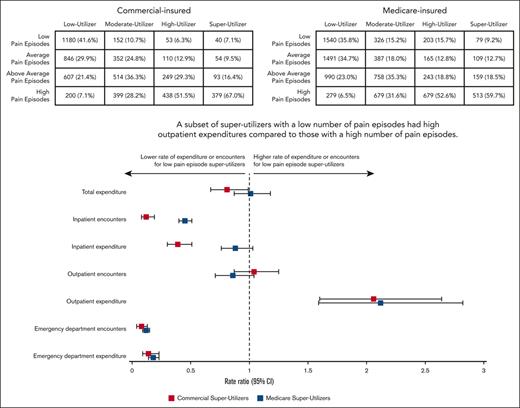
Super-utilizers had 13.37 to 43.46 times greater expenditure than low utilizers.
A subset of super-utilizers with few pain episodes had high outpatient expenditures compared with those with a high number of pain episodes.
Visual Abstract
Sickle cell disease (SCD) is a rare but costly condition in the United States. Super-utilizers have been defined as a subset of the population with high health care encounters or expenditures. Although super-utilizers have been described in other disease states, little is known about super-utilizers among adults with SCD. This study aimed to characterize the differences in expenditures, overall health care encounters, and pain episode encounters between super-utilizers (top 10% expenditures) and lower-utilizers with SCD (high, top 10%-24.9%; moderate, 25%-49.9%; and low, bottom 50% expenditures). A retrospective longitudinal cohort of adults with SCD were identified using validated algorithms in MarketScan and Medicare claim databases from 2016 to 2020. Encounters and expenditures were analyzed from inpatient, outpatient, and emergency department settings. Differences in encounters and expenditures between lower-utilizers and super-utilizers were compared using logistic regression. Among super-utilizers, differences in encounters and expenditures were compared according to incidences of pain episode encounters. The study population included 5666 patients with commercial insurance and 8600 with Medicare. Adjusted total annual health care expenditure was 43.46 times higher for super-utilizers than for low-utilizers among commercial-insured and 13.37 times higher in Medicare-insured patients. Among super-utilizers, there were patients with few pain episode encounters who had higher outpatient expenditures than patients with a high number of pain episode encounters. Our findings demonstrate the contribution of expensive outpatient care among SCD super-utilizers, in which analyses of high expenditure have largely focused on short-term care. Future studies are needed to better understand super-utilizers in the SCD population to inform the effective use of preventive interventions and/or curative therapies.
Introduction
Sickle cell disease (SCD) is a chronic disease caused by a single gene mutation in hemoglobin that affects almost all body organs throughout the lifespan.1,2 Current estimates show SCD affects >100 000 children and adults in the United States,3 and the annual health care expenditures for SCD are more than $3 billion (averaging $30 000 per person per year).4 This expenditure is largely tied to high health care encounters to address acute complications, such as episodes of intense pain (vaso-occlusive pain episodes), and long-term disease comorbidities, such as chronic kidney disease, stroke, and cardio-pulmonary complications.1 Expenditure analyses have primarily focused on pain crises as the main drivers of high expenditures among individuals with SCD.5-7
Super-utilizers are defined as individuals who use a disproportionate amount of health care resources and have been identified across multiple disease states.8-16 Adults with SCD are often identified as super-utilizers based on their high health care encounters.8,9 Because of the high expenditure associated with elevated health care encounters, there has been significant focus on identifying the characteristics of people who are super-utilizers, known as “hot spotting,”17 so that resources can be directed reactively or proactively to improve clinical outcomes and reduce long-term health care expenditures. Although interventions for super-utilizers among adults with SCD have focused on addressing health care needs and reducing expenditure,18,19 little has been done to identify the differences in cost expenditures between lower-utilizers and super-utilizers in this patient population.20,21 Furthermore, there is a lack of evidence illuminating these differences between adults with SCD who have private or public insurance. This knowledge is needed to understand the pressing health care needs of these people across payers and understand the potential impact on interventions designed to reduce expenditures. This understanding could also inform shared decision making surrounding high-risk, high-cost treatment options (eg, stem cell transplant and gene therapy) that have the potential to be curative and cost-effective for some individuals.
Applying the approach used for other chronic diseases to identify super-utilizer and lower-utilizer adults with SCD based on the total annual expenditure,15,16 we aimed to characterize the differences in overall health care encounters, distribution of expenditures across health care settings, and pain episode encounters between utilizer groups who are covered by commercial or Medicare insurance.
Methods
Data sources
The International Business Machines Corporation (IBM) Truven MarketScan commercial database and Medicare limited data sets (standard analytical files) were used to identify patients with SCD during the period between 2016 and 2020. These databases included health care encounters and expenditures related to inpatient, outpatient, and emergency department (ED) claims. Both the MarketScan and Medicare claims databases include encounter data (eg, diagnoses, procedures, expenditures, etc) as well as detailed patient information (eg, age, sex, enrollment, etc). Data related to medication use and expenditures were not used for these analyses. These data were based on claims that were received by insurance companies.
Patient selection
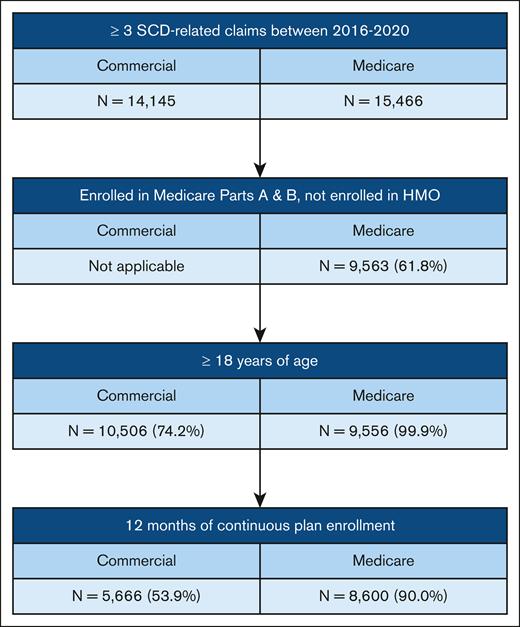
Participants were included if they had ≥3 distinct claims with an SCD-related diagnosis code between 2016 and 2020, because this method has been validated for identifying those with SCD.22 Diagnosis codes were International Classification of Diseases Tenth Edition (ICD-10) codes D57.40, D57.419, D57.1, D57.00, D57.20, D57.219, D57.80, and D57.819. Patients had to be at least 18 years of age, with age determined at first observation in the data set, and had to have 12 months of continuous plan enrollment. Medicare-insured patients had to be enrolled in Medicare parts A and B, could not be enrolled in a health maintenance organization during the study period, and had to have 12 months of observed follow-up. Multiple claims from the same day were considered 1 distinct claim. Super-utilizers were defined as those who accounted for the top 10% of expenditures, high-utilizers accounted for the next 10% to 24.9% of expenditures, moderate-utilizers accounted for the next 25% to 49.9% of expenditures, and low-utilizers accounted for the bottom 50% of expenditures.
