Key Points
FLIPI remains a prognostic index with higher discrimination for survival in patients with advanced FL treated with immunochemotherapy.
Research on FL should incorporate more precise molecular markers for both outcome prediction and optimal selection of treatment.
Abstract
Several clinical risk models have been proposed to predict the outcome of follicular lymphoma (FL). The development of next-generation sequencing technologies has allowed the integration of somatic gene mutations into clinical scores to build genotyped-based risk models, such as the m7–Follicular Lymphoma International Prognostic Index (FLIPI). We explored 4 clinical or clinicogenetic-risk models in patients with symptomatic FL who received frontline immunochemotherapy. Of 191 patients with FL grades 1 to 3a, 109 were successfully genotyped. The treatment consisted of rituximab (R) plus cyclophosphamide, vincristine, and prednisone (R-CVP)/cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) (72.5%) or R-bendamustine (R-B) (27.5%). The proportion of cases classified as high risk for FLIPI, FLIPI-2, PRIMA–prognostic index, or m7-FLIPI were 39.3%, 14%, 30.3%, and 22%, respectively. No case with low-intermediate FLIPI was upgraded in the m7-FLIPI, but 18 of the 42 high-risk patients with FLIPI were downgraded to low-risk m7-FLIPI. The sensitivity and specificity for the prediction of POD24 were highest for FLIPI. The discrimination between progression-free survival (PFS) and overall survival (OS) was the best for FLIPI (c-index: 0.644 and 0.727, respectively). When analyzed only in patients treated with R-B, m7-FLIPI showed a higher discrimination between PFS and OS. Thus, the FLIPI remains the clinical risk score with higher discrimination in patients with advanced FL treated with immunochemotherapy; however, the performance of the m7-FLIPI should be further investigated in patients treated with R-B.
Introduction
Follicular lymphoma (FL) is the second most common subtype of non-Hodgkin lymphoma in Western countries. Its clinical behavior is usually indolent with a median survival currently >15 years.1,2 However, even with modern immunochemotherapy schedules, up to 20% of patients progress or relapse within the first 2 years, and these patients have a significantly decreased overall survival (OS) rate.3-5
Several clinical risk models have been developed to predict progression-free survival (PFS) and OS, such as FLIPI,6 FLIPI-2,7 and PRIMA-PI.8 The development of next-generation sequencing (NGS) technologies has enabled the integration of somatic gene mutation information in clinical risk models to build clinicogenetic-risk models, such as m7-FLIPI and progression of disease within 24 months (POD24) prognostic index (PI).4,9 These risk models have improved pretreatment risk stratification before the initiation of frontline treatment and can also identify a high-risk group of patients who have an increased risk of developing POD24.
These studies included patients with advanced FL who were mainly treated with frontline rituximab (R), cyclophosphamide, vincristine, and prednisone (R-CVP), or rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). Rituximab and bendamustine (R-B) have become the preferred first-line therapy for symptomatic patients with FL at many centers,10 but patients treated with R-B were not included in these models. Moreover, these clinicogenetic-risk models should be validated by using real-world data.
In this study, we evaluated 4 clinical or clinicogenetic-risk models to determine their utility in previously untreated patients with symptomatic FL who were treated with R-CVP, R-CHOP, or R-B.
Methods
A cohort of 191 patients with newly diagnosed FL grades 1 to 3a from 3 Spanish tertiary hospitals was reviewed in this study, with a final number of 109 cases with successful characterization by NGS (supplemental Table 1). The diagnosis of FL was based on the World Health Organization criteria. Patients with FL grade 3b were excluded from this study. All patients fulfilled the Groupe d’Etude des Lymphomes Folliculaires high tumor burden criteria. Patients with HIV were excluded from this study. Treatment consisted of R-CVP, R-CHOP, or R-B according to standard practice. In responding patients, maintenance with rituximab for 2 years was performed according to physician or patient preference. The lymphoma response was assessed by the Lugano classification.11,12 The study was carried out in accordance with the modified Declaration of Helsinki and was approved by the ethics committees of the participating centers. Written informed consent was obtained from all patients.
