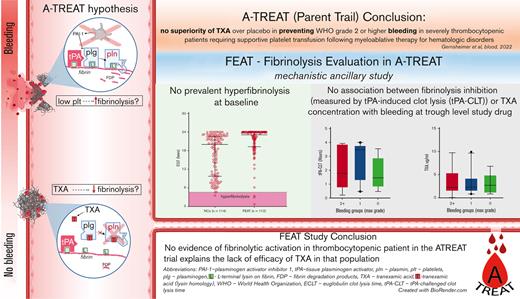
Key Points
There was no evidence of fibrinolytic activation in patients with hypoproliferative anemia.
The absence of systemic fibrinolysis may explain the absence of response to TXA.
Abstract
The American Trial Using Tranexamic Acid (TXA) in Thrombocytopenia (A-TREAT, NCT02578901) demonstrated no superiority of TXA over placebo in preventing World Health Organization (WHO) grade 2 or higher bleeding in patients with severe thrombocytopenia requiring supportive platelet transfusion following myeloablative therapy for hematologic disorders. In this ancillary study, we sought to determine whether this clinical outcome could be explained on the basis of correlative assays of fibrinolysis. Plasma was collected from A-TREAT participants (n = 115) before the initiation of study drug (baseline) and when TXA was at steady-state trough concentration (follow-up). Global fibrinolysis was measured by 3 assays: euglobulin clot lysis time (ECLT), plasmin generation (PG), and tissue-type plasminogen activator (tPA)–challenged clot lysis time (tPA-CLT). TXA was quantified in follow-up samples by tandem mass spectrometry. Baseline samples did not demonstrate fibrinolytic activation by ECLT or tPA-CLT. Furthermore, neither ECLT nor levels of plasminogen activator inhibitor-1, tPA, plasminogen, alpha2-antiplasmin, or plasmin-antiplasmin complexes were associated with a greater risk of WHO grade 2+ bleeding. TXA trough concentrations were highly variable (range, 0.7-10 μg/mL) and did not correlate with bleeding severity, despite the fact that plasma TXA levels correlated strongly with pharmacodynamic assessments by PG (Spearman r, −0.78) and tPA-CLT (r, 0.74). We conclude that (1) no evidence of fibrinolytic activation was observed in these patients with thrombocytopenia, (2) trough TXA concentrations varied significantly between patients receiving the same dosing schedule, and (3) tPA-CLT and PG correlated well with TXA drug levels.
Introduction
Antifibrinolytic agents such as tranexamic acid (TXA) have been extensively studied in numerous inherited and acquired bleeding disorders. The intense interest in TXA as a hemostatic agent has been spurred by its favorable efficacy and safety profile in bleeding related to surgery, trauma, and postpartum hemorrhage, all of which are associated with acute onset and potentially severe bleeding.1-3 More recently, a number of randomized placebo-controlled trials have addressed whether TXA is similarly beneficial in other types of bleeding, including severe gastrointestinal (GI) and nontraumatic subarachnoid or intracranial hemorrhage. However, in general, the results have been negative.4-7 The role of TXA as an adjunct to platelet transfusion in patients with thrombocytopenia with bone marrow failure was proposed many years ago, and on the basis of some early encouraging data, it was adopted by some centers as a standard of care.8,9 However, until recently, no appropriately designed and adequately powered prospective study had formally addressed this topic.10
To this end, we conducted the American Trial Using Tranexamic Acid in Thrombocytopenia (A-TREAT: NCT02578901), a multicenter, prospective, double-blinded, placebo-controlled, randomized clinical trial that compared TXA with placebo in 330 adult patients with hematologic malignancy requiring platelet transfusion support for hypoproliferative thrombocytopenia during chemotherapy or hematopoietic stem cell transplantation. We did not observe any difference in the rate of World Health Organization (WHO) grade 2 or greater (2+) bleeding or in the mean number of administered platelet transfusions. There were also no differences in the number of thrombotic events or deaths related to serious bleeding or thrombosis.11
Fibrinolysis Evaluation in A-TREAT (FEAT) was an ancillary laboratory investigation that included 115 patients enrolled in A-TREAT. The goal of FEAT was to better understand the role of fibrinolysis in bleeding and to evaluate the pharmacodynamic effects of TXA in these patients with thrombocytopenia by correlating assays of fibrinolysis in plasma with the primary clinical outcomes and with measured TXA drug levels. Specifically, the a priori study hypotheses were that (1) a baseline “hyperfibrinolytic” profile would be associated with a higher proportion of WHO grade 2+ bleeding; and (2) steady-state trough TXA levels would be associated with a “hypofibrinolytic” profile and a lower proportion of grade 2+ bleeding. The results of this ancillary study may shed light on why TXA failed to ameliorate bleeding outcomes in the A-TREAT study and, in so doing, may inform the design of future studies evaluating the role of TXA in amelioration of bleeding outcomes in various other clinical scenarios.
