Key Points
Overall, a diagnosis of MALT lymphoma was associated with moderately compromised survival, but this decrement was not universal.
Age, performance status, disease stage, and disease origin site emerged as important prognostic factors.
Abstract
There is limited understanding of the extent to which mucosa-associated lymphoid tissue (MALT) lymphoma affects a patient’s risk of death and how classically considered prognostic factors affect lymphoma-specific vs other noncancer mortality. This study analyzed major long-term outcomes of patients with MALT lymphoma and the prognostic significance of baseline clinical features. We reviewed the clinical features, treatments, disease course, and survival of 593 patients with MALT lymphoma diagnosed at Memorial Sloan Kettering between 2000 to 2012. Outcomes were analyzed using crude overall survival (OS) and relative survival (RS) by standardized mortality ratio. The median age was 60 years, 72% were at stage I/II. With a median follow-up of 9.2 years, the 10-year OS, lymphoma-specific mortality, and competing nonlymphoma mortality was 75%, 4%, and 21%, respectively; the overall standardized mortality ratio was 1.41 (95% confidence interval, 1.19-1.67; P < .001). Using multivariate analysis, older age, advanced stage, and poor performance status were independently associated with inferior OS. Several subgroups had similar RS to the normal matched population, including those with an age of ≥70 years, stage I, and skin or gastric origin. Increased lymphoma-specific death was associated with spread disease, whereas death from nonlymphoma causes was correlated with older age. Overall, a diagnosis of MALT lymphoma was associated with moderately compromised survival. Age and advanced-stage disease emerged as the most important prognostic factors. Younger patients had better OS but worse RS. Disease dissemination was the lymphoma-specific risk factor.
Introduction
Extranodal marginal zone lymphomas of mucosa-associated lymphoid tissue (MALT) are considered exceptionally indolent lymphomas. In a predominantly elderly patient population, most patients can expect that their longevity will be unaffected by disease-associated complications. However, MALT lymphoma may have a heterogenous course with increased mortality seen in association with transformation to large-cell lymphoma (2%-4% of cases), early disease progression, or lack of treatment response.1-4
The prognostic effect of various clinicodemographic features has been evaluated in large cohorts, national data sets, and clinical studies of MALT lymphoma. These outcomes provide the basis for the currently accepted prognostic factors, which include markers of disease burden and activity (stage, site, serum lactate dehydrogenase level, and presence of anemia) and patient features (performance status [PS] and age).1-5 However, these features, although efficacious in identifying patients at risk of a more challenging course, might not accurately represent the survival implication of having been diagnosed with MALT lymphoma compared with the general lymphoma-free population. In this regard, standardized relative mortality ratios (SMRs) may provide an accurate estimation of the survival implications of being diagnosed with MALT lymphoma as well as its often-associated chronic infections and autoimmune diseases.
In our previous study, we described the long-term outcomes of early-stage disease after various initial therapies, and evaluated prognostic predictors of relapse.6 This study aimed to analyze both crude and relative survival (RS) of a large cohort of patients with MALT lymphoma treated with modern approaches.
Methods
Patient cohort
After institutional review board approval, we analyzed data from patients with MALT lymphoma diagnosed between January 2000 and December 2012 at Memorial Sloan Kettering Cancer Center (N = 600). The study was conducted in accordance with the Declaration of Helsinki. This era is characterized by the standard use of rituximab and conventional-dose radiotherapy (RT) and predates the widespread introduction of targeted agents and very low–dose RT.7 We excluded cases with <1 month follow-up (n = 5) and positive HIV status (n = 2), leaving 593 evaluable patients.
Staging and treatment principles
All patients underwent baseline staging by computed tomography, positron emission tomography/computed tomography, or magnetic resonance imaging for select situations (ie, ocular/orbital MALT). A modified Ann Arbor staging system was applied to retrospectively restage all cases.8 Involvement of an extranodal site or bilateral organ (except lung or skin), with or without locoregional lymph nodes, was stage I and II, respectively. Multiple nodules in the lung limited to 1 lobe or perihilar extension associated with ipsilateral hilar adenopathy was classified as localized disease. Unilateral pleural effusion with or without lung involvement but with hilar adenopathy was considered as localized disease. Bilateral lungs and multiple distant skin involvement, multiple mucosal site (MMS) involvement with or without lymph nodes, distant adenopathy, or bone marrow (BM) involvement were classified as stage IV, building on the MMS study and the MALT-IPI study,5 including its model discovery data set9,10 and 3 validation data sets (supplemental Table 1).4,11-13 Involvement of MMS was defined as MALT lesions in ≥2 different anatomical organs independent of spleen and BM involvement.1 The MALT International Prognostic Index (MALT-IPI) was calculated per published criteria based on 3 variables: age ≥70 years, Ann Arbor stage III to IV, or elevated lactate dehydrogenase.5 To better display the prognostic effect of age on survival and cause of death (COD), we defined 3 age subgroups (<60 years vs 60-70 years vs ≥70 years) per the IPI and MALT-IPI prognostic systems.
Treatment approaches (including expectant management) were diverse. Local treatments (eg, surgery, radiation, topical treatment for cutaneous lesions, and antibiotics for gastric lesions) were typically recommended for limited-stage disease. Patients with gastric MALT were referred for RT if they had Helicobacter pylori–independent disease (biopsy did not identify H pylori) or failed antibiotic therapy for H pylori eradication based on repeat gastric biopsy. Systemic treatments were usually implemented for advanced-stage disease warranting treatment per the Groupe d'Etude des Lymphomes Folliculaires criteria14 and included anti-CD20 monoclonal immunotherapy with or without chemotherapy. Watchful waiting was defined as a documented decision to defer treatment in the medical record in the absence of treatment for at least 3 months from the date of diagnosis. Surgery was defined as complete excision of all recognized lesion(s). The principles for local RT were reported previously.6,15
Statistical analysis
Definitions and distinctions of crude overall survival (OS), RS, cumulative cause–specific mortality, and crude probability of dying from nonlymphoma causes were elaborated as per Mariotto et al.16 Crude survival is the number of living patients among patients with MALT lymphoma, it estimates the chance of remaining alive for some time after diagnosis. RS is the ratio of OS for patients with cancer to the expected survival of a comparable group of individuals that are cancer-free, it provides a measure of excess mortality experienced by patients with cancer without requiring information on the COD. Cumulative cause–specific mortality considers 2 (or more) end points, death due to cancer and death due to competing causes, and it estimates the chance of dying from the MALT lymphoma vs dying from nonlymphoma competing causes. Progression was defined per the Lugano criteria.17
Crude OS was calculated from time of diagnosis to time of death from any cause. Progression-free survival (PFS) was calculated from time of diagnosis to time of first progression or death from any cause. The crude survival (OS/PFS) was calculated with the Kaplan-Meier method and compared by log-rank test. Features independently associated with OS were identified in univariate and multivariate analyses by Cox proportional hazards regression.
