TO THE EDITOR:
The mechanisms responsible for platelet generation have been debated for more than a century. It is generally assumed that the physiological release of platelets from megakaryocytes (MKs) occurs primarily by the release of platelet-sized swellings from the tips of proplatelets.1,2 Proplatelets are long, branching membrane structures that extend from the MK surface into sinusoidal blood vessels in the bone marrow. They are also prominent in the lungs, and both MK and proplatelet fragmentation are thought to make a major contribution to in vivo platelet production. Much of the current understanding of proplatelet formation has been based on the characterization of cultured MKs, however it is notable that the structure and molecular mechanisms regulating in vitro proplatelet formation can differ significantly from what occurs in vivo.3,4
A recent elegant study from Samir Taoudi’s laboratory5 has provided comprehensive assessments of in vivo platelet biogenesis using whole-organ 3D and 4D quantitative imaging techniques during mouse embryogenesis, fetal development, and adult life. This study demonstrated that proplatelet formation in the bone marrow is uncommon (<5% of MKs), with most platelet-sized particles generated from distinct membrane structures, termed MK buds. Through a direct measurement of bud release at the whole-organ level, this study has challenged the long-held belief that proplatelets are predominant MK membrane structures generating platelets in vivo.5
The MK budding theory remains controversial. Italiano et al have raised legitimate concerns as to whether buds contain the requisite structural features of mature platelets, including α-granules, an open canalicular system, discoid morphology, and circumferential microtubule coil.6 Based on transmission electron microscopy of bone marrow MKs, Italiano et al have suggested that buds may not be platelet precursors but rather MK-derived microvesicles (MVs), which are devoid of organelles, platelet granules, and microtubule coils. These membrane structures, referred to as MK blebs, appear similarly sized to buds but conceptually seem to be based more on original descriptions of submicron microparticles derived from cultured MKs.7 This contrasts with the findings from Potts et al who demonstrated that MK buds are of similar size to circulating platelets and possess platelet granule markers, including VWF and platelet factor 4.4
Potts et al demonstrated that the majority of bone marrow MKs actively produce buds at any given time, which begs the question of why MK budding has not been widely recognized, even after decades of research. Several factors may contribute to this. One important factor has been the historical focus on the identification and characterization of proplatelets, with less attention to otherwise resting MKs. Microscopically, the buds are also not easy to visualize. Compared with the demarcation membrane system (DMS)-containing body of the MK, the peripheral margins of the MK have relatively lower levels of CD41 and CD42 expression, making it difficult to see buds without oversaturation from the DMS. Additionally, buds can only truly be appreciated using 3D imaging, and the examination of the full structure of the buds is necessary to ensure that these are not passing platelets, sections through proplatelets, or irregularities of the complex MK surface. Until recently, most studies have relied on 2D evaluation of MKs.
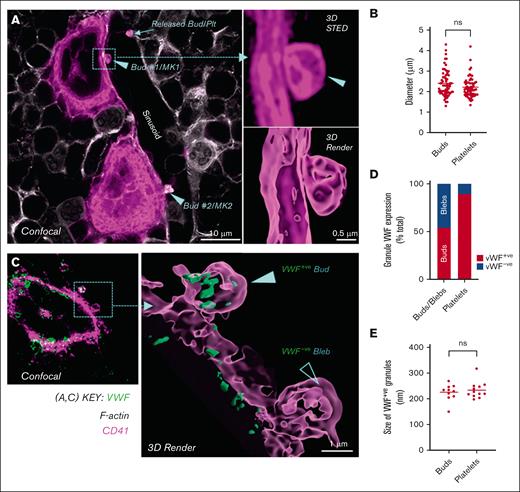
We have examined C57BL/6 bone marrow cryosections using both confocal and stimulated emission depletion (STED) microscopy, a super resolution technique that bypasses the diffraction limit of light microscopy, thereby providing superior 3D spatial resolution to confocal microscopy.8 We chose to use STED to provide sufficient resolution to evaluate subcellular structures of MKs and platelets. Mouse femurs were prepared in accordance with the method of Potts et al.5 Our studies have confirmed the infrequency of in vivo proplatelets, with only a minority (∼2%) of MKs producing sinusoid-directed cytoplasmic extensions. Consistent with the findings of Potts et al, our confocal and STED imaging of CD41 stained in situ MKs revealed that most MKs (76%) produced bud-like cytoplasmic extensions (Figure 1A). 3D STED and rendered images illustrated the bud shape and attachment to the budding MKs (Figure 1A). The average size of MK buds was ∼2.4 μm, which was comparable to that of intrasinusoidal platelets (Figure 1B).