Patients and methods
Patients and samples
Enrollment in the FEAT study was initiated in March 2017 when the first 75 subjects had been enrolled in A-TREAT at the 3 participating sites, namely the University of North Carolina, the University of Washington, and the University of Pittsburgh. The study was approved by each institutional review board, and all participants signed a separate consent form to be included in the FEAT ancillary study. The last patient was enrolled on completion of the A-TREAT study in February 2020. Patients in the TXA arm were administered oral TXA 1300 mg every 8 hours once their platelet count fell below 30 000/μL. For patients who were unable to tolerate oral medication, TXA was administered intravenously at a dose of 1 g every 8 hours. TXA dosing interval was renally adjusted daily as appropriate, according to the study protocol.11 Two blood samples were collected from participating subjects. The first (baseline) sample was obtained immediately before initiation of study drug, when the platelet count was <30 000/μL. When possible, a second (trough) sample was drawn when TXA was expected to be at a steady-state level following study drug initiation and within 2 hours before the next scheduled dose administration. The analysis population consisted of those participants with at least 1 baseline lab available.
All samples were collected in 3.2% sodium citrate in a 1:9 ratio and were processed to yield platelet-free plasma (PFP) according to International Society on Thrombosis and Haemostasis guidelines (double centrifugation at 2500g for 15 minutes at room temperature) and immediately stored at −80°C. The same operating procedure was used at all 3 study sites. After patient enrollment was completed, frozen samples were shipped to the University of North Carolina for analysis.
Reagents
Human plasminogen-free fibrinogen was purchased from Aniara Diagnostica (West Chester, OH). Ovalbumin (98% purity, grade V) was purchased from Millipore-Sigma (St. Louis, MO). Re-lipidated tissue factor (TF) (Dade Innovin, B4212-40 Siemens Healthcare, Erlangen, Germany), acetic acid (glacial, 100%, reagent grade), mineral oil (light, C16H10N2Na2O7S2, reagent grade), and 96-well microplates (polystyrene, flat bottom, nontissue culture–treated plate, Falcon) were from Fisher Scientific (Hampton, NH). Human alpha thrombin was purchased from Haematologic Technologies (Essex Junction, VT). Recombinant tissue-type plasminogen activator (tPA) was from Innovative Research (Novi, MI). Heparinase was from New England Biolabs (Ipswich, MA). Distilled water was prepared using a Direct Q3 system (Millipore-Sigma, St Louis, MO). Tris-buffered saline (10×, 500-mM Tris, 1500-mM NaCl) was prepared in house, and the pH was adjusted to 7.4. Synthetic phospholipid vesicles (phosphatidylserine: phosphatidylethanolamine: phosphatidylcholine ratios of 15:41:44) were a gift from Dougald Monroe, University of North Carolina. For kinetic optical density measurements, a Synergy H1 spectrophotometer (BioTek, Winooski, VT) was used.
Procedure to ensure absence of heparin in samples
Although the protocol specified that blood should be obtained by peripheral venipuncture rather than via a central venous catheter, a significant proportion of samples (35%) was found to contain contaminating heparin. Thus, to neutralize any contaminating heparin that might interfere with the functional studies in plasma, all samples were treated with 40 U/mL of heparinase for 15 minutes at 37°C. The efficacy of the heparinase was confirmed by demonstrating normalization of the thrombin clotting time. Notably, heparinase treatment itself had no effect on the euglobulin clot lysis time (ECLT) in control samples (data not shown).