Lymphoma-specific survival was estimated according to parameters of interest using competing risk analysis based on COD (R statistical package cmprsk). These parameters included independent predictors identified by the multivariate OS analysis and factors with clinical significance in the SMR analysis. Lymphoma-specific death was defined as death from lymphoma, large-cell transformation, or immediate treatment complications (≤3 months). We recorded diagnoses of secondary malignancies. Large-cell transformation was only considered for biopsy-confirmed cases and was not counted as a secondary malignancy.
Expected survival normalized for age, sex, and year was generated using the general US population (R survexp.us package). Observed vs expected OS was plotted using a conditional approach and summarized using SMRs of observed-to-expected deaths. SMR (ratio of observed to expected number of deaths) compared with mortality experienced by this cohort with age-, sex-, and calendar-specific mortality of the US population; SMR of >1.0 indicated worse-than-expected survival. RS was estimated as the ratio of the observed all-cause survival to the expected survival in the matched general population, which was calculated using the Ederer II method (R relsurv package).18 All analyses were performed by using R version 3.5.2 (R Foundation) and a P < .05 was considered statistically significant.
Results
Patient characteristics and treatments
A total of 593 cases were analyzed with a median patient age of 60 years and interquartile range of 50 to 70 years. The baseline characteristics are summarized in Table 1. Briefly, 26.8% of patients were aged ≥70 years, and 71.8% had limited-stage disease (Ann Arbor stage I-II). The most common anatomic sites were stomach (20.2%), skin (17.5%), lung (13.8%), and orbit (13.0%). Using MALT-IPI, 49.9% were classified as low risk, 40.3% as intermediate risk, and 9.8% as high risk.
Baseline characteristics, crude survival, and prognosis
| . | N (%) . | OS . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| 5-y (95% CI) . | 10-y (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | ||
| All patients | 593 | 89.4 (86.8-92.1) | 74.4 (70.6-79.1) | |||||
| Age group, y | <.001 | |||||||
| <60 | 282 (47.6) | 96.9 (94.8-99.0) | 87.9 (83.2-92.9) | Ref | Ref | |||
| 60-70 | 152 (25.6) | 85.1 (79.3-91.2) | 74.2 (66.6-82.7) | 2.34 (1.44-3.77) | .001 | 2.36 (1.46-3.82) | <.001 | |
| ≥70 | 159 (26.8) | 81.2 (75.1-87.9) | 53.3 (44.6-63.8) | 5.15 (3.37-7.85) | <.001 | 4.93 (3.22-7.53) | <.001 | |
| Sex | .315 | |||||||
| Male | 262 (44.2) | 88.8 (84.0-93.0) | 70.8 (64.1-78.2) | Ref | ||||
| Female | 331 (55.8) | 89.9 (86.6-93.4) | 77.4 (72.3-83.0) | 0.84 (0.60-1.18) | .315 | |||
| Stage | ||||||||
| I | 388 (65.4) | 90.8 (87.7-93.9) | 78.3 (73.4-83.6) | .007 | Ref | Ref | ||
| II | 38 (6.4) | 85.9 (75.2-98.2) | 71.6 (57.2-89.6) | 1.89 (1.03-3.50) | .041 | 1.78 (0.96-3.31) | .067 | |
| III-IV | 167 (28.2) | 87.3 (82.2-92.6) | 68.3 (60.6-77.0) | 1.67 (1.17-2.39) | .005 | 1.70 (1.18-2.43) | .004 | |
| MMS | ||||||||
| Absent | 530 (89.4) | 90.3 (87.7-93.1) | 75.1 (70.7-79.8) | .032 | Ref | .034 | ||
| Present | 63 (10.6) | 81.9 (72.8-92.2) | 71.3 (60.6-83.9) | 1.64 (1.04-2.59) | ||||
| Primary site | .059 | |||||||
| Gastric | 120 (20.2) | 94.5 (90.3-98.9) | 79.6 (70.6-89.8) | Ref | ||||
| Skin | 104 (17.5) | 96.5 (92.6-100) | 81.9 (73.3-91.4) | 0.59 (0.30-1.16) | .126 | |||
| Lung | 82 (13.8) | 88.5 (81.6-95.9) | 66.9 (55.4-80.8) | 1.14 (0.85-1.53) | .382 | |||
| Orbit | 77 (13.0) | 79.0 (69.8-89.4) | 67.3 (56.2-80.7) | 1.12 (0.92-1.37) | .240 | |||
| BM involvement∗ | .023 | |||||||
| No | 285 (48.1) | 90.5 (86.9-94.2) | 78.4 (72.8-84.5) | Ref | ||||
| Yes | 36 (6.1) | 85.5 (74.5-98.1) | 55.9 (39.7-78.6) | 2.14 (1.23-3.72) | .007 | |||