Variables
Study outcomes included annual expenditure (total expenditure / time enrolled in years) and annual number of encounters (total number of encounters / time enrolled in years). These outcomes were reported in total and in 3 health care settings: inpatient, outpatient, and ED. Health care encounters in the ED setting were identified from outpatient claims that included a claim line attributed to 1 of the following ED revenue center codes: 450, 451, 451, 456, 459, or 981.
SCD genotype was defined as the most commonly used genotype for each patient, according to ICD-10 codes shown in supplemental Table 1. In the event that a patient had 2 different genotypes used equally as often or only had codes for nonspecific SCD, they were classified as nonspecific SCD. Patient race was defined from the first instance in the data set, using 3 categories: White, Black, and other/unknown, which included categories of Asian, Hispanic, North American Native, other, and unknown. Race information was only available for Medicare-insured patients. Dual-eligible patients were defined as those with Medicare who were also eligible for Medicaid at any time during the study period. To measure the degree of comorbidity burden, the Charlson Comorbidity Index (CCI) was used.23 Patients with SCD-related comorbidities were identified using ICD-10 codes shown in supplemental Table 2.
We calculated the average annual distinct pain episode encounters (ie, in outpatient, inpatient, or ED setting) by establishing database-specific cutoffs to group patients into quartiles (ie, patients in the high pain episode–encounters group were in the highest quartile of annual number of pain episode encounters, and the above-average pain episode–encounters group were in the second highest quartile of annual number of pain episode encounters, and so on). Pain episode encounters were identified using the ICD-10 diagnosis codes provided in supplemental Table 3. Pain episode encounters without a 3-day gap were counted as 1 distinct pain episode encounter.24 The median and range of pain episode encounters for each quartile are shown in supplemental Table 4.
Statistical analysis
Descriptive statistics were presented as median (with interquartile range) for continuous measures and as frequency (relative frequency [%]) for categorical measures. For outcomes of the number of encounters, a multivariable negative binomial regression with a log link was used. Similarly, for expenditure outcomes, a multivariable gamma regression with a log link was used. In both instances, rate ratios (RRs) and their 95% confidence intervals (CIs) were provided. RRs > 1 indicated higher expenditure/encounters and RRs < 1 indicated lower expenditure/encounters than those of the reference group. RRs with a 95% CI that did not overlap with 1 were considered statistically significant compared with those of the reference group. All models were adjusted for covariates, including patient age, CCI, sex, region, and race/ethnicity (race/ethnicity controlled only in analyses of Medicare data because race/ethnicity data were not available in the commercially insured population). Statistical analyses were performed using Statistical Analysis System (SAS) version 9.4 (SAS Institute Inc, Cary, NC).
Results
A total of 5666 commercial-insured and 8600 Medicare-insured patients with SCD were included in this study (Figure 1). Characteristics of commercial- and Medicare-insured patients are presented in Table 1. The median age was 32 years for commercial-insured patients and 39 years for Medicare-insured patients. Approximately 3 in 5 patients were female (commercial, 59.8% and Medicare, 58.0%). Patients with Medicare insurance were predominantly Black (88.6%). Most patients resided in the southern region of the United States (commercial, 61.4% and Medicare, 60.0%). Hemoglobin SS was the predominate SCD genotype among all patients, and the frequency of this genotype was highest in the super-utilizer group. Super-utilizers had higher CCI than other utilizer groups (commercial super-utilizer median CCI, 2; Medicare super-utilizer median CCI, 4) and had higher frequency of SCD-related comorbidities. A majority of patients with Medicare insurance (64.6%) were dual eligible for Medicaid, and the frequency of dual-eligible patients was higher among super-utilizers.