Tumoral FL mutational analysis was performed on formalin-fixed, paraffin-embedded tissue by NGS through a DNA-targeted custom panel of 64 FL-related genes implicated in epigenetics, B-cell receptor signaling, cell survival, immune response, mTORC1 pathway, and cell migration, as previously reported (supplemental Methods; supplemental Table 2).13 The FLIPI, FLIPI-2, PRIMA-PI, and m7-FLIPI were calculated as previously described.6-9 The same cutoff as used in the original publication was applied for discrimination between the high- and low-risk groups (m7-FLIPI score ≥0.8 and <0.8, respectively).9
Statistical analyses were carried out with the statistical software R (version 4.1.0) (RStudio version 1.4.1106) using the packages survival 3.2 to 13 and ggplot2_3.3.5. In this study, PFS was calculated from the date of treatment initiation to the date of death from any cause, disease relapse or progression, or date of last contact. OS was calculated from the date of treatment initiation to the date of death from any cause or the date of last contact. POD24 was defined as progression or relapse of the disease within the first 24 months after first-line treatment initiation (modified definition).3 OS for POD24 was calculated considering survival from time of POD for the POD24 cohort, or 2 years after initial treatment of patients without POD24, according to recently proposed definitions.4 The Kaplan-Meier method was used to describe time-to-event end points, and the differences between the 2 groups were compared using the log-rank test. The differences were considered statistically significant at P < .05. The c-index was calculated using the R package survcomp 1.44.1, Akaike information criterion (AIC) with the R package flexsurv 2.1 and Gönen-Heller concordance probability estimate (CPE) with the R package CPE1.5.2. The sensitivity, specificity, and accuracy of the risk scores for time-to-event end points were estimated using the R package confusionMatrix 3.45, and circular plots were constructed using the R package circlize 0.4.14.
Results
The clinical characteristics of 109 patients are shown in Table 1. The median age at diagnosis was 58 years (range, 24-90), 56% were male, and 93.6% had stage III-IV. The median time from biopsy to initial treatment was 0.91 years (IQR, 0.35-1.7). The immunochemotherapy regimens were as follows: R-CVP in 8 patients (7.3%), R-CHOP in 71 patients (65.1%), and R-B in 30 patients (27.5%). Maintenance rituximab was administered to 95 patients (87.2%), 66 (83.5%) with R-CHOP/CVP, and 29 (96.7%) with R-B. With a median follow-up of 8 years (IQR, 5.25-11.83), PFS and OS at 8 years were 55% and 80%, respectively (47 progression events and 23 death events [10 were lymphoma related]).
Demographics and clinical characteristics of patients (n = 109)
| . | N (%) . |
|---|---|
| Follow-up duration median (IQR), mo | 96 (63-142) |
| Time from biopsy to initial treatment median (IQR), mo | 0.91 (0.35-1.68) |
| Sex | |
| Male | 61 (56.0) |
| Female | 48 (44.0) |
| Age, y | |
| ≤60 | 57 (53.3) |
| >60 | 50 (46.7) |
| Ann Arbor stage | |
| I-II | 7 (6.4) |
| III-IV | 102 (93.6) |
| B symptoms present | |
| No | 75 (68.8) |