| ECOG PS† | <.001 | |||||||
| 0-1 | 582 (98.1) | 90.4 (87.9-93.0) | 75.3 (71.1-79.8) | Ref | Ref | |||
| >1 | 11 (1.9) | 40.9 (19.4-86.3) | - | 7.93 (3.85-16.37) | <.001 | 7.02 (3.35-14.73) | <.001 | |
| LDH | .285 | |||||||
| Normal | 562 (94.8) | 90.0 (87.4-92.7) | 74.4 (70.1-79.0) | Ref | ||||
| Elevated | 31 (5.2) | 79.4 (66.0-95.5) | 66.0 (49.6-87.9) | 1.44 (0.73-2.84) | .287 | |||
| MALT-IPI risk | <.001 | |||||||
| Low | 296 (49.9) | 93.5 (90.8-96.8) | 85.3 (80.5-90.5) | Ref | ||||
| Intermediate | 239 (40.3) | 87.9 (83.7-92.4) | 69.0 (62.1-76.6) | 2.51 (1.69-3.72) | <.001 | |||
| High | 58 (9.8) | 76.7 (66.3-88.7) | 48.8 (35.8-66.4) | 4.90 (3.01-7.98) | <.001 | |||
| . | N (%) . | OS . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| 5-y (95% CI) . | 10-y (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | ||
| All patients | 593 | 89.4 (86.8-92.1) | 74.4 (70.6-79.1) | |||||
| Age group, y | <.001 | |||||||
| <60 | 282 (47.6) | 96.9 (94.8-99.0) | 87.9 (83.2-92.9) | Ref | Ref | |||
| 60-70 | 152 (25.6) | 85.1 (79.3-91.2) | 74.2 (66.6-82.7) | 2.34 (1.44-3.77) | .001 | 2.36 (1.46-3.82) | <.001 | |
| ≥70 | 159 (26.8) | 81.2 (75.1-87.9) | 53.3 (44.6-63.8) | 5.15 (3.37-7.85) | <.001 | 4.93 (3.22-7.53) | <.001 | |
| Sex | .315 | |||||||
| Male | 262 (44.2) | 88.8 (84.0-93.0) | 70.8 (64.1-78.2) | Ref | ||||
| Female | 331 (55.8) | 89.9 (86.6-93.4) | 77.4 (72.3-83.0) | 0.84 (0.60-1.18) | .315 | |||
| Stage | ||||||||
| I | 388 (65.4) | 90.8 (87.7-93.9) | 78.3 (73.4-83.6) | .007 | Ref | Ref | ||
| II | 38 (6.4) | 85.9 (75.2-98.2) | 71.6 (57.2-89.6) | 1.89 (1.03-3.50) | .041 | 1.78 (0.96-3.31) | .067 | |
| III-IV | 167 (28.2) | 87.3 (82.2-92.6) | 68.3 (60.6-77.0) | 1.67 (1.17-2.39) | .005 | 1.70 (1.18-2.43) | .004 | |
| MMS | ||||||||
| Absent | 530 (89.4) | 90.3 (87.7-93.1) | 75.1 (70.7-79.8) | .032 | Ref | .034 | ||
| Present | 63 (10.6) | 81.9 (72.8-92.2) | 71.3 (60.6-83.9) | 1.64 (1.04-2.59) | ||||
| Primary site | .059 | |||||||
| Gastric | 120 (20.2) | 94.5 (90.3-98.9) | 79.6 (70.6-89.8) | Ref | ||||
| Skin | 104 (17.5) | 96.5 (92.6-100) | 81.9 (73.3-91.4) | 0.59 (0.30-1.16) | .126 | |||
| Lung | 82 (13.8) | 88.5 (81.6-95.9) | 66.9 (55.4-80.8) | 1.14 (0.85-1.53) | .382 | |||
| Orbit | 77 (13.0) | 79.0 (69.8-89.4) | 67.3 (56.2-80.7) | 1.12 (0.92-1.37) | .240 | |||
| BM involvement∗ | .023 | |||||||
| No | 285 (48.1) | 90.5 (86.9-94.2) | 78.4 (72.8-84.5) | Ref | ||||
| Yes | 36 (6.1) | 85.5 (74.5-98.1) | 55.9 (39.7-78.6) | 2.14 (1.23-3.72) | .007 | |||
| ECOG PS† | <.001 | |||||||
| 0-1 | 582 (98.1) | 90.4 (87.9-93.0) | 75.3 (71.1-79.8) | Ref | Ref | |||
| >1 | 11 (1.9) | 40.9 (19.4-86.3) | - | 7.93 (3.85-16.37) | <.001 | 7.02 (3.35-14.73) | <.001 | |
| LDH | .285 | |||||||
| Normal | 562 (94.8) | 90.0 (87.4-92.7) | 74.4 (70.1-79.0) | Ref | ||||
| Elevated | 31 (5.2) | 79.4 (66.0-95.5) | 66.0 (49.6-87.9) | 1.44 (0.73-2.84) | .287 | |||
| MALT-IPI risk | <.001 | |||||||
| Low | 296 (49.9) | 93.5 (90.8-96.8) | 85.3 (80.5-90.5) | Ref | ||||
| Intermediate | 239 (40.3) | 87.9 (83.7-92.4) | 69.0 (62.1-76.6) | 2.51 (1.69-3.72) | <.001 | |||
| High | 58 (9.8) | 76.7 (66.3-88.7) | 48.8 (35.8-66.4) | 4.90 (3.01-7.98) | <.001 | |||
HR, hazard ratio; LDH, lactate dehydrogenase; Ref, reference.
BM status was unknown in 272 cases.
ECOG PS status was missing in 9 cases.
Treatments varied according to anatomic distribution and disease stage (Table 2). For limited-stage disease (n = 426), 52 (12.2%) were observed and 340 (79.8%) received local treatment, in which RT was given to 202 (47.4%). For stage IV disease (n = 167), 61 (36.5%) were initially observed whereas 60 (35.9%) received systemic treatment, and 46 (27.5%) received palliative local treatment. Watchful waiting was most frequently utilized for cases with multifocal skin and lung disease (n = 32, 47.1%).