Patient characteristics for each of the utilizer groups, presented as median (IQR) or frequency (%)
| Commercial . | |||||
|---|---|---|---|---|---|
| . | Total n = 5666 . | Low-utilizers n = 2833 . | Moderate-utilizers n = 1417 . | High-utilizers n = 850 . | Super-utilizers n = 566 . |
| Patient years | 3.0 (2-5) | 3.3 (2-5) | 3.0 (2-5) | 2.9 (1.8-4.5) | 2.8 (1.7-4.1) |
| Age, y | 32 (22-44) | 34 (22-45) | 32 (22-44) | 31 (21-42) | 28 (19-42) |
| Female | 3389 (59.8%) | 1672 (59.0%) | 892 (62.9%) | 505 (59.4%) | 320 (56.5%) |
| Region | |||||
| Northeast | 1115 (19.7%) | 548 (19.3%) | 275 (19.4%) | 171 (20.1%) | 121 (21.4%) |
| North Central | 751 (13.3%) | 365 (12.9%) | 200 (14.1%) | 121 (14.2%) | 65 (11.5%) |
| South | 3477 (61.4%) | 1769 (62.4%) | 863 (60.9%) | 510 (60.0%) | 335 (59.2%) |
| West | 307 (5.4%) | 143 (5.0%) | 75 (5.3%) | 45 (5.3%) | 44 (7.8%) |
| Unknown | 16 (0.3%) | 8 (0.3%) | 4 (0.3%) | 3 (0.4%) | 1 (0.2%) |
| SCD genotype | |||||
| Hb SS | 2457 (43.4%) | 797 (28.1%) | 733 (51.7%) | 530 (62.4%) | 397 (70.1%) |
| Hb SC | 1179 (20.8%) | 739 (26.1%) | 268 (18.9%) | 120 (14.1%) | 52 (9.2%) |
| Hb Sβ+/0 | 627 (11.1%) | 343 (12.1%) | 154 (10.9%) | 83 (9.8%) | 47 (8.3%) |
| Nonspecific | 1403 (24.8%) | 954 (33.7%) | 262 (18.5%) | 117 (13.8%) | 70 (12.4%) |
| CCI | 0 (0-1) | 0 (0-1) | 0 (0-2) | 1 (0-2) | 2 (1-4) |
| SCD-related comorbidity | |||||
| CVD | 624 (11.0%) | 118 (4.2%) | 170 (12.0%) | 164 (19.3%) | 172 (30.4%) |
| CHF | 543 (9.6%) | 83 (2.9%) | 129 (9.1%) | 150 (17.6%) | 181 (32.0%) |
| PH | 487 (8.6%) | 98 (3.5%) | 129 (9.1%) | 133 (15.6%) | 127 (22.4%) |
| RD | 536 (9.5%) | 122 (4.3%) | 123 (8.7%) | 127 (14.9%) | 164 (29.0%) |
| Commercial . | |||||
|---|---|---|---|---|---|
| . | Total n = 5666 . | Low-utilizers n = 2833 . | Moderate-utilizers n = 1417 . | High-utilizers n = 850 . | Super-utilizers n = 566 . |
| Patient years | 3.0 (2-5) | 3.3 (2-5) | 3.0 (2-5) | 2.9 (1.8-4.5) | 2.8 (1.7-4.1) |
| Age, y | 32 (22-44) | 34 (22-45) | 32 (22-44) | 31 (21-42) | 28 (19-42) |
| Female | 3389 (59.8%) | 1672 (59.0%) | 892 (62.9%) | 505 (59.4%) | 320 (56.5%) |
| Region | |||||
| Northeast | 1115 (19.7%) | 548 (19.3%) | 275 (19.4%) | 171 (20.1%) | 121 (21.4%) |
| North Central | 751 (13.3%) | 365 (12.9%) | 200 (14.1%) | 121 (14.2%) | 65 (11.5%) |
| South | 3477 (61.4%) | 1769 (62.4%) | 863 (60.9%) | 510 (60.0%) | 335 (59.2%) |
| West | 307 (5.4%) | 143 (5.0%) | 75 (5.3%) | 45 (5.3%) | 44 (7.8%) |
| Unknown | 16 (0.3%) | 8 (0.3%) | 4 (0.3%) | 3 (0.4%) | 1 (0.2%) |
| SCD genotype | |||||
| Hb SS | 2457 (43.4%) | 797 (28.1%) | 733 (51.7%) | 530 (62.4%) | 397 (70.1%) |
| Hb SC | 1179 (20.8%) | 739 (26.1%) | 268 (18.9%) | 120 (14.1%) | 52 (9.2%) |
| Hb Sβ+/0 | 627 (11.1%) | 343 (12.1%) | 154 (10.9%) | 83 (9.8%) | 47 (8.3%) |
| Nonspecific | 1403 (24.8%) | 954 (33.7%) | 262 (18.5%) | 117 (13.8%) | 70 (12.4%) |
| CCI | 0 (0-1) | 0 (0-1) | 0 (0-2) | 1 (0-2) | 2 (1-4) |
| SCD-related comorbidity | |||||
| CVD | 624 (11.0%) | 118 (4.2%) | 170 (12.0%) | 164 (19.3%) | 172 (30.4%) |
| CHF | 543 (9.6%) | 83 (2.9%) | 129 (9.1%) | 150 (17.6%) | 181 (32.0%) |
| PH | 487 (8.6%) | 98 (3.5%) | 129 (9.1%) | 133 (15.6%) | 127 (22.4%) |
| RD | 536 (9.5%) | 122 (4.3%) | 123 (8.7%) | 127 (14.9%) | 164 (29.0%) |
| Medicare . | |||||
|---|---|---|---|---|---|
| . | Total n = 8600 . | Low-utilizers n = 4300 . | Moderate-utilizers n = 2150 . | High-utilizers n = 1290 . | Super-utilizers n = 860 . |
| Patient years | 4.5 (3.0-4.9) | 4.5 (3.4-4.8) | 4.7 (3.2-4.9) | 4.6 (2.8-4.9) | 3.2 (1.8-4.9) |
| Age, y | 39 (29-53) | 41 (30-56) | 36 (28-51) | 37 (28-51) | 38 (28-52) |
| Female | 4985 (58.0%) | 2564 (59.6%) | 1202 (55.9%) | 742 (57.5%) | 477 (55.5%) |
| Race | |||||
| White | 401 (4.7%) | 211 (4.9%) | 93 (4.3%) | 62 (4.8%) | 35 (4.1%) |
| Black | 7621 (88.6%) | 3808 (88.6%) | 1903 (88.5%) | 1140 (88.4%) | 770 (89.5%) |
| Other/unknown | 578 (6.7%) | 281 (6.5%) | 154 (7.2%) | 88 (6.8%) | 55 (6.4%) |
| Region | |||||
| Midwest | 1432 (16.7%) | 742 (17.3%) | 354 (16.5%) | 192 (14.9%) | 144 (16.7%) |
| Northeast | 1323 (15.4%) | 653 (15.2%) | 336 (15.6%) | 215 (16.7%) | 119 (13.8%) |
| South | 5159 (60.0%) | 2575 (59.9%) | 1269 (59.0%) | 786 (60.9%) | 529 (61.5%) |
| West | 686 (8.0%) | 330 (7.7%) | 191 (8.9%) | 97 (7.5%) | 68 (7.9%) |