| Yes | 34 (31.2) |
| Eastern Cooperative Oncology Group performance status | |
| 0-1 | 102 (93.6) |
| 2-4 | 7 (6.4) |
| Hemoglobin <12 g/dL | |
| No | 84 (77.1) |
| Yes | 25 (22.9) |
| Increased lactate dehydrogenase | |
| No | 87 (79.8) |
| Yes | 19 (17.4) |
| Not known | 3 (2.7) |
| Increased B2-microglobulin | |
| No | 65 (60.6) |
| Yes | 35 (32.1) |
| Not known | 8 (7.3) |
| Involved node >6 cm | |
| No | 82 (75.2) |
| Yes | 27 (24.8) |
| Bone marrow involvement | |
| No | 46 (42.2) |
| Yes | 56 (51.4) |
| Not known | 7 (6.4) |
| FLIPI score | |
| 0-1 | 18 (16.8) |
| 2 | 43 (40.2) |
| 3-5 | 42 (39.3) |
| Not known | 4 (3.7) |
| FLIPI-2 score | |
| 0 | 12 (11.0) |
| 1-2 | 49 (45.0) |
| 3-5 | 40 (37.7) |
| Not known | 8 (7.3) |
| PRIMA-PI score | |
| Low | 37 (33.9) |
| Intermediate | 29 (26.6) |
| High | 33 (30.3) |
| Not known | 10 (9.2) |
| m7-FLIPIscore | |
| Low | 81 (74.3) |
| High | 24 (22.0) |
| Not known | 4 (3.7) |
| Histological grade | |
| 1-2 | 57 (52.3) |
| 3a | 34 (31.2) |
| Not specified | 18 (16.5) |
| Induction regimen | |
| R-CHOP | 71 (65.1) |
| R-CVP | 8 (7.3) |
| R-B | 30 (27.5) |
| Maintenance regimen | |
| No | 14 (12.8) |
| Yes | 95 (87.2) |
| . | N (%) . |
|---|---|
| Follow-up duration median (IQR), mo | 96 (63-142) |
| Time from biopsy to initial treatment median (IQR), mo | 0.91 (0.35-1.68) |
| Sex | |
| Male | 61 (56.0) |
| Female | 48 (44.0) |
| Age, y | |
| ≤60 | 57 (53.3) |
| >60 | 50 (46.7) |
| Ann Arbor stage | |
| I-II | 7 (6.4) |
| III-IV | 102 (93.6) |
| B symptoms present | |
| No | 75 (68.8) |
| Yes | 34 (31.2) |
| Eastern Cooperative Oncology Group performance status | |
| 0-1 | 102 (93.6) |
| 2-4 | 7 (6.4) |
| Hemoglobin <12 g/dL | |
| No | 84 (77.1) |
| Yes | 25 (22.9) |
| Increased lactate dehydrogenase | |
| No | 87 (79.8) |
| Yes | 19 (17.4) |
| Not known | 3 (2.7) |
| Increased B2-microglobulin | |
| No | 65 (60.6) |
| Yes | 35 (32.1) |
| Not known | 8 (7.3) |
| Involved node >6 cm | |
| No | 82 (75.2) |
| Yes | 27 (24.8) |
| Bone marrow involvement | |
| No | 46 (42.2) |
| Yes | 56 (51.4) |
| Not known | 7 (6.4) |
| FLIPI score | |
| 0-1 | 18 (16.8) |
| 2 | 43 (40.2) |
| 3-5 | 42 (39.3) |
| Not known | 4 (3.7) |
| FLIPI-2 score | |
| 0 | 12 (11.0) |
| 1-2 | 49 (45.0) |
| 3-5 | 40 (37.7) |
| Not known | 8 (7.3) |
| PRIMA-PI score | |
| Low | 37 (33.9) |
| Intermediate | 29 (26.6) |
| High | 33 (30.3) |
| Not known | 10 (9.2) |
| m7-FLIPIscore | |
| Low | 81 (74.3) |
| High | 24 (22.0) |
| Not known | 4 (3.7) |
| Histological grade | |
| 1-2 | 57 (52.3) |
| 3a | 34 (31.2) |
| Not specified | 18 (16.5) |
| Induction regimen | |
| R-CHOP | 71 (65.1) |
| R-CVP | 8 (7.3) |
| R-B | 30 (27.5) |
| Maintenance regimen | |
| No | 14 (12.8) |
| Yes | 95 (87.2) |
Data are median (IQR) or n/N (%). Percentages might not add up to 100% because of rounding.
IQR, interquartile range.
All patients presented mutations, with a median of 6 mutations (range, 2-23) per patient. The mutation frequency distribution is shown in supplemental Figure 1. The frequency of mutated genes included in the m7-FLIPI score were EZH2 (23%), ARID1A (17%), MEF2B (19%), EP300 (19%), FOXO1 (10%), CREBBP (75%), and CARD11 (17%).