Treatments according to lesion dissemination
| . | Localized (n = 426, 71.8%) . | Disseminated (n = 167, 28.2%) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Stage I . | Stage II . | Stage III-IV . | ||||||
| A single lesion (involvement) in extranodal site (n = 375) . | Multifocal involvement of a single/pared extranodal site∗ (n = 13) . | One site + regional LN(s) (n = 38) . | Multiple distant skin involvement (n = 51) . | Bilateral lung involvement (n = 17) . | A single site + nonlocoregional nodes/BM involvement (n = 36) . | MMS involvement (n = 35) . | Multiple sites + LN(s) (n = 28) . | |
| Observation | 45 (12.0) | 1 (7.7) | 6 (15.8) | 22 (43.1) | 10 (58.8) | 12 (33.3) | 9 (25.7) | 8 (28.6) |
| Local treatment | ||||||||
| Total | 308 (82.1) | 10 (76.9) | 22 (57.9) | 23 (45.1) | 4 (23.5) | 5 (13.9) | 10 (28.6) | 4 (14.3) |
| RT | 185 (49.3) | 6 (46.2) | 11 (28.9) | 9 (17.6) | 0 | 1 (2.8) | 7 (20.0) | 3 (10.7) |
| Surgery | 105 (28.0) | 2 (15.4) | 8 (21.1) | 3 (5.9) | 4 (23.5) | 3 (8.3) | 2 (5.7) | 1 (3.6) |
| Site specific | 18 (4.8) | 2 (15.4) | 3 (7.9) | 11 (21.6) | 0 | 1 (2.8) | 1 (2.9) | 0 |
| Systemic treatment | ||||||||
| Total | 22 (5.9) | 2 (15.4) | 10 (26.3) | 6 (11.8) | 3 (17.6) | 19 (52.8) | 16 (45.7) | 16 (57.1) |
| Anti-CD20 antibody | 16 (4.3) | 1 (7.7) | 2 (5.3) | 3 (5.9) | 1 (5.9) | 7 (19.4) | 10 (28.6) | 7 (25.0) |
| Chemoimmunotherapy | 6 (1.6) | 1 (7.7) | 8 (21.1) | 3 (5.9) | 2 (11.8) | 12 (33.3) | 6 (17.1) | 9 (32.1) |
| . | Localized (n = 426, 71.8%) . | Disseminated (n = 167, 28.2%) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Stage I . | Stage II . | Stage III-IV . | ||||||
| A single lesion (involvement) in extranodal site (n = 375) . | Multifocal involvement of a single/pared extranodal site∗ (n = 13) . | One site + regional LN(s) (n = 38) . | Multiple distant skin involvement (n = 51) . | Bilateral lung involvement (n = 17) . | A single site + nonlocoregional nodes/BM involvement (n = 36) . | MMS involvement (n = 35) . | Multiple sites + LN(s) (n = 28) . | |
| Observation | 45 (12.0) | 1 (7.7) | 6 (15.8) | 22 (43.1) | 10 (58.8) | 12 (33.3) | 9 (25.7) | 8 (28.6) |
| Local treatment | ||||||||
| Total | 308 (82.1) | 10 (76.9) | 22 (57.9) | 23 (45.1) | 4 (23.5) | 5 (13.9) | 10 (28.6) | 4 (14.3) |
| RT | 185 (49.3) | 6 (46.2) | 11 (28.9) | 9 (17.6) | 0 | 1 (2.8) | 7 (20.0) | 3 (10.7) |
| Surgery | 105 (28.0) | 2 (15.4) | 8 (21.1) | 3 (5.9) | 4 (23.5) | 3 (8.3) | 2 (5.7) | 1 (3.6) |
| Site specific | 18 (4.8) | 2 (15.4) | 3 (7.9) | 11 (21.6) | 0 | 1 (2.8) | 1 (2.9) | 0 |
| Systemic treatment | ||||||||
| Total | 22 (5.9) | 2 (15.4) | 10 (26.3) | 6 (11.8) | 3 (17.6) | 19 (52.8) | 16 (45.7) | 16 (57.1) |
| Anti-CD20 antibody | 16 (4.3) | 1 (7.7) | 2 (5.3) | 3 (5.9) | 1 (5.9) | 7 (19.4) | 10 (28.6) | 7 (25.0) |
| Chemoimmunotherapy | 6 (1.6) | 1 (7.7) | 8 (21.1) | 3 (5.9) | 2 (11.8) | 12 (33.3) | 6 (17.1) | 9 (32.1) |
LN, lymph node.
Excluding bilateral lungs and multiple distant skin involvement (classified as stage IV, whereas bilateral organ involvement in other anatomical sites is defined as stage I disease).
Among 222 patients treated with RT, 66.7% received a dose of 30 Gy. Other commonly prescribed doses included 36 to 40 Gy (14.5%), 24 Gy (11.0%), and 4 Gy (5.0%). For patients treated with systemic therapy (n = 94), 47 received rituximab (R) monotherapy and 47 received chemoimmunotherapy; common regimens included R-fludarabine (27.1%), rituximab, cyclophosphamide, vincristine, prednisone; 22.9%, and rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; 20.8%.
Long-term survival and events
The median follow-up was 9.2 years among survivors. Of 593 patients, 40.1% (n = 238) had documented disease progression/relapse during the analyzed period. The median time to first progression was 4.8 years (interquartile range, 1.8-8.8 years). The 5-year and 10-year PFS was 63.7% and 56.6%, respectively, for the entire cohort; 70.4% and 62.8%, respectively, for limited-stage disease; and 47.6% and 44.3%, respectively, for advanced-stage disease.
Among the 113 cases with initial watchful waiting as the primary management, 58 progressed (51.3%) and 44 subsequently received antitumor treatments (38.9%). The median time to initiation of treatment was 19.4 months (range, 3-146.2 months). The time to initiation of treatment is displayed in supplemental Figure 1, using death as the competing risk. The 5-year and 10-year PFS were 52.8% and 43.0%, respectively, for the entire cohort; 56.2% and 42.6%, respectively, for limited-stage disease; and 49.9% and 43.4%, respectively, for advanced-stage disease.