| SCD genotype | |||||
| Hb SS | 5590 (65.0%) | 2407 (56.0%) | 1577 (73.3%) | 943 (73.1%) | 663 (77.1%) |
| Hb SC | 928 (10.8%) | 646 (15.0%) | 165 (7.7%) | 77 (6.0%) | 40 (4.7%) |
| HB Sβ+/0 | 643 (7.5%) | 383 (8.9%) | 133 (6.2%) | 78 (6.0%) | 49 (5.7%) |
| Nonspecific | 1439 (16.7%) | 864 (20.1%) | 275 (12.8%) | 192 (14.9%) | 108 (12.6%) |
| CCI | 1 (0-3) | 0 (0-1) | 1 (0-3) | 3 (1-5) | 4 (2-6) |
| SCD-related comorbidity | |||||
| CVD | 1232 (14.3%) | 321 (7.5%) | 366 (17.0%) | 269 (20.9%) | 276 (32.1%) |
| CHF | 2234 (26.0%) | 485 (11.3%) | 627 (29.2%) | 602 (46.7%) | 520 (60.5%) |
| PH | 1923 (22.4%) | 397 (9.2%) | 591 (27.5%) | 514 (39.8%) | 421 (49.0%) |
| RD | 2341 (27.2%) | 564 (13.1%) | 613 (28.5%) | 635 (49.2%) | 529 (61.5%) |
| Dual eligible | 5552 (64.6%) | 2504 (58.2%) | 1497 (69.6%) | 914 (70.9%) | 637 (74.1%) |
| Medicare . | |||||
|---|---|---|---|---|---|
| . | Total n = 8600 . | Low-utilizers n = 4300 . | Moderate-utilizers n = 2150 . | High-utilizers n = 1290 . | Super-utilizers n = 860 . |
| Patient years | 4.5 (3.0-4.9) | 4.5 (3.4-4.8) | 4.7 (3.2-4.9) | 4.6 (2.8-4.9) | 3.2 (1.8-4.9) |
| Age, y | 39 (29-53) | 41 (30-56) | 36 (28-51) | 37 (28-51) | 38 (28-52) |
| Female | 4985 (58.0%) | 2564 (59.6%) | 1202 (55.9%) | 742 (57.5%) | 477 (55.5%) |
| Race | |||||
| White | 401 (4.7%) | 211 (4.9%) | 93 (4.3%) | 62 (4.8%) | 35 (4.1%) |
| Black | 7621 (88.6%) | 3808 (88.6%) | 1903 (88.5%) | 1140 (88.4%) | 770 (89.5%) |
| Other/unknown | 578 (6.7%) | 281 (6.5%) | 154 (7.2%) | 88 (6.8%) | 55 (6.4%) |
| Region | |||||
| Midwest | 1432 (16.7%) | 742 (17.3%) | 354 (16.5%) | 192 (14.9%) | 144 (16.7%) |
| Northeast | 1323 (15.4%) | 653 (15.2%) | 336 (15.6%) | 215 (16.7%) | 119 (13.8%) |
| South | 5159 (60.0%) | 2575 (59.9%) | 1269 (59.0%) | 786 (60.9%) | 529 (61.5%) |
| West | 686 (8.0%) | 330 (7.7%) | 191 (8.9%) | 97 (7.5%) | 68 (7.9%) |
| SCD genotype | |||||
| Hb SS | 5590 (65.0%) | 2407 (56.0%) | 1577 (73.3%) | 943 (73.1%) | 663 (77.1%) |
| Hb SC | 928 (10.8%) | 646 (15.0%) | 165 (7.7%) | 77 (6.0%) | 40 (4.7%) |
| HB Sβ+/0 | 643 (7.5%) | 383 (8.9%) | 133 (6.2%) | 78 (6.0%) | 49 (5.7%) |
| Nonspecific | 1439 (16.7%) | 864 (20.1%) | 275 (12.8%) | 192 (14.9%) | 108 (12.6%) |
| CCI | 1 (0-3) | 0 (0-1) | 1 (0-3) | 3 (1-5) | 4 (2-6) |
| SCD-related comorbidity | |||||
| CVD | 1232 (14.3%) | 321 (7.5%) | 366 (17.0%) | 269 (20.9%) | 276 (32.1%) |
| CHF | 2234 (26.0%) | 485 (11.3%) | 627 (29.2%) | 602 (46.7%) | 520 (60.5%) |
| PH | 1923 (22.4%) | 397 (9.2%) | 591 (27.5%) | 514 (39.8%) | 421 (49.0%) |
| RD | 2341 (27.2%) | 564 (13.1%) | 613 (28.5%) | 635 (49.2%) | 529 (61.5%) |
| Dual eligible | 5552 (64.6%) | 2504 (58.2%) | 1497 (69.6%) | 914 (70.9%) | 637 (74.1%) |
Low-utilizers: bottom 50% of costs; moderate-utilizers: top 25% to 49.9% of costs; high-utilizers: top 10% to 24.9% of costs; super-utilizers: top 10% of costs.
CHF, congestive heart failure; CVD, cerebrovascular disease; Hb SS: hemoglobin SS; Hb SC, hemoglobin SC; HB Sβ+/0, hemoglobin Sβ+/0 thalassemia; IQR, interquartile range; PH, pulmonary hypertension; RD, renal disease.
Average overall encounters and expenditures for commercial-insured and Medicare-insured patients with SCD are presented in Table 2. Median total annual expenditure for commercial-insured patients was $11 400 compared with $21 000 for Medicare-insured patients. For commercial-insured patients, the median total expenditure for super-utilizers was 36.6 times higher than for low-utilizers and 18.0 times higher for Medicare-insured patients. There were fewer inpatient encounters among low-utilizers (median, 0.0 and 0.5 for commercial-insured and Medicare-insured, respectively) than among super-utilizers (median, 2.6 and 6.8 for commercial-insured and Medicare-insured, respectively). The median number of outpatient encounters for super-utilizers was 4.9 times greater than that of low-utilizers among commercial-insured patients, whereas the median number of outpatient encounters for super-utilizers was 3.3 times greater than that of low-utilizers among Medicare-insured patients. The median number of ED encounters for super-utilizers and low-utilizers, respectively, were 1.3 and 0.2 in commercial-insured patients and 4.7 and 1.1 in Medicare-insured patients.