Development and validation of liquid chromatography tandem mass spectrometric assay to quantify TXA concentration in plasma
To quantify trough TXA concentrations, we developed and validated a liquid chromatography tandem mass spectrometric assay. A 1-step protein precipitation extraction using acidified acetonitrile was optimized for preparation of plasma samples to avoid the logistical complications and analytical variability/dilution introduced from derivatization reactions commonly used to quantify TXA (eg, perchloric acid precipitation with NaOH neutralization). Chromatographic separation was achieved using Waters XBridge Amide column (3.5 μm × 2.1 mm × 50 mm). TXA and an isotopically labeled TXA (internal standard) were detected by multiple reaction monitoring using electrospray ionization in positive mode. The final validated assay was linear over the concentration range of 0.2 to 400 μg/mL (linearity [R2] = 0.9977; lower limit of detection = 0.12 μg/mL; lower limit of quantification ∼ 0.2 μg/mL). Analytical robustness was evaluated across multiple independent preparations by analyzing replicate samples (n = 6) at 4 concentrations within the linear concentration range (including lower limit of detection of 0.12 μg/mL). The intra- and interday precision values were below 10%, and accuracy was better than 4% (range, 97%-104%).
Euglobulin clot lysis time
ECLT was performed as previously described.12 In brief, PFP was diluted 1:10 in distilled water and acidified to pH 5.9, followed by incubation for 30 minutes on ice. After centrifugation (1000 × g, 6 minutes), the supernatant was removed, and the residue was dissolved to half of the original plasma volume in tris-buffered saline. This euglobulin fraction was then mixed with plasminogen-free fibrinogen (250 μg/mL) and ovalbumin (4%) and clotted with thrombin (17.6 nM). To prevent desiccation, mineral oil was added to the top of each well. Optical density changes were then read in a microplate reader (λ = 405 nm, 37°C) for 24 hours. The ECLT (representing the time from half maximal clotting to half maximal lysis) was the primary end point. We previously determined that intra- and interassay coefficients of variation for the ECLT were 2.5% and 9.9%, respectively.12
The tPA-challenged clot lysis assay (tPA-CLT) was performed in 96-well microplates and turbidity was read in a spectrophotometer (λ = 405 nm, 37°C), as previously described.12 In brief, coagulation and fibrinolysis were simultaneously initiated in PFP by recalcification, addition of TF (1 pM), phosphatidylserine: phosphatidylcholine: phosphatidylethanolamine phospholipid vesicles (20 μM), and tPA (0.6 nM for assays on the baseline samples). However, in the follow-up samples, this assay was used primarily to assess the inhibitory effect of TXA on fibrinolysis. Thus, a higher tPA concentration (16 nM) was required to produce clot lysis in the range of trough TXA levels encountered. Lysis was observed for 24 hours. To prevent the clot from drying out, mineral oil was added on the top of each well. The tPA-CLT (the time from half maximal clotting to half maximal lysis) was recorded as the primary end point. We previously determined that intra- and interassay coefficients of variation for the tPA-CLT assay were 4.8% and 9.4%, respectively.12
For both the ECLT and tPA-CLT assays, data were obtained using Gen5 software and processed using Shiny apps developed by Colin Longstaff.13 We previously defined reference ranges for both assays in our laboratory. Thus, for the ECLT, the 2.5th to 97.5th percentile range in healthy individuals was 4.6 to 23.5 hours, whereas for the tPA-CLT assay (with 0.6 nM tPA concentration), it was 1.6 to 23.97 hours.12
PG assay
Plasmin generation (PG) was measured using a previously reported method with a calibrated, automated design.14 TF, phospholipids, and tPA were added to PFP in reaction wells; calibrator wells contained plasma and α2-macroglobulin/plasmin complex. Reactions were initiated by automatically dispensing CaCl2 and fluorogenic substrate to each well. Fluorescence curves were used to calculate descriptive parameters, including lag time, velocity, peak of PG, and endogenous plasmin potential (area under the curve), as previously described.14
D-dimer levels were measured as fibrinogen-equivalent units by each participating center’s clinical laboratory using the Hemosil IL D-dimer HS500 assay at the University of North Carolina and the Stago STA Liatest D-Di assay at the University of Pittsburgh and University of Washington.
Plasma-free tPA, total plasminogen activator inhibitor-1 (PAI-1), α2 antiplasmin, and plasmin-α2 antiplasmin complexes were detected using commercially available ELISA kits from Molecular Innovations (Novi, MI), according to the manufacturer’s instructions.
