TO THE EDITOR:
Survival outcomes are improving rapidly in multiple myeloma (MM). Thus, having overall survival as primary end point in clinical trials became impractical because of the time needed to record putative differences, the large study design to reach statistical significance, and the possible interference of selective access to new drugs at relapse. Accordingly, the recent approval of new drugs into the treatment armamentarium of MM has been typically achieved using progression-free survival (PFS) as the primary end point of clinical trials.
In patients who are newly diagnosed and transplant-eligible (NDTE), median PFS is surpassing 5 years with 3-drug combinations.1-4 With quadruplets, PFS rates at 3 years are >80%.5,6 Unprecedented PFS has also been observed in newly diagnosed patients who are transplant-ineligible (NDTI)7-9 and with relapsed/refractory MM (RRMM).10-16 That notwithstanding, some patients have tumors that are primarily resistant to therapy, and disease relapse remains frequent in those who respond. Novel regimens are therefore needed to increase cure rates, but new studies will not have a (positive or negative) readout for many years if alternative end points to PFS are not identified.
A surrogate end point is defined as a proxy outcome that can substitute for and predict a final patient-relevant outcome.17 One such candidate to predict improved survival outcomes could be the depth of response achieved in different treatment arms. Assessment of measurable residual disease (MRD) is one of the most relevant prognostic factors and outperforms conventional methods of response assessment in MM.18 However, the role of MRD as a surrogate of treatment efficacy for potential use as an end point in clinical trials, remains uncertain.19,20
In this study, we sought to analyze the correlation between MRD-negative rates defined according to the International Myeloma Working Group response criteria21 and PFS benefit in randomized clinical trials across the spectrum of MM treatment. For this purpose, we searched for studies describing PFS and MRD data in >50 patients in each treatment arm, using next-generation flow cytometry (NGF) or next-generation sequencing (NGS) methods with a reported minimum sensitivity of 10−5, in Medline articles and abstracts published in the meetings of the American Society of Clinical Oncology (ASCO), American Society of Hematology (ASH), European Hematology Association (EHA) and the International Myeloma Society (IMS), since 2016 to December 2022. The number of arms was not a criterion. Two independent investigators selected the articles and abstracts for potential inclusion; disagreements were resolved by a third investigator. The only notable exclusion among randomized clinical trials reporting PFS and MRD data was ICARIA (NCT02990338), because only 18 samples from 16 patients out of the 307 enrolled were analyzed for MRD.14 MRD-negative rates were calculated for the intent-to-treat population, regardless of the depth of response. Patients with missing data were considered as MRD positive. The most recent updated analysis of each study was used for the collection of PFS and MRD data.
The strength of the association between the treatment effect on PFS and the treatment effect on MRD-negative rates was quantified with the trial-level coefficient of determination (R2trial) and the 95% confidence interval (CI). The weighted least square regression method was performed. The analyses were weighted by the trial sample size. The criteria for R2trial interpretation were set a priori as poor (R2trial<0.4), moderate (R2trial≥0.4 and R2trial≤0.8), and good to excellent correlation (R2trial>0.8). A P value <.05 was considered statistically significant. Statistical analyses were performed using R 4.2.2 (R Core Team [2022]; R: a language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria) and Stata version 14 (StataCorp LP, 2015, Stata Statistical Software: release 14, College Station, TX).
Fifteen clinical trials were identified, 6 in NDTE, 3 in NDTI, and 6 in RRMM (Table 1). All studies except GRIFFIN5 were phase 3, and all except the GRIFFIN5 and CASSIOPEIA6 used PFS as the primary end point (supplemental Table 1). MRD was assessed using NGF and NGS in 4 and 11 trials, respectively (Table 1). The primary end point was met in 12 of the 15 studies, and 8 new drugs or regimens were approved for the treatment of patients with MM based on the positive results of these trials (supplemental Table 1).