Average annual health care encounters and expenditure outcomes presented as median (IQR)
| Commercial . | |||||
|---|---|---|---|---|---|
| . | Total n = 5666 . | Low-utilizers n = 2833 . | Moderate-utilizers n = 1417 . | High-utilizers n = 850 . | Super-utilizers n = 566 . |
| Total expenditure (kUSD) | 11.4 (3.7-31.5) | 3.7 (1.7-6.6) | 18.4 (14.5-23.9) | 48.5 (39.4-60.7) | 135.5 (100.8-204.5) |
| Group total (mUSD) | 190.3 | 12.4 | 27.4 | 43.1 | 107.4 |
| Inpatient | |||||
| No. of encounters | 0.2 (0.0-0.8) | 0.0 (0.0-0.0) | 0.5 (0.2-0.8) | 1.2 (0.6-2.0) | 2.6 (1.0-4.8) |
| Expenditure (kUSD) | 2.4 (0.0-15.2) | 0.0 (0.0-0.0) | 9.1 (2.9-14.1) | 28.5 (14.7-39.8) | 80.8 (41.3-136.6) |
| Outpatient | |||||
| No. of encounters | 11.5 (6.0-21.7) | 7.2 (4.2-11.6) | 14.4 (8.6-22.4) | 21.5 (13.8-32.8) | 35.3 (24.0-54.8) |
| Expenditure (kUSD) | 5.0 (2.1-11.5) | 2.5 (1.3-4.3) | 7.5 (4.4-12.4) | 14.6 (7.7-29.8) | 41.5 (20.4-89.6) |
| ED | |||||
| No. of encounters | 0.5 (0.0-1.3) | 0.2 (0.0-0.7) | 0.8 (0.2-1.6) | 1.0 (0.3-2.3) | 1.3 (0.4-3.6) |
| Expenditure (kUSD) | 0.3 (0.0-1.2) | 0.1 (0.0-0.5) | 0.5 (0.0-1.5) | 0.8 (0.0-2.1) | 1.3 (0.1-4.4) |
| Commercial . | |||||
|---|---|---|---|---|---|
| . | Total n = 5666 . | Low-utilizers n = 2833 . | Moderate-utilizers n = 1417 . | High-utilizers n = 850 . | Super-utilizers n = 566 . |
| Total expenditure (kUSD) | 11.4 (3.7-31.5) | 3.7 (1.7-6.6) | 18.4 (14.5-23.9) | 48.5 (39.4-60.7) | 135.5 (100.8-204.5) |
| Group total (mUSD) | 190.3 | 12.4 | 27.4 | 43.1 | 107.4 |
| Inpatient | |||||
| No. of encounters | 0.2 (0.0-0.8) | 0.0 (0.0-0.0) | 0.5 (0.2-0.8) | 1.2 (0.6-2.0) | 2.6 (1.0-4.8) |
| Expenditure (kUSD) | 2.4 (0.0-15.2) | 0.0 (0.0-0.0) | 9.1 (2.9-14.1) | 28.5 (14.7-39.8) | 80.8 (41.3-136.6) |
| Outpatient | |||||
| No. of encounters | 11.5 (6.0-21.7) | 7.2 (4.2-11.6) | 14.4 (8.6-22.4) | 21.5 (13.8-32.8) | 35.3 (24.0-54.8) |
| Expenditure (kUSD) | 5.0 (2.1-11.5) | 2.5 (1.3-4.3) | 7.5 (4.4-12.4) | 14.6 (7.7-29.8) | 41.5 (20.4-89.6) |
| ED | |||||
| No. of encounters | 0.5 (0.0-1.3) | 0.2 (0.0-0.7) | 0.8 (0.2-1.6) | 1.0 (0.3-2.3) | 1.3 (0.4-3.6) |
| Expenditure (kUSD) | 0.3 (0.0-1.2) | 0.1 (0.0-0.5) | 0.5 (0.0-1.5) | 0.8 (0.0-2.1) | 1.3 (0.1-4.4) |
| Medicare . | |||||
|---|---|---|---|---|---|
| . | Total n = 8600 . | Low-utilizers n = 4300 . | Moderate-utilizers n = 2150 . | High-utilizers n = 1290 . | Super-utilizers n = 860 . |
| Total expenditure (kUSD) | 21.0 (6.8-51.1) | 6.8 (2.7-12.8) | 32.7 (26.5-40.4) | 67.2 (58.9-77.9) | 122.5 (103.8-150.7) |
| Group total (mUSD) | 312.6 | 34.6 | 72.8 | 88.6 | 116.6 |
| Inpatient | |||||
| No. of encounters | 1.4 (0.4-3.4) | 0.5 (0.2-1.0) | 2.5 (1.4-3.6) | 4.4 (2.4-6.4) | 6.8 (4.3-9.9) |
| Expenditure (kUSD) | 12.9 (3.3-34.6) | 3.7 (0.1-8.8) | 23.7 (16.9-31.0) | 49.7 (33.1-60.8) | 94.0 (71.9-118.9) |
| Outpatient | |||||
| No. of encounters | 8.7 (3.8-16.4) | 5.8 (2.6-10.6) | 10.6 (4.9-18.1) | 15.6 (7.8-23.9) | 18.9 (10.5-28.2) |
| Expenditure (kUSD) | 1.8 (0.5-7.7) | 0.8 (0.3-2.1) | 3.1 (0.9-11.0) | 8.7 (2.2-31.1) | 17.9 (3.9-38.4) |
| ED | |||||
| No. of encounters | 1.6 (0.6-4.0) | 1.1 (0.4-2.4) | 1.9 (0.8-4.8) | 3.0 (1.3-7.7) | 4.7 (1.6-14.9) |
| Expenditure (kUSD) | 0.9 (0.3-2.5) | 0.5 (0.1-1.2) | 1.2 (0.4-2.9) | 2.1 (0.8-5.2) | 4.0 (1.3-11.3) |
| Medicare . | |||||
|---|---|---|---|---|---|
| . | Total n = 8600 . | Low-utilizers n = 4300 . | Moderate-utilizers n = 2150 . | High-utilizers n = 1290 . | Super-utilizers n = 860 . |
| Total expenditure (kUSD) | 21.0 (6.8-51.1) | 6.8 (2.7-12.8) | 32.7 (26.5-40.4) | 67.2 (58.9-77.9) | 122.5 (103.8-150.7) |
| Group total (mUSD) | 312.6 | 34.6 | 72.8 | 88.6 | 116.6 |
| Inpatient | |||||
| No. of encounters | 1.4 (0.4-3.4) | 0.5 (0.2-1.0) | 2.5 (1.4-3.6) | 4.4 (2.4-6.4) | 6.8 (4.3-9.9) |
| Expenditure (kUSD) | 12.9 (3.3-34.6) | 3.7 (0.1-8.8) | 23.7 (16.9-31.0) | 49.7 (33.1-60.8) | 94.0 (71.9-118.9) |
| Outpatient | |||||
| No. of encounters | 8.7 (3.8-16.4) | 5.8 (2.6-10.6) | 10.6 (4.9-18.1) | 15.6 (7.8-23.9) | 18.9 (10.5-28.2) |
| Expenditure (kUSD) | 1.8 (0.5-7.7) | 0.8 (0.3-2.1) | 3.1 (0.9-11.0) | 8.7 (2.2-31.1) | 17.9 (3.9-38.4) |
| ED | |||||
| No. of encounters | 1.6 (0.6-4.0) | 1.1 (0.4-2.4) | 1.9 (0.8-4.8) | 3.0 (1.3-7.7) | 4.7 (1.6-14.9) |
| Expenditure (kUSD) | 0.9 (0.3-2.5) | 0.5 (0.1-1.2) | 1.2 (0.4-2.9) | 2.1 (0.8-5.2) | 4.0 (1.3-11.3) |
Low-utilizers: bottom 50% of costs; moderate-utilizers: top 25% to 49.9% of costs; high-utilizers: top 10% to 24.9% of costs; super-utilizers: top 10% of costs.