Transformation to large-cell lymphoma was observed in 16 patients (2.7%). The median time of transformation was 34.4 months (range 9.3-122.5 months). After systemic treatment, 2 patients died of other causes (34 and 46 months, respectively), 3 patients died of lymphoma within 2 years (median survival time, 12 months). In total, 213 (35.9%) patients had a synchronous or metachronous second malignancy (tumor in situ in skin or cervix, n = 20, 3.4%; hematological malignancy unrelated to MALT lymphoma, n = 31, 5.2%; solid tumor, n = 132, 22.3%; multiple tumor types, n = 30, 5.1%).
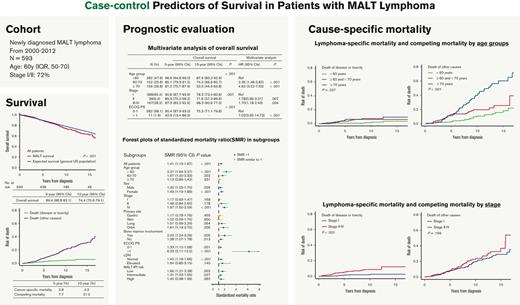
In total, 137 patients had died, of which 24 deaths (17.5%) were lymphoma specific. The 5- and 10-year OS were 89.4% (95% confidence interval [CI], 86.8-92.1) and 74.7% (95% CI, 70.6-79.1), respectively (Figure 1A). The 5- and 10-year lymphoma-specific mortality was 2.8% and 4.3%, respectively, and the cumulative mortality of other competing causes (deaths without recurrence/progression or second non-MALT lymphoma malignancies) was 7.7% and 21.0%, respectively (Figure 1B).
Survival and mortality of the entire cohort. (A) The crude OS of MALT lymphoma vs expected survival in the general US population. (B) The cumulative lymphoma-specific and competing mortality.
Survival and mortality of the entire cohort. (A) The crude OS of MALT lymphoma vs expected survival in the general US population. (B) The cumulative lymphoma-specific and competing mortality.
Patients with MALT lymphoma had a significantly increased risk of death compared with baseline mortality rates in the US population matched for age, sex, and calendar period, with an overall SMR of 1.41 (95% CI, 1.19-1.67; P < .001; Figure 1A).
Prognostic evaluation with crude survival analysis
Several clinicodemographic factors were significantly associated with OS in univariate prognostic analysis including age group (<60 vs 60-70 vs >70), stage, East Cooperative Oncology Group (ECOG) PS (0-1 vs >1), and BM involvement (Table 1). Prognosis by involvement pattern is listed in Table 3, stage IV with MMS or bilateral lung involvement had the worst prognosis.
Prognosis analysis by involvement patterns
| . | . | N (%) . | 5-y OS . | 10-y OS . | P value (vs stage I) . | SMR . | P value for SMR . |
|---|---|---|---|---|---|---|---|
| Stage I | One lesion | 375 (63.2) | 91.4 (88.3-84.5) | 77.7 (72.5-83.3) | Ref. | 1.18 (0.94-1.49) | .156 |
| Stage I | Multifocal involvement of a single/pared extranodal site | 13 (2.2) | 80.0 (58.7-100) | 80.0 (58.7-100) | .457 | 0.94 (0.23-3.74) | .925 |
| Stage II | One site + regional LN(s) | 38 (6.4) | 85.9 (75.2-98.2) | 71.6 (57.2-89.6) | .041 | 1.48 (0.84-2.60) | .178 |
| Stage IV | Multiple distant skin involvement | 51 (8.6) | 95.6 (89.9-100) | 71.8 (58.1-88.8) | .852 | 1.46 (0.81-2.64) | .207 |
| Stage IV | Bilateral lung involvement | 17 (2.9) | 76.0 (58.0-99.6) | 43.2 (20.5-91.0) | .002 | 3.78 (1.89-7.76) | <.001 |
| Stage IV | One site + distant LN(s) | 36 (6.1) | 90.8 (81.3-100) | 67.6 (51.9-88.0) | .175 | 1.48 (0.80-2.75) | .216 |
| Stage IV | Multiple sites | 35 (5.9) | 79.5 (67.0-94.3) | 73.5 (60.0-90.0) | .038 | 2.31 (1.34-3.98) | .003 |
| Stage IV | Multiple sites + LN(s) | 28 (4.7) | 85.1 (72.6-99.7) | 67.7 (51.3-89.2) | .056 | 2.32 (1.21-4.46) | .012 |
| . | . | N (%) . | 5-y OS . | 10-y OS . | P value (vs stage I) . | SMR . | P value for SMR . |
|---|---|---|---|---|---|---|---|
| Stage I | One lesion | 375 (63.2) | 91.4 (88.3-84.5) | 77.7 (72.5-83.3) | Ref. | 1.18 (0.94-1.49) | .156 |
| Stage I | Multifocal involvement of a single/pared extranodal site | 13 (2.2) | 80.0 (58.7-100) | 80.0 (58.7-100) | .457 | 0.94 (0.23-3.74) | .925 |
| Stage II | One site + regional LN(s) | 38 (6.4) | 85.9 (75.2-98.2) | 71.6 (57.2-89.6) | .041 | 1.48 (0.84-2.60) | .178 |
| Stage IV | Multiple distant skin involvement | 51 (8.6) | 95.6 (89.9-100) | 71.8 (58.1-88.8) | .852 | 1.46 (0.81-2.64) | .207 |
| Stage IV | Bilateral lung involvement | 17 (2.9) | 76.0 (58.0-99.6) | 43.2 (20.5-91.0) | .002 | 3.78 (1.89-7.76) | <.001 |
| Stage IV | One site + distant LN(s) | 36 (6.1) | 90.8 (81.3-100) | 67.6 (51.9-88.0) | .175 | 1.48 (0.80-2.75) | .216 |
| Stage IV | Multiple sites | 35 (5.9) | 79.5 (67.0-94.3) | 73.5 (60.0-90.0) | .038 | 2.31 (1.34-3.98) | .003 |
| Stage IV | Multiple sites + LN(s) | 28 (4.7) | 85.1 (72.6-99.7) | 67.7 (51.3-89.2) | .056 | 2.32 (1.21-4.46) | .012 |
LN, lymph node.