Randomized clinical trial describing PFS and MRD data in >50 patients, using NGF and sequencing methods with a reported minimum sensitivity of 10−5, in Medline articles and ASCO/ASH/EHA/IMS abstracts published until 2 December 2022.
| Clinical trial (phase) . | NCT . | Disease setting . | Randomization . | MRD method . | MRD-ve rates (%) . | Median PFS (mo) . | Reference . |
|---|---|---|---|---|---|---|---|
| ATLAS (3) | NCT02659293 | NDTE | KRD vs R alone | NGS | 53% vs 31% | 59.1 vs 41.4 | 1 |
| CASSIOPEIA (3) | NCT02541383 | NDTE | DaraVTD vs VTD | NGF | 64% vs 43.5% | NR vs 51.5 | 6 |
| DETERMINATION (3) | NCT01208662 | NDTE | VRD-ASCT vs VRD alone | NGS | 13% vs 12% | 67.5 vs 46.2 | 2 |
| GEM2012MENOS65 (3) | NCT01916252 | NDTE | VRD-BuMel vs VRD-Mel | NGF | 58% vs 55.5% | NR vs 75.3 | 4 |
| GRIFFIN (2) | NCT02874742 | NDTE | DaraVRD vs VRD | NGS | 64% vs 30% | NR vs NR | 5 |
| IFM 2009 (3) | NCT01191060 | NDTE | VRD-ASCT vs VRD alone | NGS | 21% vs 15% | 50.0 vs 36.0 | 3 |
| ALCYONE (3) | NCT02195479 | NDTI | DaraVMP vs VMP | NGS | 28% vs 7% | 36.4 vs 19.3 | 8 |
| CLARION (3) | NCT01818752 | NDTI | KMP vs VMP | NGF | 5% vs 5% | 22.3 vs 22.1 | 9 |
| MAIA (3) | NCT02252172 | NDTI | DaraRD vs RD | NGS | 31% vs 10% | 62.0 vs 34.3 | 7 |
| APOLLO (3) | NCT03180736 | RRMM | DaraPD vs PD | NGS | 9% vs 2% | 12.4 vs 6.9 | 15 |
| BOSTON (3) | NCT03110562 | RRMM | SelVD vs VD | NGF | 5% vs 4% | 13.9 vs 9.5 | 16 |
| CANDOR (3) | NCT03158688 | RRMM | DaraKD vs KD | NGS | 18% vs 4% | 28.6 vs 15.2 | 12 |
| CASTOR(3) | NCT02136134 | RRMM | DaraVD vs VD | NGS | 15% vs 2% | 16.7 vs 7.1 | 11 |
| IKEMA (3) | NCT03275285 | RRMM | IsaKD vs KD | NGS | 30% vs 13% | NR vs 19.2 | 13 |
| POLLUX (3) | NCT02076009 | RRMM | DaraRD vs RD | NGS | 33% vs 17% | 45.0 vs 17.5 | 10 |
| Clinical trial (phase) . | NCT . | Disease setting . | Randomization . | MRD method . | MRD-ve rates (%) . | Median PFS (mo) . | Reference . |
|---|---|---|---|---|---|---|---|
| ATLAS (3) | NCT02659293 | NDTE | KRD vs R alone | NGS | 53% vs 31% | 59.1 vs 41.4 | 1 |
| CASSIOPEIA (3) | NCT02541383 | NDTE | DaraVTD vs VTD | NGF | 64% vs 43.5% | NR vs 51.5 | 6 |
| DETERMINATION (3) | NCT01208662 | NDTE | VRD-ASCT vs VRD alone | NGS | 13% vs 12% | 67.5 vs 46.2 | 2 |
| GEM2012MENOS65 (3) | NCT01916252 | NDTE | VRD-BuMel vs VRD-Mel | NGF | 58% vs 55.5% | NR vs 75.3 | 4 |
| GRIFFIN (2) | NCT02874742 | NDTE | DaraVRD vs VRD | NGS | 64% vs 30% | NR vs NR | 5 |
| IFM 2009 (3) | NCT01191060 | NDTE | VRD-ASCT vs VRD alone | NGS | 21% vs 15% | 50.0 vs 36.0 | 3 |
| ALCYONE (3) | NCT02195479 | NDTI | DaraVMP vs VMP | NGS | 28% vs 7% | 36.4 vs 19.3 | 8 |