IQR, interquartile range; kUSD, US dollars in thousands; mUSD, US dollars in millions.
The adjusted RRs of annual health care encounters and expenditures for patients with SCD with commercial insurance or Medicare insurance are presented in Table 3. Total expenditure was 43.46 times higher for super-utilizers than for low-utilizers among commercial-insured patients and 13.37 times higher among Medicare-insured patients after adjusting for participant demographics and clinical characteristics. Similar differences were present in outpatient expenditure (commercial, 25.41; Medicare, 12.30) and inpatient expenditure (commercial, 27.65; Medicare, 12.69). Conversely, differences between ED expenditure for super-utilizers compared with low-utilizers were smaller and more comparable between the 2 populations (commercial, 7.38; Medicare, 8.25). Adjusted RRs comparing low-, moderate-, and high-utilizer groups are presented in supplemental Table 5. Adjusted RRs comparing utilizer groups among Medicare-insured patients based on dual-eligibility status are presented in supplemental Table 6. Differences in encounters and expenditure between super-utilizers and low-utilizers were largely similar between Medicare-insured patients who were dual eligible for Medicaid and those who were not. The most noticeable discrepancies between groups included smaller differences in outpatient expenditure and larger differences in ED encounters and expenditure among dual-eligible patients between super-utilizers and low-utilizers than patients who were not dual eligible.
Adjusted RRs (95% CI) of annual health care encounters and expenditure outcomes between utilizer groups
| Commercial . | |||
|---|---|---|---|
| . | Super-utilizers vs low-utilizers . | Super-utilizers vs moderate-utilizers . | Super-utilizers vs high-utilizers . |
| Total expenditure | 43.46 (41.03-46.03) | 9.92 (10.55-9.33) | 3.79 (4.05-3.54) |
| Inpatient | |||
| No. of encounters | 39.99 (34.81-45.96) | 6.01 (6.58-5.49) | 2.45 (2.67-2.26) |
| Expenditure | 27.65 (25.74-29.71) | 9.86 (10.51-9.25) | 3.68 (3.94-3.44) |
| Outpatient | |||
| No. of encounters | 5.60 (5.29-5.92) | 3.02 (3.21-2.84) | 1.85 (1.98-1.74) |
| Expenditure | 25.41 (23.64-27.30) | 8.76 (9.47-8.10) | 3.75 (4.09-3.45) |
| ED | |||
| No. of encounters | 8.49 (7.63-9.44) | 3.58 (3.99-3.21) | 2.19 (2.46-1.95) |
| Expenditure | 7.38 (6.62-8.23) | 3.66 (4.11-3.27) | 2.15 (2.44-1.90) |
| Commercial . | |||
|---|---|---|---|
| . | Super-utilizers vs low-utilizers . | Super-utilizers vs moderate-utilizers . | Super-utilizers vs high-utilizers . |
| Total expenditure | 43.46 (41.03-46.03) | 9.92 (10.55-9.33) | 3.79 (4.05-3.54) |
| Inpatient | |||
| No. of encounters | 39.99 (34.81-45.96) | 6.01 (6.58-5.49) | 2.45 (2.67-2.26) |
| Expenditure | 27.65 (25.74-29.71) | 9.86 (10.51-9.25) | 3.68 (3.94-3.44) |
| Outpatient | |||
| No. of encounters | 5.60 (5.29-5.92) | 3.02 (3.21-2.84) | 1.85 (1.98-1.74) |
| Expenditure | 25.41 (23.64-27.30) | 8.76 (9.47-8.10) | 3.75 (4.09-3.45) |
| ED | |||
| No. of encounters | 8.49 (7.63-9.44) | 3.58 (3.99-3.21) | 2.19 (2.46-1.95) |
| Expenditure | 7.38 (6.62-8.23) | 3.66 (4.11-3.27) | 2.15 (2.44-1.90) |
| Medicare . | |||
|---|---|---|---|
| . | Super-utilizers vs low-utilizers . | Super-utilizers vs moderate-utilizers . | Super-utilizers vs high-utilizers . |
| Total expenditure | 13.37 (12.68-14.09) | 3.51 (3.71-3.33) | 1.86 (1.97-1.75) |
| Inpatient | |||
| No. of encounters | 8.91 (8.45-9.39) | 2.69 (2.81-2.58) | 1.59 (1.66-1.53) |
| Expenditure | 12.69 (12.04-13.39) | 3.84 (4.05-3.64) | 2.05 (2.17-1.93) |
| Outpatient | |||
| No. of encounters | 2.75 (2.57-2.93) | 1.60 (1.71-1.50) | 1.21 (1.30-1.13) |
| Expenditure | 12.30 (11.14-13.57) | 3.02 (3.34-2.73) | 1.47 (1.63-1.32) |
| ED | |||
| No. of encounters | 7.07 (6.46-7.74) | 3.34 (3.66-3.05) | 1.78 (1.96-1.62) |
| Expenditure | 8.25 (7.55-9.02) | 3.74 (4.10-3.42) | 1.97 (2.17-1.79) |
| Medicare . | |||
|---|---|---|---|
| . | Super-utilizers vs low-utilizers . | Super-utilizers vs moderate-utilizers . | Super-utilizers vs high-utilizers . |
| Total expenditure | 13.37 (12.68-14.09) | 3.51 (3.71-3.33) | 1.86 (1.97-1.75) |
| Inpatient | |||
| No. of encounters | 8.91 (8.45-9.39) | 2.69 (2.81-2.58) | 1.59 (1.66-1.53) |
| Expenditure | 12.69 (12.04-13.39) | 3.84 (4.05-3.64) | 2.05 (2.17-1.93) |
| Outpatient | |||
| No. of encounters | 2.75 (2.57-2.93) | 1.60 (1.71-1.50) | 1.21 (1.30-1.13) |
| Expenditure | 12.30 (11.14-13.57) | 3.02 (3.34-2.73) | 1.47 (1.63-1.32) |
| ED | |||
| No. of encounters | 7.07 (6.46-7.74) | 3.34 (3.66-3.05) | 1.78 (1.96-1.62) |
| Expenditure | 8.25 (7.55-9.02) | 3.74 (4.10-3.42) | 1.97 (2.17-1.79) |
Low-utilizers: bottom 50% of costs; moderate-utilizers: top 25% to 49.9% of costs; high-utilizers: top 10% to 24.9% of costs; super-utilizers: top 10% of costs.