Multivariable analyzes included variables identified as statistically significant by univariate analysis or with clinical significance in the SMR analysis: age (<60 vs 60-70 vs ≥70), Ann Arbor stage (I vs II vs III-IV), MMSs (absent vs present), BM involvement (no vs yes), ECOG PS (0-1 vs >1), primary site (gastric vs skin vs lung vs orbit vs others). Three factors remained significantly associated with OS: age group, disease stage, and ECOG PS (Table 1). MALT-IPI was not an independent risk factor in this model.
Prognostic evaluation with RS analysis
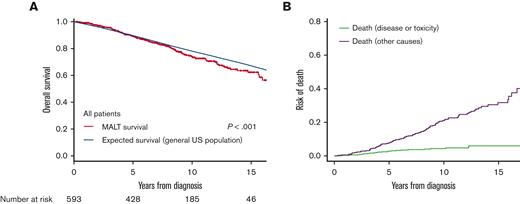
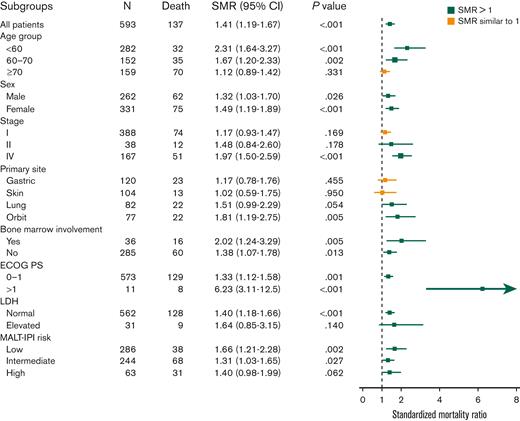
Most of the analyzed subgroups identified by clinical factors had modest but significantly increased risk of death compared with the general population in SMR analysis (SMR ranging between 1-2.5; Figure 2). Of note, patients with an ECOG PS of ≥2 had a very high incremental risk (SMR, 6.23; P < .001). However, we also identified several subgroups with similar mortality to the matched generalized population as evidenced by SMR values near 1 with nonsignificant P values. These included patients aged ≥70 years (SMR, 1.12; P = .331, Figure 3A), stage I (SMR, 1.17; P = .169, Figure 3B), primary cutaneous origin (SMR, 1.02; P = .95, Figure 3C), and primary gastric origin (SMR, 1.17; P = .455, Figure 3D). This analysis highlighted the discriminating effects of age, stage, origin site, and ECOG PS on the end point of RS. In contrast to the prognostic effect of increasing age on OS as the end point, the SMR decreased across increasing-age subgroups, ranging from 2.36-fold greater than the general population in the subgroup aged <60 years, to 1.67-fold in the subgroup aged 60 to 70 years, to 1.12-fold (and nonsignificant) for the group aged ≥70 years. The nonlymphoma SMR analysis also showed a higher nonlymphoma mortality risk in the younger subgroup (supplemental Table 2).
Forest plots of SMR in MALT lymphoma subgroups. Error bars show 95% CIs. The relative mortality effect was interpreted based on SMR, CIs, and sample sizes, and visualized in different colors. LDH, lactate dehydrogenase.
Forest plots of SMR in MALT lymphoma subgroups. Error bars show 95% CIs. The relative mortality effect was interpreted based on SMR, CIs, and sample sizes, and visualized in different colors. LDH, lactate dehydrogenase.
The crude OS vs expected survival in subgroups with normal life expectancy. (A) Age of ≥70 years (SMR, 1.12; P = .331); (B) stage I disease (SMR, 1.17; P = .184); (C) skin origin (SMR, 1.02; P = .95); and (D) stomach origin (SMR,1.17; P = .455).
The crude OS vs expected survival in subgroups with normal life expectancy. (A) Age of ≥70 years (SMR, 1.12; P = .331); (B) stage I disease (SMR, 1.17; P = .184); (C) skin origin (SMR, 1.02; P = .95); and (D) stomach origin (SMR,1.17; P = .455).
Cumulative COD according to factors of interest
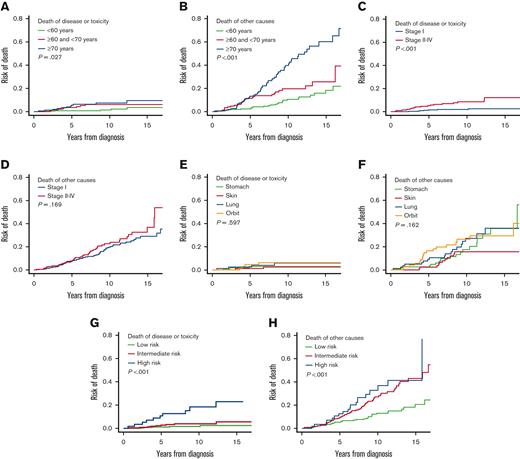
The cumulative COD analysis results according to parameters of interest are displayed in Table 4 and Figure 4. ECOG PS was not analyzed because of the small case numbers in the group in which PS ≥2. MALT-IPI was also analyzed as it represented a MALT lymphoma–specific comprehensive prognostic model (Figure 4G-H).