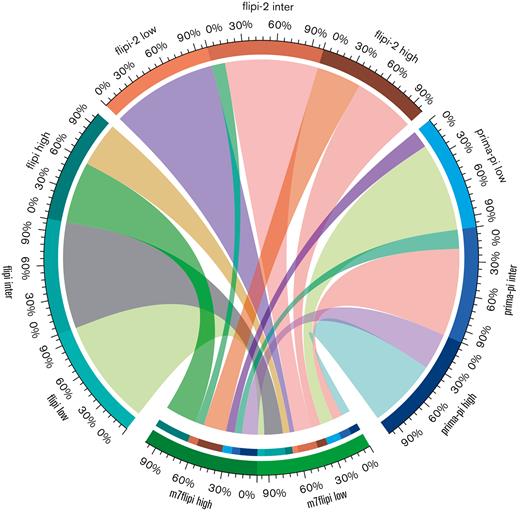
The proportion of patients according to the 4 risk scores is shown in Table 1. Risk stratification could not be calculated in 4, 8, 10, and 4 patients for the FLIPI, FLIPI-2, PRIMA-PI, and m7-FLIPI scores, respectively. In the distribution of patients among risk categories in the 4 risk scores, it is noteworthy that the proportion of cases classified as low risk in the FLIPI was only 16.8%, and, conversely, the proportion of cases allocated to the high-risk group in the FLIPI-2 was only 14%. No patient with a low-intermediate FLIPI score was upgraded in the m7-FLIPI. However, 18 of the 42 high-risk patients with FLIPI were downgraded to low-risk m7-FLIPI. The risk categories of FLIPI, FLIPI-2, and PRIMA-PI compared with m7-FLIPI are shown in Figure 1.
Risk categories among the clinical scores FLIPI, FLIPI-2, and PRIMA-PI compared with the clinicogenetic score m7-FLIPI. Note that FLIPI, FLIPI-2, and PRIMA-PI have 3 categories and m7-FLIPI has only 2 risk categories. Patients are given in percentages. Inter: intermediate.
Risk categories among the clinical scores FLIPI, FLIPI-2, and PRIMA-PI compared with the clinicogenetic score m7-FLIPI. Note that FLIPI, FLIPI-2, and PRIMA-PI have 3 categories and m7-FLIPI has only 2 risk categories. Patients are given in percentages. Inter: intermediate.
Response and early relapse
The rate of complete remission was 84.3% for the whole series and 85.9% and 80.0% for patients treated with R-CVP/R-CHOP and R-B, respectively (P = .556). A 77-year-old patient was not evaluated for early death due to myocardial infarction after 2 cycles of R-CHOP. The FLIPI was the only score that showed statistically significant differences in complete remission rates between the risk categories (Table 2; supplemental Table 3). Twenty-two patients (20.4%) experienced an early relapse and were considered as POD24. The FLIPI showed statistically significant differences, with patients in the high-risk category having a higher proportion of POD24 (34.1%) (supplemental Table 3). However, patients within the low- or intermediate-risk subsets of the FLIPI had a similar proportion of POD24 (11.1% and 13.1%, respectively). The PRIMA-PI was also significant, but identified patients in the intermediate category as those at the highest risk of POD24 (34.5%) (Table 2). POD24 in this cohort of patients was predictive of a shorter OS (P < .0001) (supplemental Figure 2).