MK budding is prominent in the bone marrow of mice. C57BL/6 mouse femoral bone marrow cryosections (10 and 40 μm) were immunostained with anti-CD41 (pink, for platelets and MKs) and anti-VWF (green, for granule content), co-stained with phalloidin (white, for F-actin), and then subjected to confocal and STED microscopy (Leica SP8; 93× glycerol objective; NA 1.3; z-step size, 80 nm). The images were deconvoluted using Huygens Software (ver. 20.04), processing was done using ImageJ (Ver. 1.53c), and surface renders were created using Imaris (ver. 9.8) using deconvolved fluorescent images. (A) Representative 2D confocal image (left panel) showing 2 MKs (MK1 and MK2) budding into a sinusoid. Their respective buds (Bud #1 and Bud #2), being released into the sinusoid (white) are indicated by arrow heads (▶). Buds were differentiated from transiting platelets by clear evidence of attachment to MKs based on the presence of CD41 joining the bud to the MK body. A released intrasinusoidal platelet is indicated by an arrow, which is not in direct proximity to a MK, differentiating it from a bud. Right panels depict a CD41+ STED optical section (top) and a 3D render created from the z-stack of the entire bud (bottom) of Bud #1 from MK1 (images were taken from one representative C57BL/6 bone marrow of n = 3, scale bar indicated). (B) Dot plot showing the size of buds and platelets in sinusoids, expressed as their maximal transverse diameter, from the evaluation of individual optical sections (50-60 buds and platelets from n = 3, C57BL/6 bone marrow), acquired using STED microscopy. (C) Representative confocal (left panel) and 3D STED render (right panel) images showing VWF+ buds (green; with granules) and VWF– blebs (without α-granules) on MKs. (D) Graph showing the percentage of VWF+ buds and VWF– blebs on MKs (64 budding MKs analyzed from n = 3, C57BL/6 bone marrows). (E) Graph showing the size of VWF+ granules within buds and platelets in the sinusoids. Data represent the mean granule size in 11 buds and 9 platelets. Statistical analysis (B, E) was performed using an unpaired t-test (GraphPad prism, v9.1.2).
MK budding is prominent in the bone marrow of mice. C57BL/6 mouse femoral bone marrow cryosections (10 and 40 μm) were immunostained with anti-CD41 (pink, for platelets and MKs) and anti-VWF (green, for granule content), co-stained with phalloidin (white, for F-actin), and then subjected to confocal and STED microscopy (Leica SP8; 93× glycerol objective; NA 1.3; z-step size, 80 nm). The images were deconvoluted using Huygens Software (ver. 20.04), processing was done using ImageJ (Ver. 1.53c), and surface renders were created using Imaris (ver. 9.8) using deconvolved fluorescent images. (A) Representative 2D confocal image (left panel) showing 2 MKs (MK1 and MK2) budding into a sinusoid. Their respective buds (Bud #1 and Bud #2), being released into the sinusoid (white) are indicated by arrow heads (▶). Buds were differentiated from transiting platelets by clear evidence of attachment to MKs based on the presence of CD41 joining the bud to the MK body. A released intrasinusoidal platelet is indicated by an arrow, which is not in direct proximity to a MK, differentiating it from a bud. Right panels depict a CD41+ STED optical section (top) and a 3D render created from the z-stack of the entire bud (bottom) of Bud #1 from MK1 (images were taken from one representative C57BL/6 bone marrow of n = 3, scale bar indicated). (B) Dot plot showing the size of buds and platelets in sinusoids, expressed as their maximal transverse diameter, from the evaluation of individual optical sections (50-60 buds and platelets from n = 3, C57BL/6 bone marrow), acquired using STED microscopy. (C) Representative confocal (left panel) and 3D STED render (right panel) images showing VWF+ buds (green; with granules) and VWF– blebs (without α-granules) on MKs. (D) Graph showing the percentage of VWF+ buds and VWF– blebs on MKs (64 budding MKs analyzed from n = 3, C57BL/6 bone marrows). (E) Graph showing the size of VWF+ granules within buds and platelets in the sinusoids. Data represent the mean granule size in 11 buds and 9 platelets. Statistical analysis (B, E) was performed using an unpaired t-test (GraphPad prism, v9.1.2).