Cumulative mortalities according to significant factors
| . | Cancer-specific mortality (%) . | Competing mortality (%) . | ||||
|---|---|---|---|---|---|---|
| 5 y . | 10 y . | P value . | 5 y . | 10 y . | P value . | |
| All | 2.8 | 4.3 | 7.7 | 21.0 | ||
| Age group, y | .027 | <.001 | ||||
| <60 | 0.8 | 1.4 | 2.3 | 10.6 | ||
| 60-70 | 3.6 | 6.1 | 11.4 | 19.7 | ||
| ≥70 | 5.6 | 7.4 | 13.3 | 39.7 | ||
| Stage | <.001 | .169 | ||||
| I | 1.5 | 1.9 | 7.7 | 19.7 | ||
| II-IV | 5.2 | 8.3 | 7.8 | 22.7 | ||
| Site of origin | .597 | .162 | ||||
| Gastric | 2.9 | 2.9 | 2.6 | 17.5 | ||
| Skin | 1.1 | 2.5 | 2.4 | 15.6 | ||
| Lung | 2.5 | 5.9 | 9.1 | 27.2 | ||
| Orbit | 4.5 | 6.2 | 16.5 | 26.5 | ||
| MALT-IPI risk | <.001 | <.001 | ||||
| Low | 1.1 | 1.6 | 5.3 | 13.0 | ||
| Intermediate | 2.8 | 3.9 | 9.3 | 27.1 | ||
| High | 10.7 | 18.4 | 12.6 | 32.8 | ||
| . | Cancer-specific mortality (%) . | Competing mortality (%) . | ||||
|---|---|---|---|---|---|---|
| 5 y . | 10 y . | P value . | 5 y . | 10 y . | P value . | |
| All | 2.8 | 4.3 | 7.7 | 21.0 | ||
| Age group, y | .027 | <.001 | ||||
| <60 | 0.8 | 1.4 | 2.3 | 10.6 | ||
| 60-70 | 3.6 | 6.1 | 11.4 | 19.7 | ||
| ≥70 | 5.6 | 7.4 | 13.3 | 39.7 | ||
| Stage | <.001 | .169 | ||||
| I | 1.5 | 1.9 | 7.7 | 19.7 | ||
| II-IV | 5.2 | 8.3 | 7.8 | 22.7 | ||
| Site of origin | .597 | .162 | ||||
| Gastric | 2.9 | 2.9 | 2.6 | 17.5 | ||
| Skin | 1.1 | 2.5 | 2.4 | 15.6 | ||
| Lung | 2.5 | 5.9 | 9.1 | 27.2 | ||
| Orbit | 4.5 | 6.2 | 16.5 | 26.5 | ||
| MALT-IPI risk | <.001 | <.001 | ||||
| Low | 1.1 | 1.6 | 5.3 | 13.0 | ||
| Intermediate | 2.8 | 3.9 | 9.3 | 27.1 | ||
| High | 10.7 | 18.4 | 12.6 | 32.8 | ||
The cumulative cause–specific mortalities according to factors of interest. (A) Lymphoma-specific mortality and (B) competing mortality by age groups; (C) lymphoma-specific mortality and (D) competing mortality by disease stage; (E) lymphoma-specific mortality and (F) competing mortality by site of origin grouping; (G) lymphoma-specific mortality and (H) competing mortality by MALT-IPI groups.
The cumulative cause–specific mortalities according to factors of interest. (A) Lymphoma-specific mortality and (B) competing mortality by age groups; (C) lymphoma-specific mortality and (D) competing mortality by disease stage; (E) lymphoma-specific mortality and (F) competing mortality by site of origin grouping; (G) lymphoma-specific mortality and (H) competing mortality by MALT-IPI groups.
Competing deaths (deaths without recurrence/progression and second non-MALT lymphoma malignancies) contributed heavily to the increasing all-cause mortality over time (Table 4; Figure 1B). The cumulative lymphoma-specific mortality remained as low as <5% at 10 years in subgroups aged <60 years, with stage I disease, with skin and gastric origin, or a low to intermediate MALT-IPI score, but was relatively higher (>5% at 10 years) in subgroups aged ≥60 years, with stage II-IV disease, lung and orbit origin, or a high initial MALT-IPI score. Patient age showed a dramatic effect on nonlymphoma competing mortality but not on lymphoma-specific mortality. The competing mortality curves of the 3 age subgroups (<60 vs 60-70 vs ≥70) separated dramatically at the 5- and 10-year landmarks (Figure 4B); whereas the curves of lymphoma-specific mortality were similar across age subgroups throughout follow-up (Figure 4A). In contrast to age, disease stage showed a significant effect on lymphoma-specific mortality: stage II-IV was associated with an increased lymphoma-specific mortality throughout follow-up (Figure 4C; Table 4); whereas the competing mortality was similar to the stage I group (Figure 4D). Origin site showed no effects in either lymphoma-specific mortality or competing mortality (Figure 4E-F); whereas MALT-IPI showed significant effect on both lymphoma-specific and competing mortalities (Figure 4G-H).
Discussion
This study estimates the excess mortality associated with the diagnosis of MALT lymphoma and its various presentations compared with the general lymphoma-free age-matched US population. We demonstrate that a diagnosis of MALT is associated with a modest increase in relative mortality (1.4-fold). Overall, 10-year survival was 75% with <20% of all deaths due to lymphoma or its complications (an absolute lymphoma-specific mortality of 4%). The data are in keeping with other large series2,9,19-21 including baseline characteristics such as age distribution and commonly involved sites, as well as demonstration of a typically indolent disease course. The 5-year PFS was 64% in our cohort, which aligns with estimates of 60% to 66% from other prospective or retrospective series.1,11 Long-term surveillance is essential for MALT lymphoma watchful waiting, as the disease harbors a persistent and ongoing risk of relapse. Conversely, large-cell transformation (2.3% in our cohort and 2%-3.6% in literature2,9,11,22) and lymphoma-specific mortality were low; 10-year lymphoma-specific mortality was 4.3% in our cohort. In the literature, the 5-year lymphoma-specific mortality was ∼5% to 7%,4,11 supporting the fact that most of the lymphoma progression events do not contribute directly to mortality.
The RS estimates employed in the present study quantitatively demonstrate the excess mortality risks attributable to MALT lymphoma. We found a pooled SMR of 1.4, indicating that this population had a 40% higher risk of death than the general population; Alderuccio et al21 recorded a SMR of 1.19 with stage I MALT lymphoma in SEER database. This is markedly lower compared with other lymphomas, including classical Hodgkin lymphoma (HL) (6.1 in the British Columbia database), aggressive diffuse large B-cell lymphoma (DLBCL) (2.88 in the Molecular Epidemiology Resource (MER) of the University of Iowa/Mayo Clinic Lymphoma Specialized Program and North Central Cancer Treatment Group NCCTG-N0489 cohort), and even indolent follicular lymphoma (2.55 in the Spanish Lymphoma Oncology Group study).23-25 Our results and recently published SEER results confirm that MALT lymphoma carries a modest but statistically significant compromise to life expectancy. Importantly, this impediment is nuanced, as evidenced by a holistic interpretation of the various survival analyses.