Outcomes according to the 4 risk scores
| . | Complete remission . | POD24 . | PFS at 6 y . | OS at 6 y . | ||||
|---|---|---|---|---|---|---|---|---|
| n/total (%) . | P . | n/total (%) . | P . | % (95% confidence interval) . | P . | % (95% confidence interval) . | P . | |
| 91/108 (84.5) . | NA . | 22/108 (20.4) . | NA . | 60.5 (51.6-70.9) . | NA . | 88.2 (82.0-94.7) . | NA . | |
| FLIPI score | 0.029 | 0.013 | 0.00072 | 0.00057 | ||||
| 0-2 | 57/63 (90.5) | 8/63 (12.7) | 71.9 (60.7-85.1) | 100 | ||||
| 3-5 | 30/41 (73.2) | 14/41 (34.1) | 39.3 (26.8-57.7) | 70.6 (57.9-86.2) | ||||
| FLIPI-2 score | 0.591 | 1 | 0.35 | 0.046 | ||||
| 0-2 | 51/60 (85.0) | 13/60 (21.7) | 61.7 (50.0-76.2) | 92.8 (86.1-100) | ||||
| 3-5 | 32/40 (80.0) | 9/40 (22.5) | 53.3 (39.5-71.9) | 79.1 (67.1-93.2) | ||||
| PRIMA-PI score | 0.145 | 1 | 0.21 | 0.17 | ||||
| Low-intermediate | 58/66 (87.9) | 13/66 (19.7) | 61.1 (49.8-75.0) | 91.5 (84.6-99.0) | ||||
| High | 24/32 (75.0) | 7/32 (21.9) | 52.3 (37.1-73.5) | 77.9 (64.6-93.9) | ||||
| m7-FLIPI score | 0.065 | 0.392 | 0.076 | 0.06 | ||||
| Low | 70/80 (87.5) | 15/80 (18.8) | 62.7 (52.4-75.1) | 92.0 (86.0-98.4) | ||||
| High | 17/24 (70.8) | 7/24 (29.2) | 45.8 (29-7-70.8) | 74.1 (58.1-94.4) | ||||
| . | Complete remission . | POD24 . | PFS at 6 y . | OS at 6 y . | ||||
|---|---|---|---|---|---|---|---|---|
| n/total (%) . | P . | n/total (%) . | P . | % (95% confidence interval) . | P . | % (95% confidence interval) . | P . | |
| 91/108 (84.5) . | NA . | 22/108 (20.4) . | NA . | 60.5 (51.6-70.9) . | NA . | 88.2 (82.0-94.7) . | NA . | |
| FLIPI score | 0.029 | 0.013 | 0.00072 | 0.00057 | ||||
| 0-2 | 57/63 (90.5) | 8/63 (12.7) | 71.9 (60.7-85.1) | 100 | ||||
| 3-5 | 30/41 (73.2) | 14/41 (34.1) | 39.3 (26.8-57.7) | 70.6 (57.9-86.2) | ||||
| FLIPI-2 score | 0.591 | 1 | 0.35 | 0.046 | ||||
| 0-2 | 51/60 (85.0) | 13/60 (21.7) | 61.7 (50.0-76.2) | 92.8 (86.1-100) | ||||
| 3-5 | 32/40 (80.0) | 9/40 (22.5) | 53.3 (39.5-71.9) | 79.1 (67.1-93.2) | ||||
| PRIMA-PI score | 0.145 | 1 | 0.21 | 0.17 | ||||
| Low-intermediate | 58/66 (87.9) | 13/66 (19.7) | 61.1 (49.8-75.0) | 91.5 (84.6-99.0) | ||||
| High | 24/32 (75.0) | 7/32 (21.9) | 52.3 (37.1-73.5) | 77.9 (64.6-93.9) | ||||
| m7-FLIPI score | 0.065 | 0.392 | 0.076 | 0.06 | ||||
| Low | 70/80 (87.5) | 15/80 (18.8) | 62.7 (52.4-75.1) | 92.0 (86.0-98.4) | ||||
| High | 17/24 (70.8) | 7/24 (29.2) | 45.8 (29-7-70.8) | 74.1 (58.1-94.4) | ||||
Complete remission and POD24 was not calculable in 1 patient. Risk stratification could not be calculated in 4, 8, 10, and 4 patients for the FLIPI, FLIPI-2, PRIMA-PI, and m7-FLIPI scores, respectively.
NA, not applicable.
To evaluate the performance of the 4 risk scores, given that the m7-FLIPI has 2 risk categories, the other PIs were merged into 2 categories such that the proportion of high-risk patients was similar among them (supplemental Table 4).
The sensitivity for the prediction of POD24 was highest for FLIPI (87%) and lowest for FLIPI-2 (78%), specificity was highest for FLIPI (34%) and lowest for PRIMA-PI (21%), and accuracy was highest for m7-FLIPI (69%) and lowest for FLIPI-2 (56%) (Table 3).