Statistical analysis
All continuous variables are described with the mean and standard deviation (SD), as well as the median and quartiles. Categorical variables are expressed as percentages. The confidence interval for the proportion of participants with hyperfibrinolysis as measured by the ECLT assay was estimated using the inverted score test with a continuity correction. Statistical significance in the mean difference between 2 groups was estimated by the t test. When >2 groups were analyzed, a one-way ANOVA test was used (with heteroskedasticity-consistent standard errors). A linear regression model with heteroskedasticity-consistent standard errors was used to assess the association between trough TXA levels and select covariates. Significance is presented as P values, with P < .05 considered statistically significant. Correlation was assessed by Spearman rank-order and presented as Spearman r correlation coefficient. On days where participants had a missing bleeding assessment, it was assumed that no grade 2 or higher bleed occurred. No adjustments were made for multiple comparisons. All analyses were conducted in IBM SPSS Statistics version 26.0.0.0 (IBM Inc, Armonk, NY), GraphPad Prism version 7.05 (GraphPad Software Inc, San Diego, CA), R version 4.1.2 (R Foundation, Vienna, Austria), or SAS 9.4 (SAS Institute Inc, Cary, NC).
Results
In total, 126 of the 356 enrolled A-TREAT study subjects provided informed consent and were coenrolled in FEAT. At least 1 baseline laboratory test was available for 115 participants. Demographic data and myeloablative treatment modality are shown in Table 1. Overall, 47% of patients were undergoing allogeneic stem cell transplantation, 31.3% were undergoing autologous transplantation, and 21.7% were receiving chemotherapy. The FEAT participants had a higher mean BMI than non-FEAT A-TREAT subjects, as well as different primary diagnoses and treatment strata (supplemental Table 1). Notably, however, the proportion of FEAT vs non-FEAT participants who experienced grade 2+ bleeding (45% vs 46%, respectively) was identical. Similarly, the frequency of the primary end point in FEAT subjects who were randomized to placebo vs TXA did not differ. Specifically, 25 of 58 subjects (43.1%) in the placebo group, and 26 out of 57 subjects (45.6%) in the TXA group experienced a grade 2+ bleed.
Demographics of the FEAT study cohort
| Demographics . | N = 115 . |
|---|---|
| Age, mean (SD) | 52.8 (12.6) |
| Sex, n (%) | |
| Male | 53 (46.1) |
| Female | 62 (53.9) |
| BMI, mean (SD) | 30.7 (7.6) |
| Race, n (%) | |
| American Indian/Alaska Native | 0 (0) |
| Asian | 3 (2.6) |
| Native Hawaiian/Pacific Islander | 0 (0) |
| Black/African American | 12 (10.4) |
| White | 94 (81.7) |
| More than 1 | 1 (0.9) |
| Unknown/not reported | 4 (3.5) |
| Ethnicity, n (%) | |
| Hispanic | 4 (3.5) |
| Non-Hispanic | 89 (77.4) |
| Unknown/not reported | 22 (19.1) |
| Disease status, n (%) | |
| Leukemia, in remission | 33 (28.7) |
| Leukemia, active disease | 16 (13.9) |
| Other | 66 (57.4) |
| Therapeutic group, n (%) | |
| Allogeneic transplant | 54 (47) |
| Autologous transplant | 36 (31.3) |
| Chemo/immunotherapy | 25 (21.7) |
| Demographics . | N = 115 . |
|---|---|
| Age, mean (SD) | 52.8 (12.6) |
| Sex, n (%) | |
| Male | 53 (46.1) |
| Female | 62 (53.9) |
| BMI, mean (SD) | 30.7 (7.6) |
| Race, n (%) | |
| American Indian/Alaska Native | 0 (0) |
| Asian | 3 (2.6) |
| Native Hawaiian/Pacific Islander | 0 (0) |
| Black/African American | 12 (10.4) |
| White | 94 (81.7) |
| More than 1 | 1 (0.9) |
| Unknown/not reported | 4 (3.5) |
| Ethnicity, n (%) | |
| Hispanic | 4 (3.5) |
| Non-Hispanic | 89 (77.4) |
| Unknown/not reported | 22 (19.1) |
| Disease status, n (%) | |
| Leukemia, in remission | 33 (28.7) |
| Leukemia, active disease | 16 (13.9) |
| Other | 66 (57.4) |
| Therapeutic group, n (%) | |
| Allogeneic transplant | 54 (47) |
| Autologous transplant | 36 (31.3) |
| Chemo/immunotherapy | 25 (21.7) |
BMI, body mass index.