| CLARION (3) | NCT01818752 | NDTI | KMP vs VMP | NGF | 5% vs 5% | 22.3 vs 22.1 | 9 |
| MAIA (3) | NCT02252172 | NDTI | DaraRD vs RD | NGS | 31% vs 10% | 62.0 vs 34.3 | 7 |
| APOLLO (3) | NCT03180736 | RRMM | DaraPD vs PD | NGS | 9% vs 2% | 12.4 vs 6.9 | 15 |
| BOSTON (3) | NCT03110562 | RRMM | SelVD vs VD | NGF | 5% vs 4% | 13.9 vs 9.5 | 16 |
| CANDOR (3) | NCT03158688 | RRMM | DaraKD vs KD | NGS | 18% vs 4% | 28.6 vs 15.2 | 12 |
| CASTOR(3) | NCT02136134 | RRMM | DaraVD vs VD | NGS | 15% vs 2% | 16.7 vs 7.1 | 11 |
| IKEMA (3) | NCT03275285 | RRMM | IsaKD vs KD | NGS | 30% vs 13% | NR vs 19.2 | 13 |
| POLLUX (3) | NCT02076009 | RRMM | DaraRD vs RD | NGS | 33% vs 17% | 45.0 vs 17.5 | 10 |
DaraKD, daratumumab-carfilzomib-dexamethasone; DaraPD, daratumumab-pomalidomide-dexamethasone; DaraRD, daratumumab-lenalidomide-dexamethasone; DaraVD, daratumumab-bortezomib-dexamethasone; DaraVMP, daratumumab-bortezomib-melphalan-prednisone; DaraVRD, daratumumab-bortezomib-lenalidomide-dexamethasone; DaraVTD, daratumumab-bortezomib-thalidomide-dexamethasone; IsaKD, isatuximab-carfilzomib-dexamethasone; KD, carfilzomib-dexamethasone; KRD12, carfilzomib-lenalidomide-dexamethasone (12 cycles); NCT, National Clinical Trial (Clinicaltrials.gov Identification Number); NGF, next-generation flow; NGS, next-generation sequencing; NR, not reached; PD, pomalidomide-dexamethasone; RD, lenalidomide-dexamethasone; SelVD, selinexor-bortezomib-dexamethasone; VD, bortezomib-dexamethasone; VMP, bortezomib-melphalan-prednisone; VRD, bortezomib-lenalidomide-dexamethasone; VRd-BuMel, bortezomib-lenalidomide-dexamethasone-busulfan-melphalan; VRd-Mel, bortezomib-lenalidomide-dexamethasone-melphalan; VTD, bortezomib-thalidomide-dexamethasone.
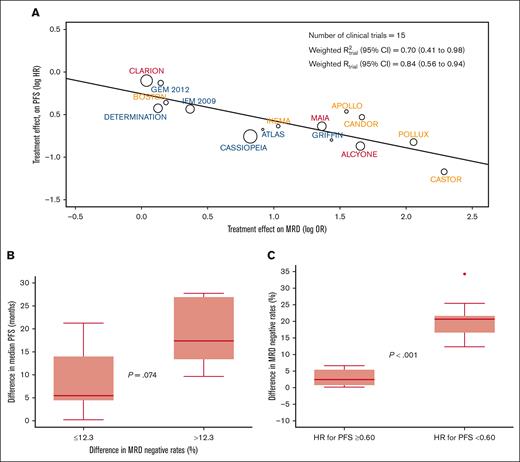
The weighted trial-level R2 (95% CI) observed in the aggregated analysis of the 15 clinical trials was 0.70 (95% CI, 0.41-0.98) (Figure 1A). Sensitivity analyses using a leave-one-out approach showed consistent R2trial levels ranging from 0.62 to 0.78 (supplemental Figure 1). Thus, MRD-negative rates in MM randomized clinical trials are moderately correlated with a treatment effect in PFS. The R2trial observed in this study is inferior to the 0.97 reported in a previous study using data from 6 trials19 and slightly superior to the 0.62 of a recent meta-analysis of 13 trials,20 some of which using MRD methods other than NGF and NGS. Thus, the improved sensitivity and specificity of next-generation methods may be paramount for a more accurate prediction of treatment effect based on MRD-negative rates.