Performance metrics of the 4 risk scores for prediction of POD24 and discrimination of PFS and OS
| . | Prediction of POD24 . | Discrimination (PFS) . | Discrimination (OS) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity . | Specificity . | Accuracy . | c-index . | AIC . | CPE . | c-index . | AIC . | CPE . | |
| FLIPI score | |||||||||
| 0-2/3-5 | 0.6364 | 0.6707 | 0.6635 | 0.644 | 546.2091 | 0.6085 | 0.727 | 316.917 | 0.6535 |
| FLIPI-2 score | |||||||||
| 0-2/3-5 | 0.4091 | 0.6026 | 0.56 | 0.521 | 543.3556 | 0.5329 | 0.602 | 327.5089 | 0.5949 |
| PRIMA-PI score | |||||||||
| Low-intermediate/high | 0.3500 | 0.6795 | 0.6122 | 0.537 | 532.7998 | 0.5418 | 0.586 | 326.2398 | 0.5616 |
| m7-FLIPI score | |||||||||
| Low/high | 0.3182 | 0.7927 | 0.6923 | 0.561 | 556.3833 | 0.5478 | 0.602 | 329.8374 | 0.5665 |
| . | Prediction of POD24 . | Discrimination (PFS) . | Discrimination (OS) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity . | Specificity . | Accuracy . | c-index . | AIC . | CPE . | c-index . | AIC . | CPE . | |
| FLIPI score | |||||||||
| 0-2/3-5 | 0.6364 | 0.6707 | 0.6635 | 0.644 | 546.2091 | 0.6085 | 0.727 | 316.917 | 0.6535 |
| FLIPI-2 score | |||||||||
| 0-2/3-5 | 0.4091 | 0.6026 | 0.56 | 0.521 | 543.3556 | 0.5329 | 0.602 | 327.5089 | 0.5949 |
| PRIMA-PI score | |||||||||
| Low-intermediate/high | 0.3500 | 0.6795 | 0.6122 | 0.537 | 532.7998 | 0.5418 | 0.586 | 326.2398 | 0.5616 |
| m7-FLIPI score | |||||||||
| Low/high | 0.3182 | 0.7927 | 0.6923 | 0.561 | 556.3833 | 0.5478 | 0.602 | 329.8374 | 0.5665 |
PFS and OS
Forty-two patients relapsed (38.9%), and 23 (21.1%) died during the follow-up period. The median PFS was 9.1 years, and the median OS was not reached.
According to the original description, the FLIPI, FLIPI-2, and PRIMA-PI scores showed differences in PFS (0.0031, 0.025, and 0.019, respectively); however, the m7-FLIPI score did not reach statistical significance (0.076) (Figure 2). Regarding OS, FLIPI and FLIPI-2 showed statistically significant differences among the originally described groups (0.0026 and 0.048, respectively), but PRIMA-PI and m7-FLIPI scores did not (0.28 and 0.06, respectively) (Figure 3). Cox regression analyses for PFS and OS according to the mutation status of the 7 genes included in the m7-FLIPI are described in supplemental Table 5.
When PFS and OS were compared between the low- and high-risk categories of the 4 risk scores, the FLIPI was significantly better in pairwise comparisons (supplemental Table 6).
To evaluate the performance of the 4 risk indices, we assessed the discrimination between PFS and OS, that is, the ability to anticipate PFS and OS, respectively. The Harrell c-index for PFS and OS was the best for the FLIPI (0.644, P = .001; 0.727, P = .001, respectively), and the other 3 scores reached similar results (Table 3). The AIC for PFS was best for PRIMA-PI (532), similar to the other 3 risk scores (543-556). The AIC for OS was the best for FLIPI (316), with higher values for the other 3 scores (326-329). Therefore, FLIPI had a higher level of parsimony (the ability to eliminate unnecessarily complicated models, including too many parameters for accurate estimation). CPE was the highest with FLIPI for both PFS and OS, indicating a higher concordance than the other 3 regimens, including m7-FLIPI (Table 3).
Subgroup analyses
Finally, we assessed the impact of the 4 scores on PFS and OS in the group of patients treated with R-CVP/R-CHOP and R-B (supplemental Tables 7 and 8). The discrimination parameters for PFS and OS were better for the FLIPI score than for the other 3 scores in patients treated with R-CVP/R-CHOP. In contrast, m7-FLIPI had a higher discrimination for PFS and OS in the group of patients treated with R-B when compared with the other 3 scores. However, these results should be interpreted with caution given the limited number of patients treated with R-B.