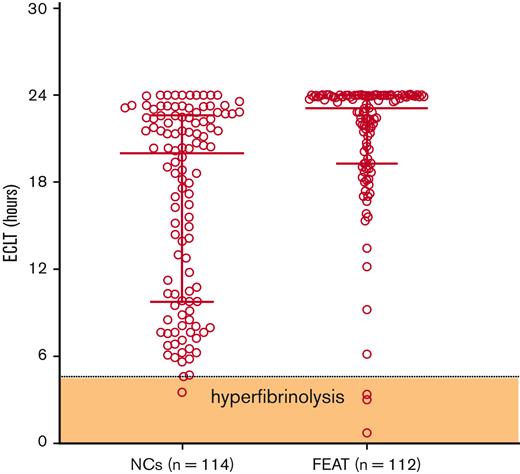
To evaluate whether the presence of a hyperfibrinolytic profile was associated with a higher proportion of WHO grade 2+ bleeding, baseline samples were tested for evidence of hyperfibrinolysis, which we previously defined as ECLT < 4.6 hours, the 2.5th percentile of the reference range.15Figure 1 demonstrates that the majority of ECLTs fell within the reference range, with only 3 samples (2.7%; 95% confidence interval [95% CI], 0.7%-8.4%) demonstrating a “hyperfibrinolytic” profile. Thus, these data do not support the presence of hyperfibrinolysis in the plasma fraction of these patients. Although the ECLT is acknowledged to be uniquely sensitive to the balance of endogenous tPA relative to PAI-1 in plasma, it is not sensitive to levels of antifibrinolytic regulatory molecules, such as PAI-1 and thrombin activatable fibrinolysis inhibitor.16,17 Because the tPA-CLT assay is considered to be much more sensitive to endogenous antifibrinolytic capacity in plasma, we also measured tPA-CLT in the same baseline samples. The distribution of values between study subjects and normal controls was unremarkable, as shown in supplemental Figure 1. Furthermore, no difference in tPA-CLT was noted between subjects who experienced grade 2+ bleeding and those who did not (Table 2). Also consistent with the lack of baseline hyperfibrinolysis, none of the individual fibrinolytic parameters were associated with WHO grade 2+ bleeding. Indeed, the sole parameter that was associated with grade 2+ bleeding was a low baseline hematocrit (P < .01), a known risk factor for bleeding that is assumed to be due to reduced platelet margination and adhesion within the intravascular compartment18,19 (Table 2). Thus, in aggregate, these data suggest that bleeding was independent of the presence of endogenous fibrinolytic activation at baseline.
Baseline ECLT in FEAT participants compared with NCs. The shaded zone labeled “hyperfibrinolysis” is defined by the 2.5th percentile of the reference range. ∗∗∗∗P < .0001. NCs, normal controls.
Baseline ECLT in FEAT participants compared with NCs. The shaded zone labeled “hyperfibrinolysis” is defined by the 2.5th percentile of the reference range. ∗∗∗∗P < .0001. NCs, normal controls.
Baseline analytes and bleeding outcomes
| Baseline assays and future bleeding (activated subjects) . | No WHO grade 2+ bleeding . | WHO grade 2+ bleeding . | P value . | |
|---|---|---|---|---|
| Platelets × 103/mm3 | N | 64 | 51 | |
| Mean (SD) | 20.5 (5) | 21 (9) | .72 | |
| Median (Q1, Q3) | 20 (17, 24) | 20 (17, 24) | ||
| Hematocrit % | N | 64 | 51 | |
| Mean (SD) | 28 (4.3) | 25.5 (2.5) | <.01 | |
| Median (Q1, Q3) | 28 (25, 30) | 25.7 (24, 27) | ||
| Serum creatinine (mg/dL) | N | 64 | 51 | |
| Mean (SD) | 0.7 (0.2) | 0.7 (0.2) | .64 | |
| Median (Q1, Q3) | 0.7 (0.6, 0.8) | 0.7 (0.5, 0.9) | ||
| ANC × 103/mm3 | N | 62 | 51 | |