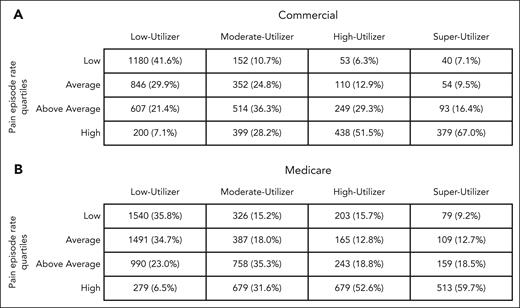
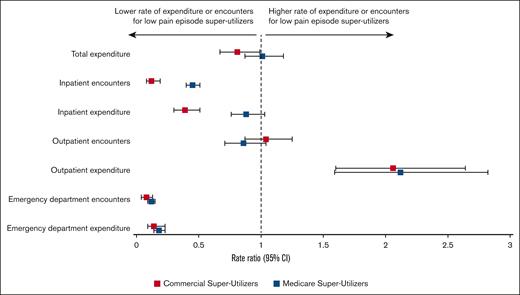
For both commercial- and Medicare-insured patients, a higher percentage of super-utilizers experienced high numbers of pain episode encounters, compared with lower-utilizer groups (Figure 2). Among super-utilizers, RRs comparing annual health care encounters and expenditures between the highest pain episode–encounters group with the lowest pain episode–encounter groups are presented in Figure 3. There was no detectable association between the rate of pain episode encounters and total expenditures among super-utilizers. In the inpatient setting, there was a strong association between the average annual rate of pain episode encounters and the number of inpatient admissions for both commercial- and Medicare-insured super-utilizers. More specifically, commercial-insured super-utilizers with a low annual rate of pain episode encounters had an 88% lower rate of inpatient encounters (RR, 0.12; 95% CI, 0.08-0.19), and those with Medicare had a 55% lower rate of inpatient encounters (RR, 0.45; 95% CI, 0.40-0.51) than super-utilizers with the highest annual rate of pain episode encounters. Furthermore, among commercial-insured super-utilizers, those with a low annual rate of pain episode encounters had a 61% lower rate of inpatient expenditure (RR, 0.39; 95% CI, 0.30-0.51). Similarly, a strong association between average annual pain episode encounters and ED encounters was noted. Specifically, ED encounter rates were 92% lower (RR, 0.08; 95% CI, 0.04-0.13) among commercial-insured super-utilizers with low pain episode encounters and 88% lower (RR, 0.12; 95% CI, 0.09-0.15) among those with Medicare with low pain episode encounters than among those in the respective high pain episode encounter groups. Similar findings were reported for ED expenditure (commercial, 86% lower; RR, 0.14, 95% CI, 0.09-0.23; Medicare, 82% lower; RR, 0.18; 95% CI, 0.14-0.23). Conversely, super-utilizers with a low annual rate of pain episode encounters had higher rates of outpatient expenditure, irrespective of payer, with those with commercial insurance showing a 106% higher expenditure (RR, 2.06; 95% CI, 1.60-2.64) and those with Medicare showing a 112% higher expenditure (RR, 2.12; 95% CI, 1.59-2.82). Comparisons between super-utilizers with average and above-average pain episodes, in comparison with those with high pain episodes, are shown in supplemental Table 7.
Number of patients in each pain episode rate quartile, among each utilizer group for (A) Commerical insurance and (B) Medicare insurance.
Number of patients in each pain episode rate quartile, among each utilizer group for (A) Commerical insurance and (B) Medicare insurance.
Adjusted RRs of annual health care encounters and expenditure outcomes between low vs high pain episode encounter groups among super-utilizers.
Adjusted RRs of annual health care encounters and expenditure outcomes between low vs high pain episode encounter groups among super-utilizers.
Discussion
Health care expenditures are frequently distributed unequally across populations, including adults with SCD.7 This study served to improve our understanding of how health care encounters, expenditures, and pain episode encounters for super-utilizer adults with SCD compared with lower-utilizer adults with SCD across payers. For individuals in our study population, health care expenditures were >43 times higher for super-utilizers than for low-utilizers among those with commercial insurance and >13 times higher among those with Medicare insurance. Encounters were also higher in all settings for super-utilizers than for lower-utilizers, but among super-utilizers, individuals with differing numbers of pain episode encounters had different encounter and expenditure profiles.
The difference in expenditures between super-utilizers and low-utilizers among patients with Medicare (13.37) vs patients with commercial insurance (43.46) may be because patients with Medicare were in worse health or because of the differences in reimbursement policies. The low-utilizer group with Medicare may have higher expenditures owing to having worse health and more comorbidities than the commercial population, because eligibility for Medicare for those aged <65 years requires presence of a disability, minimizing the differences in comparison with the super-utilizer group. Furthermore, the median age of the Medicare-insured patients was higher than that of the commercial-insured patients, which could affect the differences we saw in our results, especially in overall expenditures and encounters. This higher median age in Medicare is likely because of manifestations of SCD that worsen with age,25,26 leading to a disability and allowing for entry into Medicare before the age of 65 years. A second hypothesis to explain the differences in magnitude between insurance groups is that our reported differences reflect Medicare reimbursement policies, which typically involve lower payment rates than commercial insurance.27 Therefore, the super-utilizer Medicare group may not have as high expenditures as the super-utilizer commercial group.