Discussion
Our study assessed the clinical value and statistical performance of the 4 most broadly applied risk scores in a cohort of patients with FL treated in the real-world setting. In our cohort of patients with symptomatic FL treated with immunochemotherapy and with a median follow-up of 8 years, we showed that the FLIPI remains a PI with higher discrimination for survival. Nevertheless, the predictive value of the FLIPI was better for R-CVP/R-CHOP than for R-B.
For performance analysis, we applied binary categories to FLIPI (low/intermediate risk vs high risk), FLIPI2 (0-2 vs 3-5 risk factors), and PRIMA-PI (low/intermediate risk vs high risk) because several studies have not observed significant differences in failure-free survival between low- and intermediate-risk patients,14-16 and also to allow a more direct comparison with the m7-FLIPI, which only has 2 categories. In our cohort, 42% of the patients with high-risk FLIPI were reclassified into the low-risk m7-FLIPI category. This is similar to the original publication of m7-FLIPI,9 but in contrast to that recently reported in a cohort of patients with advanced FL randomized to consolidation with high-dose therapy and autologous stem cell transplantation, where only 9% of patients with high-risk FLIPI were reclassified to low-risk m7-FLIPI.16 This might be related, at least in part, to the dependence on the age of the FLIPI and m7-FLIPI.17 These well-known clinicogenetic-risk scores are not appropriate to stratify younger patient groups.16 Moreover, the role of age as a relevant element of PIs for FL has been questioned because decreased survival in older patients is significantly owing to an increased rate of nonrelapse deaths.14 Recently, the PRIMA-PI, an age-independent tool, was found to identify a smaller cohort of high-risk FL cases than FLIPI or FLIPI-2, but in this study, patients were treated exclusively with R-CHOP.17 In our series, the performance of PRIMA-PI did not improve FLIPI both in R-CVP/R-CHOP or R-B subgroups. However, the limited number of patients treated with R-B in our study precluded definitive conclusions.
In our cohort, m7-FLIPI showed prognostic value in patients with FL treated with first-line rituximab-based chemotherapy including R-B, although it was not as powerful as FLIPI, similar to the observation in patients with FL receiving rituximab without chemotherapy.18 Interestingly, m7-FLIPI had higher discrimination for PFS and OS in the group of patients treated with R-B compared with the other 3 scores. This is in contrast with a recent analysis of the GALLIUM trial, in which the m7-FLIPI was prognostic in patients treated with rituximab-based regimens but not in those receiving obinutuzumab-based regimens.19 Moreover, when analyzed by chemotherapy regimen, the m7-FLIPI was prognostic in patients receiving CHOP/CVP-based treatment but not in the bendamustine-based treatment. These results should be interpreted with caution, given the limited number of patients treated with R-B in both studies.
When evaluating the ability to predict POD24, FLIPI had the highest sensitivity and specificity, however, the m7-FLIPI showed the best accuracy among the 4 risk scores. Regarding the comparison of the performance metrics, all 4 risk scores displayed similar calibration. In addition, our study supports the association between POD24 and OS after first-line immunochemotherapy (R-CVP, R-CHOP, and R-B), an assertion widely validated by multiple studies and recently confirmed in a pooled analysis involving 5225 patients with FL.20
Recently, other risk scores have also been proposed. We did not evaluate the FLEX score, developed to improve the identification of high-risk patients in the GALLIUM trial, and later validated it using data from the SABRINA trial because we did not perform natural killer cell count analysis in peripheral blood in routine practice.21 A novel score model called PReDiCt-FL has also been developed considering the mutation status of 11 genes, and cases with high risk according to this novel score have longer failure-free survival when treated with high dose intensification followed by autologous transplant. However, no differences were observed for OS.16
In addition to mutation-based scores, other genetic models have been proposed. A gene expression profiling predictive model using 23 genes reflecting both B-cell biology and the tumor microenvironment was built for patients with advanced FL treated with rituximab-based chemotherapy. This score identified 2 groups of patients with FL with remarkably different PFS independently of rituximab maintenance and FLIPI score.22 Recently, a transcriptomic predictor based on machine learning models have been developed and validated for OS in FL.23
To the best of our knowledge, this is the first study to directly compare FLIPI, FLIPI-2, PRIMA-PI, and m7-FLIPI in patients treated with R-B. At many centers, the standard treatment for patients with advanced FL has been switched to R-B. Our study provides a careful analysis of patients with FL treated with R-CVP/R-CHOP and R-B in a real-world setting. However, apart from its retrospective nature, some limitations of this analysis included the relatively small sample size. Moreover, 43% of the initially identified cases could not be included in the final analysis, mainly because of exhausted blocks or sequencing failure. The latter might have had some impact on the outcomes of the study, because clinical characteristics differed from the final selected cohort. The long median follow-up of our real-world series, which is essential for adequate evaluation of survival in FL, resulted in a considerable number of samples unsuitable for performing NGS studies. In addition, we observed a higher number of NGS failures in the older samples. However, this study included patients with a long follow-up period after treatment with conventional R-CVP/R-CHOP. The follow-up period for patients treated with R-B was shorter because of the later approval of this regimen in our country.