| Mean (SD) | 0.2 (0.7) | 0.5 (1.1) | .21 | |
| Median (Q1, Q3) | 0 (0, 0.1) | 0 (0, 0.2) | ||
| Clauss fibrinogen (mg/dL) | N | 30 | 30 | |
| Mean (SD) | 400.7 (174.7) | 445.7 (151.1) | .29 | |
| Median (Q1, Q3) | 401.5 (279.2, 485.5) | 422 (335.5, 575.5) | ||
| D-dimer (μg/mL) | N | 64 | 51 | |
| Mean (SD) | 2 (5.1) | 1.6 (3.3) | .57 | |
| Median (Q1, Q3) | 0.9 (0.5, 1.9) | 0.8 (0.4, 1.4) | ||
| ECLT (h)∗ | N | 61 | 49 | |
| Mean (SD) | 20.6 (5.5) | 21.9 (2.9) | .11 | |
| Median (Q1, Q3) | 23.1 (18.9, 24.0) | 23.7 (21.2, 24.0) | ||
| tPA-CLT (h)∗ | N | 62 | 51 | |
| Mean (SD) | 3.5 (2.4) | 3.3 (1.9) | .53 | |
| Median (Q1, Q3) | 2.8 (2.3, 3.4) | 2.7 (2.3, 3.0) | ||
| Total PAI-1 (ng/mL) | N | 62 | 50 | |
| Mean (SD) | 24.2 (25.9) | 24.2 (23) | 1.00 | |
| Median (Q1, Q3) | 16 (9.1, 28.8) | 17.8 (7.3, 30.9) | ||
| tPA (IU/mL) | N | 62 | 50 | |
| Mean (SD) | 0.4 (0.6) | 0.3 (0.6) | .73 | |
| Median (Q1, Q3) | 0.2 (0.1, 0.4) | 0.1 (0.1, 0.3) | ||
| Plasminogen (μg/mL) | N | 57 | 37 | |
| Mean (SD) | 119.7 (76.8) | 115.2 (83.6) | .79 | |
| Median (Q1, Q3) | 97.7 (68.4, 161.9) | 78.4 (59.9, 139.9) | ||
| PAP complexes (μg/mL) | N | 57 | 37 | |
| Mean (SD) | 1.2 (1.1) | 1.1 (1) | .79 | |
| Median (Q1, Q3) | 0.8 (0.5, 1.5) | 0.8 (0.5, 1.4) | ||
| Α2AP (μg/mL) | N | 57 | 37 | |
| Mean (SD) | 223.1 (191) | 250.4 (211.5) | .53 | |
| Median (Q1, Q3) | 179.8 (102, 277) | 180.9 (100.4, 333.7) | ||
| Baseline assays and future bleeding (activated subjects) . | No WHO grade 2+ bleeding . | WHO grade 2+ bleeding . | P value . | |
|---|---|---|---|---|
| Platelets × 103/mm3 | N | 64 | 51 | |
| Mean (SD) | 20.5 (5) | 21 (9) | .72 | |
| Median (Q1, Q3) | 20 (17, 24) | 20 (17, 24) | ||
| Hematocrit % | N | 64 | 51 | |
| Mean (SD) | 28 (4.3) | 25.5 (2.5) | <.01 | |
| Median (Q1, Q3) | 28 (25, 30) | 25.7 (24, 27) | ||
| Serum creatinine (mg/dL) | N | 64 | 51 | |
| Mean (SD) | 0.7 (0.2) | 0.7 (0.2) | .64 | |
| Median (Q1, Q3) | 0.7 (0.6, 0.8) | 0.7 (0.5, 0.9) | ||
| ANC × 103/mm3 | N | 62 | 51 | |
| Mean (SD) | 0.2 (0.7) | 0.5 (1.1) | .21 | |
| Median (Q1, Q3) | 0 (0, 0.1) | 0 (0, 0.2) | ||
| Clauss fibrinogen (mg/dL) | N | 30 | 30 | |
| Mean (SD) | 400.7 (174.7) | 445.7 (151.1) | .29 | |
| Median (Q1, Q3) | 401.5 (279.2, 485.5) | 422 (335.5, 575.5) | ||
| D-dimer (μg/mL) | N | 64 | 51 | |
| Mean (SD) | 2 (5.1) | 1.6 (3.3) | .57 | |
| Median (Q1, Q3) | 0.9 (0.5, 1.9) | 0.8 (0.4, 1.4) | ||
| ECLT (h)∗ | N | 61 | 49 | |
| Mean (SD) | 20.6 (5.5) | 21.9 (2.9) | .11 | |
| Median (Q1, Q3) | 23.1 (18.9, 24.0) | 23.7 (21.2, 24.0) | ||
| tPA-CLT (h)∗ | N | 62 | 51 | |
| Mean (SD) | 3.5 (2.4) | 3.3 (1.9) | .53 | |
| Median (Q1, Q3) | 2.8 (2.3, 3.4) | 2.7 (2.3, 3.0) | ||
| Total PAI-1 (ng/mL) | N | 62 | 50 | |
| Mean (SD) | 24.2 (25.9) | 24.2 (23) | 1.00 | |
| Median (Q1, Q3) | 16 (9.1, 28.8) | 17.8 (7.3, 30.9) | ||
| tPA (IU/mL) | N | 62 | 50 | |
| Mean (SD) | 0.4 (0.6) | 0.3 (0.6) | .73 | |