Age is a universally poor prognostic factor for OS in lymphoma prognostic models, such as IPI in DLBCL,26 Follicular Lymphoma International Prognostic Index (FLIPI) in follicular lymphoma,27 International Prognostic Score (IPS) in advanced Hodgkin lymphoma,28 and the MALT-IPI.5 The underling mechanisms may relate to the treatment tolerance and complications, the differential tumor biology, or competing medical risks. Therefore, different strategies are needed when optimizing treatment, as reflected by the applicability of age-adjusted models.26 In our study, older age proved to be a significant poor prognostic factor in multivariate OS modeling but a better factor in SMR analysis. This suggests that older age is associated with poorer outcomes when comparing with younger patients. However, in the context of the diagnosis itself, being diagnosed with MALT lymphoma at a younger age was associated with an increased all-cause and nonlymphoma mortality compared with the healthy population, whereas the disease is less likely to affect longevity in older patients. The increased nonlymphoma mortality might be related to late or delayed complications of MALT lymphoma (including known associated etiological autoimmune conditions or chronic infections) or its treatment during longer-term follow-up in the younger patient group because they obviously have a longer life expectancy. Similarly, elevated nonlymphoma mortalities of infection, and gastrointestinal, respiratory, blood, and vascular disease have also been documented in HL and DLBCL patients with long-term follow-up.29,30 A slightly increased lymphoma-specific mortality in old patients might be because of poorer tolerability of treatment. This observation indicates that great care should be taken not to over-treat these patients because for most the lymphoma is unlikely to compromise life expectancy; the treatment goal in elderly patients might be to relieve symptoms with the least aggressive treatment. For many, very low–dose RT to dominant or painful sites might represent a promising strategy in this regard, as previous studies have demonstrated MALT to be exquisitely sensitive to radiation doses as low as 4 Gy, which are associated with minimal to no toxicity.7,31
MALT lymphomas originate from, and often progress within, MALT sites in multiple organs. In the literature, presence of multiple lesions predicted increased high-grade transformation rate,1,3 lower PFS,6 and lower OS.1 In the current study, prognosis was analyzed according to disease dissemination and involved sites with detailed clinical scenarios. This study documented that stage II to IV disease was associated with a higher risk of lymphoma-related mortality but not competing mortalities. From a SEER Medicare database study of gastric MALT lymphoma, stage was also strongly prognostic for lymphoma-related death.32 These results suggest that disease stage could serve as a lymphoma-specific prognostic factor.
The stomach, skin, lung, and orbit represent the 4 most common MALT lymphoma sites and accounted for approximately two-thirds of the cases in this cohort. This distribution was in agreement with other large series, although the specific percentages were slightly different across studies, possibly owing to the referral pattern and local epidemiology.2,5,9 Sites of origin not only associate with diverse disease epidemiology but also affect treatment patterns. In our study, certain sites demonstrated prognostic significance for SMR but not in multivariate OS analysis. Cutaneous MALT lymphoma had the lowest SMR among all evaluated subgroups, indicating the best life expectancy and lowest excess mortality risk. This agrees with the RS results from the SEER database of early-stage MALT lymphoma.19,21 Outcome associations have been more heterogeneous for noncutaneous sites in both our study and in the literature. Gastric disease also had a low SMR in our model, which was not documented by the SEER early-stage study.19,21 The more favorable PFS and tendency for less dissemination of gastric compared with extragastric MALT lymphomas partially explain the low SMR in our study.2,6,12 This study did not evaluate the relative risks of other relatively uncommon sites owing to the small case numbers. In general, we and others have demonstrated an important effect of anatomic site in MALT lymphoma.
In the present cohort, treatments were highly individualized based on disease distribution, organ involved, and patient interests. A significant proportion of cases received primary local treatment and initial active surveillance in both localized and disseminated cases, respectively. Our study was neither designed nor powered to address the effects of management decision. However, the descriptive treatment information of our study mirrored the situations and challenges in the current MALT lymphoma treatment paradigms; recommendations were mainly based on retrospective cohorts and largely inferred from follicular lymphomas. Only recently were prospective trials of systemic regimens carried out in disseminated disease.9,22,33-35 Nonetheless, the low lymphoma-specific mortality and excellent survival recorded by our and other series should be a consideration for current and future treatment algorithms.2,9,11,19
Our study is limited by its retrospective and single-institutional nature but is based on a comprehensive data set and a long follow-up. Furthermore, it relates to the era that predates the introduction of multiple novel US Food and Drug Administration–approved agents, which may further reduce the already low lymphoma-specific mortality associated with MALT lymphoma.
In conclusion, the present study demonstrated the indolent disease course of MALT lymphoma and its modest compromised effect on survival. A presentation with disseminated disease was the main driver of reduced lymphoma-specific survival whereas younger age was surprisingly associated with an increase in nonlymphoma mortality.
Acknowledgment
This research was funded through the Connecticut Cancer Foundation and Memorial Sloan Kettering Cancer Center Support grant (P30 CA008748).
Authorship
Contribution: S.Q., B.S.I., and J.Y. designed the study; S.Q. and X.L. analyzed the data and made the figures; S.Q., B.I., A.N., S.T., and J.Y. wrote the manuscript; J.Y. contributed to the study coordination; and all authors contributed to data collection and interpretation and approved the manuscript.
Conflict-of-interest disclosure: B.S.I. has received an honorarium from GT Medical Technologies, Inc. The remaining authors declare no competing financial interests.
Correspondence: Joachim Yahalom, Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center, 530 East 74th St, New York, NY 10021; e-mail: yahalomj@mskcc.org.
Reference
Author notes
∗B.S.I. and J.Y. are joint senior authors.
Data are available on request from the corresponding author, Joachim Yahalom (yahalomj@mskcc.org).
The full-text version of this article contains a data supplement.