Treatment effect in PFS according to MRD-negative rates. (A) The weighted trial-level R2 (95% CI) observed in the aggregated analysis of the 15 clinical trials. PFS hazard ratio (HR) and MRD odds ratio (OR) values were natural log transformed. Circle size is proportional to the number of patients included in the study. (B) Clinical trials stratified according to the median difference of MRD-negative rate differences observed between experimental vs control arms, and corresponding median (p25-p75) difference in median PFS. Trials with differences in MRD-negative rates <12.3%: APOLLO, BOSTON, CLARION, DETERMINATION, and IFM 2009. Trials with differences in MRD-negative rates ≥12.3%: ALCYONE, ATLAS, CANDOR, CASTOR, MAIA, and POLLUX. P value for the comparison of median PFS improvement between trials with differences in MRD-negative rates <12.3% vs ≥12.3%: 0.074. The median PFS was not available for 1 or more intervention groups of the following trials: CASSIOPEIA, GEM2012MENOS65, GRIFFIN, and IKEMA; therefore, these trials were not included in this analysis. (C) Difference in MRD-negative rates between experimental vs control arms in clinical trials demonstrating with an HR lower vs equal or greater than 0.60 (supplemental Table 3). Trials with a HR ≥0.60: APOLLO, BOSTON, CLARION, DETERMINATION, GEM2012MENOS65, and IFM 2009. Trials with a HR <0.60: ALCYONE, CANDOR, CASSIOPEIA, CASTOR, GRIFFIN, IKEMA, MAIA and POLLUX.
Treatment effect in PFS according to MRD-negative rates. (A) The weighted trial-level R2 (95% CI) observed in the aggregated analysis of the 15 clinical trials. PFS hazard ratio (HR) and MRD odds ratio (OR) values were natural log transformed. Circle size is proportional to the number of patients included in the study. (B) Clinical trials stratified according to the median difference of MRD-negative rate differences observed between experimental vs control arms, and corresponding median (p25-p75) difference in median PFS. Trials with differences in MRD-negative rates <12.3%: APOLLO, BOSTON, CLARION, DETERMINATION, and IFM 2009. Trials with differences in MRD-negative rates ≥12.3%: ALCYONE, ATLAS, CANDOR, CASTOR, MAIA, and POLLUX. P value for the comparison of median PFS improvement between trials with differences in MRD-negative rates <12.3% vs ≥12.3%: 0.074. The median PFS was not available for 1 or more intervention groups of the following trials: CASSIOPEIA, GEM2012MENOS65, GRIFFIN, and IKEMA; therefore, these trials were not included in this analysis. (C) Difference in MRD-negative rates between experimental vs control arms in clinical trials demonstrating with an HR lower vs equal or greater than 0.60 (supplemental Table 3). Trials with a HR ≥0.60: APOLLO, BOSTON, CLARION, DETERMINATION, GEM2012MENOS65, and IFM 2009. Trials with a HR <0.60: ALCYONE, CANDOR, CASSIOPEIA, CASTOR, GRIFFIN, IKEMA, MAIA and POLLUX.
Subanalysis according to the inclusion of an autologous stem cell transplantation in clinical trials showed a similar weighted R2trial in NDTE (0.74; 95% CI, 0.24-1.00) and NDTI/RRMM (0.83; 95% CI, 0.57-1.00) (supplemental Figure 2). The estimates of R2trial in sensitivity analyses using a leave-one-out approach ranged from 0.66 to 0.83 in NDTE and from 0.62 to 0.88 in NDTI/RRMM. Additional subanalysis showed similar weighted R2trial in clinical trials enrolling patients with NDMM (0.75) and RRMM (0.63) (supplemental Figure 3), as well as in those using NGF (0.97) and NGS (0.65) for MRD assessment (supplemental Figure 4). Although preliminary because of the reduced number of trials in each subanalysis, these findings suggest that similar to its prognostic value,18 the role of MRD as a surrogate end point could be explored in all treatment scenarios.
Beyond the moderate weighted R2trial observed in this study, it would be valuable to have an estimation of the magnitude of differences in MRD negativity that is needed for the prediction of improved PFS. The median difference in MRD-negative rates between 2-arm comparisons in the 11 clinical trials in which the median PFS was reached in the 2 arms (Table 1), was 12.3%. Interestingly, the difference in median PFS between 2 arms in trials in which the MRD-negative rates were equal or less vs greater than 12.3%, was of 5.5 vs 17 months (P = .07), respectively (Figure 1B). Next, we compared the difference in MRD-negative rates between experimental and control arms in clinical trials demonstrating a reduction in the risk of progression and/or death >40% vs those who did not show any reduction (supplemental Table 4). The median (p25-p75) difference in MRD-negative rates was significantly higher in clinical trials with a hazard ratio <0.60 vs ≥0.60; 21% (17%-22%) vs 2.5% (0.75%-5.4%), respectively (P < .001). Interestingly, there was nearly no overlap between p25 and p75 difference in MRD-negative rates observed in the former vs the later studies (Figure 1C). Taking together the 2 analysis, it appears that a cutoff of ∼10% difference in MRD-negative rates between treatment arms may predict improved PFS (supplemental Table 3).