| Median (Q1, Q3) | 0.2 (0.1, 0.4) | 0.1 (0.1, 0.3) | ||
| Plasminogen (μg/mL) | N | 57 | 37 | |
| Mean (SD) | 119.7 (76.8) | 115.2 (83.6) | .79 | |
| Median (Q1, Q3) | 97.7 (68.4, 161.9) | 78.4 (59.9, 139.9) | ||
| PAP complexes (μg/mL) | N | 57 | 37 | |
| Mean (SD) | 1.2 (1.1) | 1.1 (1) | .79 | |
| Median (Q1, Q3) | 0.8 (0.5, 1.5) | 0.8 (0.5, 1.4) | ||
| Α2AP (μg/mL) | N | 57 | 37 | |
| Mean (SD) | 223.1 (191) | 250.4 (211.5) | .53 | |
| Median (Q1, Q3) | 179.8 (102, 277) | 180.9 (100.4, 333.7) | ||
tPA concentration = 0.6 nM.
ANC, absolute neutrophil count; A2AP, alpha2 antiplasmin; PAP, plasmin-α2 antiplasmin.
Measured for a maximum of 24 hours.
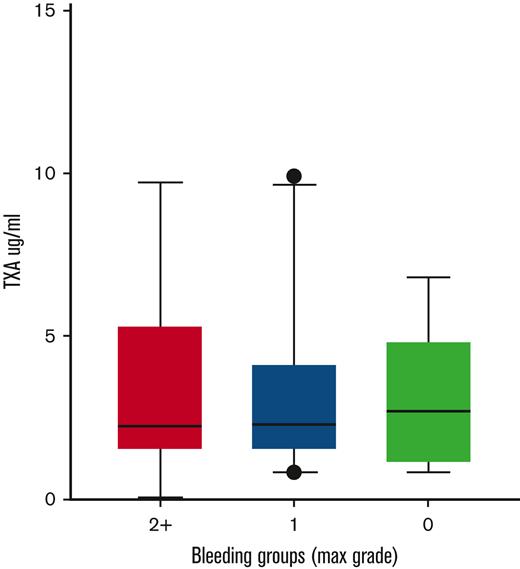
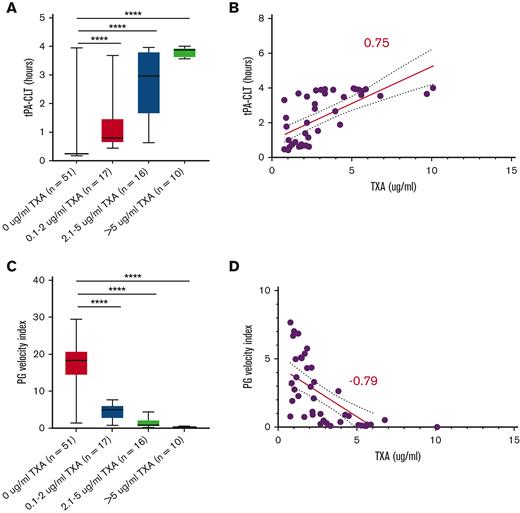
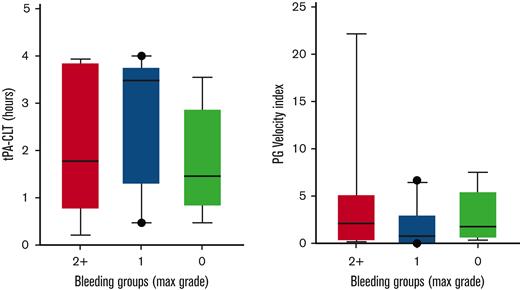
Follow-up samples containing TXA (or placebo) at steady-state trough concentrations were available from 94 subjects. Samples were obtained within 2 hours before the next scheduled TXA administration at a mean of 5 ± 1.2 (mean ± SD) days following study drug initiation. These samples were used to address the hypothesis that steady-state trough TXA concentration would be associated with a “hypofibrinolytic” laboratory profile and a lower proportion of WHO grade 2+ bleeding. As expected, no TXA was detected in patients randomized to placebo. A high interpatient variability of TXA trough concentrations was noted in the active treatment arm (range, 0.7-10 μg/mL). However, no correlation was noted between maximal bleeding grade and plasma TXA levels (Figure 2). Although TXA levels were not correlated with baseline serum creatinine concentration or BMI, serum creatinine measured on the day that blood was drawn for determining TXA levels was highly correlated (P < .001). More specifically, each mg/dL increase in serum creatinine was associated with a 4.76-μg/mL increase in trough TXA level. Of the 91 patients in whom route of drug administration was recorded, 40 (44%) received oral study drug, 47 (52%) received IV drug, and 4 (4%) received a mix of both oral and IV drug on the day of the trough sample. Notably, however, the route of administration (oral or IV) was not associated with trough TXA level (P = .61). Because the ECLT is essentially insensitive to TXA, we selected 2 other global assays of fibrinolysis, namely the tPA-CLT and the PG assays, as pharmacodynamic readouts. Both require the addition of exogenous tPA to clotted plasma and are sensitive to TXA as well as levels of endogenous antifibrinolytic molecules. As shown in Figure 3, a stepwise increase in tPA-CLT correlated with increasing trough TXA concentration across the range of drug levels encountered (Spearman r, 0.75; 95% CI, 0.56-0.85). Similarly, as previously noted,14 the PG velocity index was negatively correlated with TXA concentration (Spearman r, −0.79; 95% CI, −0.88 to −0.62) (Figure 3). However, neither tPA-CLT nor PG velocity index were associated with maximal bleeding severity (Figure 4).