To address whether MK buds have ultrastructural features similar to those of platelets, we evaluated the internal content of buds for the α-granule protein von Willebrand factor (VWF) (Figure 1C). 3D STED microscopy revealed that 55% of platelet-sized buds contained VWF (Figure 1D). The VWF+ clusters in buds were similar in size to those within the platelets (Figure 1E). Integrins αIIbβ3 and F-actin are 2 highly expressed internal platelet proteins. An internal pool of αIIbβ3 is contained within α-granules and the open canalicular system, whereas F-actin is present in the membrane skeleton and throughout the cytoplasm of platelets.9-11 STED microscopy confirmed abundant CD41 (αIIb) and F-actin lining the surface and internal structure of buds (Figure 2Ai). In contrast, no VWF or F-actin was present in the membrane blebs, nor was there internal staining of CD41 (Figure 2Aii). VWF+ buds were more homogenous in size than blebs, with a mean diameter and size distribution similar to those of platelets (2.37 μm ± 0.43 μm). Blebs however, ranged in size between 1 to 6 μm (3.04 μm ± 1.39 μm) and had a more heterogeneous morphology, ranging from compact bud-like morphology to elongated tubular structures. Italiano et al had proposed that MK buds may represent MVs. We confirmed that MVs were abundant in the bone marrow adjacent to MKs (Figure 2B). However, CD41+ MVs were much smaller than buds (<1 μm) and did not contain VWF.
MK buds are distinguishable from blebs and MVs. C57BL/6 mouse femoral cryosections were stained with antibodies against CD41 (pink), VWF (green), or phalloidin (white), and imaged using STED microscopy. (A) Representative 2D STED images showing MK buds with internal VWF, CD41, and F-actin (i), in contrast to hollow MK blebs (ii). (i, ii) Left hand panels depict merged, low magnification images, whereas the remaining panels depict a magnified bud (i) and hollow bleb; (ii) with individual fluorescence channels for VWF, CD41, and F-actin at high magnification. (B) Representative 3D STED (left panels) and 3D render (right panels) images showing VWF– MK MVs (top panel) and VWF+ MK buds (lower panel). Note: The top left panel contains an inset with highlighted MVs magnified by 3.5×, and the CD41 intensity increased to illustrate MVs.
MK buds are distinguishable from blebs and MVs. C57BL/6 mouse femoral cryosections were stained with antibodies against CD41 (pink), VWF (green), or phalloidin (white), and imaged using STED microscopy. (A) Representative 2D STED images showing MK buds with internal VWF, CD41, and F-actin (i), in contrast to hollow MK blebs (ii). (i, ii) Left hand panels depict merged, low magnification images, whereas the remaining panels depict a magnified bud (i) and hollow bleb; (ii) with individual fluorescence channels for VWF, CD41, and F-actin at high magnification. (B) Representative 3D STED (left panels) and 3D render (right panels) images showing VWF– MK MVs (top panel) and VWF+ MK buds (lower panel). Note: The top left panel contains an inset with highlighted MVs magnified by 3.5×, and the CD41 intensity increased to illustrate MVs.