This analysis has limitations which, to some extent, are a consequence of the challenges that current MRD datasets have for surrogacy analysis in MM. First, information about how many patients MRD was evaluated is missing in some studies; therefore, the protection of the randomization allocation of treatments on the treatment effect estimated on MRD may not be systematically guaranteed. Second, there is considerable heterogeneity in disease stage, therapies, time points and patient selection for MRD assessment. Thus, the ongoing meta-analyses based on individual patient-level data are mandatory to determine if MRD is a statistically robust surrogate of treatment efficacy, defined according to PFS benefit (ie, i2TEAMM). Meanwhile, this study suggests moderate value and could provide guidance on the MRD methods that should be used in the patient-level analysis, as well as the definition of MRD end points in MM. Because early tumor–based end points are heavily weighted to assess efficacy, and the impacts of late toxicity and other postprogression events are not captured by MRD assessment,22 the i2TEAMM should also investigate the impact of treatment effect on MRD and its relationship with patients’ quality of life and overall survival.
Acknowledgments: This study was supported by grants from the Centro de Investigación Biomédica en Red – Área de Oncología del Instituto de Salud Carlos III (CIBERONC; CB16/12/00369); Instituto de Salud Carlos III/Subdirección General de Investigación Sanitaria (FIS number PI19/01451); the Cancer Research UK (C355/A26819), Fundación Científica Asociación Española Contra el Cáncer (FCAECC) and Associazione Italiana per la Ricerca sul Cancro (AIRC) under the Accelerator Award Program EDITOR; the CRIS Cancer Foundation (PR_EX_2020-02), the Black Swan Research Initiative of the International Myeloma Foundation; and the Riney Family Multiple Myeloma Research Program Fund.
Contribution: B.P., A.Z., J.M.N-C., P.R.-O., Q.S., N.C.M., B.G.M.D., and J.S.-M. contributed to the conception and design, collection and assembly of data, data analysis and interpretation; and all authors drafted and reviewed the manuscript, approved the final version for submission, and vouch for data accuracy and completeness.
Conflict-of-interest disclosure: B.P. reports honoraria for lectures from and membership on advisory boards with Adaptive, Amgen, Bristol Myers Squibb (BMS)-Celgene, Gilead, GlaxoSmithKline (GSK), Janssen, Oncopeptides, Roche, Sanofi and Takeda; unrestricted grants from BMS-Celgene, EngMab, GSK, Roche, Sanofi, and Takeda; and consultancy for BMS-Celgene, Janssen and Sanofi. P.R.-O. declares honoraria for lectures from and membership on advisory boards with Amgen, Sanofi, GSK, Janssen, BMS-Celgene, Regeneron and Pfizer. Q.S. reports consulting/advisory role from Yiviva Inc, Boehringer Ingelheim Pharmaceuticals, Inc, Regeneron Pharmaceuticals, Inc, Hoosier Cancer Research Network, Kronos Bio, and Mirati Therapeutics Inc; honorarium/speaker role from Chugai Pharmaceutical Co, Ltd (to self); and research funds from Celgene/BMS, Roche/Genentech, Janssen, Novartis (to institution). N.C.M. reports consultancy or advisory role with Adaptive, Amgen, BMS, Celgene, Janssen, Novartis, Pfizer, Legend and Takeda; advisory board activity with honoraria or consultation fees from Takeda; and stock for Oncopep and DCT. B.G.M.D. reports receiving consultancy from Amgen, BMS, Janssen, and Takeda. J.S.-M. reports consulting or advisory role with Amgen, Celgene, Takeda, BMS, Merck, Novartis, Sanofi, Janssen, Roche, AbbVie, GSK, Karyopharm Therapeutics, Secura Bio, Regeneron, and HaemaLogiX. The remaining authors declare no competing interests.
Correspondence: Bruno Paiva, Clinica Universidad de Navarra, Centro de Investigacion Medica Aplicada, Instituto de Investigacion Sanitaria de Navarra, Centro de Investigación Biomédica en Red de Oncología CB16/12/00369, Pamplona 31008, Spain; email: bpaiva@unav.es.
References
Author notes
∗B.P. and A.Z. have contributed equally to this study and are first authors.
Data will be shared upon reasonable request from the corresponding author, Bruno Paiva (bpaiva@unav.es).
The full-text version of this article contains a data supplement.