In conclusion, our data from a real-world cohort with a long follow-up show that currently available scores provide information to predict outcomes in patients with FL, but the FLIPI remains the best tool for identifying patients at high risk. The observation that m7-FLIPI may perform better than FLIPI in R-B–treated patients remains to be confirmed. Efforts should now be directed toward the development of tools that may help in selecting the optimal treatment in a more precise way.
Acknowledgments
This study was supported in part by grants from the Instituto de Salud Carlos III (ISCIII) and cofunded by the European Union (FIS-FEDER PI15/0459, FIS-FEDER PI19/00034, GILEAD GLD18/00117, 2017SGR205, and PT20/00023) and Xarxa de Banc de Tumors de Catalunya, sponsored by Pla Director d’Oncologia de Catalunya. The authors also acknowledge the biobank of the Fundación MD Anderson International supported by the Instituto de Salud Carlos III (PT17/0015/0008).
Authorship
Contribution: J.J.R.-S. performed the research and statistical analysis, analyzed and interpreted the results, and wrote the manuscript; C.F.-R. performed the research, collected and analyzed the next-generation sequencing (NGS) data, and wrote the manuscript; L.B., R.D.-F., S.P., A.F., B.S., E.G., and J.S. collected and analyzed the clinical data; L.F.-I. and M.L. collected and analyzed the NGS data; J.G. performed the bioinformatic analysis of the NGS data; R.R., J.F.G., and L.C. performed the histologic diagnostic; B.B. and A.G. designed the research, analyzed and interpreted the results, and wrote the manuscript; A.S. designed and performed the research, analyzed and interpreted the results, performed the statistical analysis, and wrote the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: A.S. received research funding from Roche, AbbVie, and Gilead Sciences; served on speaker’s bureau for Roche and Janssen Pharmaceuticals; and provided consultancy for Janssen Pharmaceuticals and Bristol Myers Squibb/Celgene. B.B. received research funding from Roche, AstraZeneca, and Novartis; provided consultancy for Thermo Fisher Scientific, Qiagen, Roche, AstraZeneca, Merck-Serono, Novartis, and Bristol Myers Squibb; and served on speaker’s bureau for Thermo Fisher Scientific, Qiagen, Roche, AstraZeneca, Biocartis, Merck-Serono, Novartis, and Bristol Myers Squibb. B.S. provided consultancy for Amgen, Novartis, and Takeda and served on speaker’s bureau for Amgen, Gilead Sciences, Novartis, Shire, and Takeda.
Correspondence: Antonio Salar, Department of Hematology, Hospital del Mar Medical Research Institute (IMIM), Passeig Marítim 25-29, 08003 Barcelona, Spain; e-mail: asalar@psmar.cat.
References
Author notes
∗J.J.R.-S. and C.F.-R. contributed equally to this study.
The sequencing data reported in this article have been deposited in the European Genome-phenome Archive database (accession number EGAD00001009647).
Data are available on request from corresponding author, Antonio Salar (asalar@parcdesalutmar.cat).
The full-text version of this article contains a data supplement.