to the statistical analysis of this project.
Medical writing support was provided by Loren M. DeVito of Citrus Health Group, which was in accordance with Good Publication Practice (2022) guidelines and funded by ADC Therapeutics SA (Murray Hill, NJ). The LOTIS-2 (ADCT-402-201) study was funded by ADC Therapeutics SA.
Contribution: P.F.C., W.A., J.P.A., K.M.A., M.H., B.H., J.R., M.S., A. Stathis, P.L.Z., B.K., A. Spira, and C.C.-S. were principal investigators and contributed to provision of patient care, data analysis and interpretation, development and revision of the manuscript, and provision of final approval of the submitted content; Y.Q., L.L., L.C., Y.W., and Z.C.X. contributed to statistical analyses, data analysis and interpretation, development and critical revision of the manuscript, and provision of final approval of the submitted content; X.Z. contributed to data analysis and interpretation, development and critical revision of the manuscript, and provision of final approval of the submitted content; and all authors had full access to all the data in the study, approved the decision to submit for publication, and have agreed to be accountable for all aspects of the work, including its accuracy and integrity.
Conflict-of-interest disclosure: L.C., Y.Q., and Z.C.X. are employees of ADC Therapeutics with equity and stock options in the company. L.L. and Y.W. report equity and stock options in ADC Therapeutics and were affiliated with ADC Therapeutics at the time the work was conducted. B.K. has received consulting fees from ADC Therapeutics. A. Spira has received institutional research support from ADC Therapeutics. J.R. has received honoraria from ADC Therapeutics for taking part in advisory boards and is an ADC Therapeutics stockholder. P.F.C. reports grants from ADC Therapeutics, during the conduct of the study; grants and personal fees from Genentech; and personal fees from ADC Therapeutics, Amgen, Celgene, Kite Pharmaceuticals, Seattle Genetics, TG Therapeutics, and Verastem, outside of the submitted work. W.A. reports grants from Nurix Therapeutics and personal fees from ADC Therapeutics, Kymera, and Nurix outside of the submitted work. K.M.A. reports clinical research support from University College London Hospitals Biomedical Research Centre, and personal fees from BeiGene, Celgene, Gilead, Roche, and Takeda outside of the submitted work. M.H. reports grants from Astellas Pharma, Spectrum Pharmaceuticals, and Takeda, and personal fees from AbGenomics, ADC Therapeutics, AstraZeneca, BeiGene, Celgene, Incyte Corporation, Janssen, Omeros, Pharmacyclics, Sanofi Genzyme, TeneoBio, and Verastem outside of the submitted work. B.H. reports grants from ADC Therapeutics during the conduct of the study, and personal fees from ADC Therapeutics, AstraZeneca, and Bristol Myers Squibb outside of the submitted work. M.S. reports grants from ADC Therapeutics during the conduct of the study, and personal fees from Amgen and Celgene outside of the submitted work. A. Stathis reports grants from ADC Therapeutics during the conduct of the study; grants from Bayer, Eli Lilly, MEI Pharma, Merck, Novartis, Pfizer, and Roche; and personal fees from AbbVie and PharmaMar outside of the submitted work. P.L.Z. reports personal fees from ADC Therapeutics, Bristol Myers Squibb, Celgene, Celltrion, Eusapharma, Gilead, Immune Design, Janssen-Cilag, Kyowa Kirin, Merck Sharp & Dohme, Portola, Roche, Sandoz, Sanofi, Servier, and Verastem outside of the submitted work. C.C.-S. reports grants from ADC Therapeutics during the conduct of the study; grants from Rhizen Pharmaceuticals; and personal fees from ADC Therapeutics, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Genenta Science, Janssen Oncology, Karyopharm, Merck Sharp & Dohme, Novartis, Roche, Sanofi, and Servier outside of the submitted work. J.P.A. reports personal fees from ADC Therapeutics and Genentech outside of the submitted work, and has an immediate family member who has served on advisory boards from Agios Pharmaceuticals, Forma Therapeutics, Foundation Medicine, Inovio Pharmaceuticals, and Puma Biotechnology. X.Z. declares no competing financial interests.
Correspondence: Mehdi Hamadani, Division of Hematology and Oncology, BMT & Cellular Therapy Program, Medical College of Wisconsin, 9200 W Wisconsin Ave, Milwaukee, WI 53226; email: mhamadani@mcw.edu.
References
Author notes
Summary data for the LOTIS-2 study, from which these subanalyses were performed, are available at www.clinicaltrials.gov (NCT03589469).
Proposals requesting deidentified participant data collected for the study following publication can be sent to ADC Therapeutics (clinical.trials@adctherapeutics.com). The requests will be evaluated on a case-by-case basis.
The full-text version of this article contains a data supplement.