Particularly novel findings of our study were the differences in expenditures in the outpatient, inpatient, and ED settings among super-utilizers with high and low numbers of pain episode encounters. Understanding the differences in health care encounters and expenditures among super-utilizer groups is critical to inform actions to address health care needs and mitigate expenditures among adults with SCD. Super-utilizers with high pain episode encounters had greater expenditure in the inpatient and ED settings than those with low pain episode encounters, as others have shown.5,7 However, our analyses demonstrated that high expenditures were not always attributed to high rates of pain episode encounters. Notably, a super-utilizer subgroup had a below-average number of pain episode encounters yet higher outpatient expenditures. In the absence of these pain episode encounters, their high expenditures were likely due to SCD-related comorbidities and complications, such as heart, lung, and end-stage kidney disease, which can be costly to manage in the outpatient setting. For example, others have documented the high expenditure of patients with SCD with end-organ damage, particularly among those with renal failure or stroke,28-30 including high outpatient expenditures attributed to treatment and supportive care (eg, special education for children experiencing disabilities caused by silent cerebral infarcts).31 Future work to understand how clusters of comorbidities affect the differences in expenditures and encounters between the utilizer groups is of interest. Complimentary to understanding these differences among super-utilizers, there is also the small but interesting subgroup of those who are low-utilizers despite having high rates of pain episodes. Future studies could investigate how pain episodes are managed in this population.
Understanding super-utilizers based on health care encounters and expenditure is especially important when access to new medications and curative therapy is limited32,33 and the cost-effectiveness of these treatments is unknown. Novel therapies such as transplant and gene therapy have the potential to lower expenditures, especially if pursued by those who are super-utilizers with high rates of vaso-occlusive pain episodes, because data suggest these can reduce vaso-occlusive pain episodes that require short-term health care use.29,34,35 However, there are few disease-modifying therapies that have been shown to significantly lower the comorbidity burden of SCD.36 Additional research is needed to examine whether these novel therapies are also able to improve or reverse costly SCD comorbidities. Furthermore, evaluating which interventions may convert super-utilizers to lower-utilizers will improve our understanding of the potential cost savings of these interventions. Targeting super-utilizers, or hot spotting, with other interventions that address social determinants of health, patient engagement, and health literacy could demonstrate dramatic reduction in health care expenditures and significantly improve health outcomes of this subset of the population.37
There are limitations that must be considered when interpreting the results of this study. First, this study used claims data and identified study participants using ICD codes, which may include coding errors in identifying SCD and discriminating between SCD genotypes. However, we used algorithms that have been previously validated to identify individuals with SCD,38,39 and our cohort had costs similar to those reported in other studies.5,40 Second, we excluded patients from our analyses who had less than 12 months of continuous enrollment, which eliminated a significant portion of patients identified with SCD, particularly among the commercially insured. Although there may be some selection bias from requiring 12 months of continuous enrollment, we were evaluating annual expenditures; thus, only those with a complete year of data could be included to ensure that their total costs for the year were not underestimated. This selection criterion is also common among studies analyzing annual expenditures and encounters in this patient population, and the portion of patients excluded because of this criterion is in line with other studies.41 Third, we did not include Medicaid-insured patients, who represent the largest proportion of individuals with SCD,42 experience unique challenges accessing health care, and may have a different profile of individuals with high expenditures. We did, however, include individuals who were dual eligible for Medicare and Medicaid, which accounted for a significant proportion of our Medicare-insured study population. Fourth, our study could not differentiate day-hospital encounters from outpatient, inpatient, or ED encounters. We recognize that day hospitals are a care setting particularly relevant for SCD pain management, but the accuracy of identifying this care setting would require additional information because these data sets do not bill for day hospitals in the same way. These variable billing strategies make it difficult to assess whether the care was delivered in a day-hospital or other setting. Other data sets, such as the Center for Disease Control and Prevention’s SCD Data Collection program or the American Society of Hematology Research Collective Datahub,43,44 may be better suited to understand the use of this care setting in future analyses. Finally, the lack of incorporating societal expenditures, such as absenteeism, job loss, and supportive care needs, was another limitation of our analyses.31,45-47 The annual health care expenditures we reported were high, but the expenditures to society are also likely significant. For example, most Medicare-insured individuals in our study population were aged <65 years, suggesting they experience disabilities that qualify them for Medicare, which could drive expenditure disparities between super-utilizers and lower-utilizers even higher.
We are in a new era of treatment for SCD, with additional options including new medications and curative therapy. Curative therapy is expensive, but it may prove to be economically beneficial and improve quality of life over the lifetime for some individuals with SCD.35 Optimally, everyone with SCD would be cured with no adverse outcomes with these new therapies, but these therapies are unlikely to be available to the entire population in the near future and there remain significant risks with these therapies that may not be acceptable to all individuals. Economic evaluation of new treatment options should consider super-utilizers in their sensitivity analyses to demonstrate differences in potential cost-effectiveness within these subsets of the population while considering health outcomes relevant to both pain episodes and SCD-related comorbidities.
Acknowledgments
The authors are grateful to John Lawrence for his assistance with this study.
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (K23HL141447), and the National Center for Advancing Translational Sciences (UL1TR002733).
Authorship
Contribution: S.R.M., C.C., J.M.H., and R.M.C. designed the research; C.C. and J.M.H. conducted analyses; S.R.M., C.C., J.M.H., and R.M.C. analyzed results; S.R.M., C.C., S.H.O., S.C., C.J.L., J.M.H., and R.M.C. wrote and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert Cronin, Division of General Internal Medicine, The Ohio State University, Suite 2335 Martha Morehouse Medical Plaza 2050 Kenny Rd, Columbus, OH 43221; email: robert.cronin@osumc.edu.
References
Author notes
Data will not be shared or made publicly available as the data sets used for our analyses (Medicare limited data set and International Business Machines Corporation (IBM) Truven MarketScan commercial database) are accessed under data use agreements.
The full-text version of this article contains a data supplement.