Overall, our findings are consistent with those of Potts et al and support the notion that MK budding is a common process that is likely to represent a viable mechanism for platelet biogenesis in the bone marrow. They also help reconcile some of the concerns raised by Italiano et al, that a subset of buds do not contain ultrastructural features consistent with those of platelets. In addition to in vivo proplatelets, our studies confirmed that there are at least 3 distinct membrane structures derived from MKs in the bone marrow (Figure 3). First, VWF+ buds, containing internal αIIbβ3 and F-actin, had a size distribution consistent with that of platelets. Second, VWF– membrane blebs, which appear to be similar to buds, lack internal CD41 and F-actin. These structures are likely to represent membrane blebs formed at the rim of MKs, as described by Italiano et al. Finally, MVs which are discrete submicron CD41+ structures scattered throughout the marrow, likely similar to those previously observed in circulation by Flaumenhaft et al.7
Schematic of 2 platelet production mechanisms and MK membrane structures in bone marrow. Bone marrow MKs release platelets via 2 distinct mechanisms: (1) Proplatelets: long and irregularly shaped cytoplasmic protrusions that extend from the surface of MKs into sinusoids. Proplatelets can undergo further fragmentation prior to being converted into circulating platelets; and (2) Membrane buds: platelet-sized membrane protrusions directly released from the MK surface into sinusoids. Budding MKs are more frequently detected (70%-80%) in the bone marrow than proplatelets (2%). Buds are membrane structures distinct from MK membrane blebs and MVs. Buds contain typical platelet granule proteins (VWF) as well as internal CD41 (integrin αIIbβ3) and filamentous actin, all of which are absent in blebs and microvesicles. Created using BioRender.com.
Schematic of 2 platelet production mechanisms and MK membrane structures in bone marrow. Bone marrow MKs release platelets via 2 distinct mechanisms: (1) Proplatelets: long and irregularly shaped cytoplasmic protrusions that extend from the surface of MKs into sinusoids. Proplatelets can undergo further fragmentation prior to being converted into circulating platelets; and (2) Membrane buds: platelet-sized membrane protrusions directly released from the MK surface into sinusoids. Budding MKs are more frequently detected (70%-80%) in the bone marrow than proplatelets (2%). Buds are membrane structures distinct from MK membrane blebs and MVs. Buds contain typical platelet granule proteins (VWF) as well as internal CD41 (integrin αIIbβ3) and filamentous actin, all of which are absent in blebs and microvesicles. Created using BioRender.com.
Our studies have confirmed the existence of MK membrane buds and demonstrated that they contain ultrastructural features consistent with those of platelets. They highlight the importance of using multiple platelet markers and imaging modalities to classify the diverse membrane structures derived from MKs. It remains to be seen how buds convert from circular structures into flat, discoid platelets in circulation and what cytoskeletal mechanics drive bud formation and generation of the microtubule marginal band. The mechanism by which dysregulated budding leads to qualitative and quantitative disorders of platelets will be an important area for future investigation.
Acknowledgments: The authors thank Simone Schoenwaelder for assistance in preparing and proofreading the manuscript. The authors acknowledge the technical and scientific assistance of Sydney Microscopy & Microanalysis, University of Sydney Node of Microscopy Australia.
This work was supported by the Kanematsu Research Award (Royal College of Pathologists of Australasia) (M.L.E.) and New South Wales Health (NSW, Australia) Cardiovascular Capacity Building Grants (S.P.J.). S.P.J. is supported by the National Health and Medical Research Council of the Australia Investigator Grant (Leadership 3, No.1176016).
Contribution: M.L.E., I.A., and R.S. performed the experiments; M.L.E., I.A., and Y.Y. analyzed the results and made the figures; and M.L.E., Y.Y., and S.P.J. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shaun P. Jackson, The Heart Research Institute, Level 3, Building D17, Charles Perkins Centre, University of Sydney, Orphan School Creek Rd, Camperdown, NSW 2006, Australia; e-mail: shaun.jackson@sydney.edu.au.
References
Author notes
Data are available on request from the corresponding author, Shaun P. Jackson (shaun.jackson@sydney.edu.au).