Trough TXA concentration correlates with tPA-induced fibrinolysis (A-B) and inversely with PG (C-D). ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Trough TXA concentration correlates with tPA-induced fibrinolysis (A-B) and inversely with PG (C-D). ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Global fibrinolysis measurements at trough study drug concentration were not associated with changes in bleeding severity.
Global fibrinolysis measurements at trough study drug concentration were not associated with changes in bleeding severity.
Discussion
Bleeding is a morbid and potentially life-threatening complication of severe hypoproliferative thrombocytopenia in patients with hematologic malignancy undergoing myeloablative therapies.20,21 In the current standard of care, patients receive prophylactic platelet transfusions triggered by platelet counts <10 000/μL. However, the incidence of WHO grade 2 or higher bleeding in this population remains consistently high, in the range of 43% to 71%.22,23 The observation that bleeding is largely independent of platelet counts >5000/μL but that platelet transfusion can reduce grade 2 bleeding overall suggests that an interaction between platelet function and other, as yet unidentified modifier(s), determines bleeding risk. This rationale led us to perform the A-TREAT trial to test the hypothesis that (hyper)fibrinolysis, possibly related to reduced availability of platelet-associated PAI-1, augments thrombocytopenic bleeding. The FEAT study was subsequently launched to obtain and analyze plasma samples from A-TREAT participants who were recruited in the later phase of the trial.
The efficacy and safety of TXA in inherited and acquired bleeding disorders has been a subject of investigation since the development of TXA as a potent inhibitor of PG more than 50 years ago.1 As a measure of the interest in this topic, PubMed searches for “tranexamic acid and placebo-controlled trial” or “tranexamic acid and meta-analysis” produced 708 and 491 hits, respectively (23 May 2022). However, perception regarding the generalizability of the hemostatic efficacy of TXA remains a matter of debate.2,7 Data from large international clinical trials that have focused on major causes of hemorrhagic deaths, such as trauma or postpartum hemorrhage, have shown that administration of TXA within 3 hours of bleeding onset reduced death due to bleeding without increasing the risk of thromboembolic events.24-27 Conversely, an equally large international study addressing the role of TXA in GI bleeding (the HALT-IT trial), demonstrated no benefit of TXA on death from acute GI bleeding, with a higher incidence of venous thromboembolic events.4,28 Although providing high-quality clinical evidence for or against the use of TXA, these pragmatic clinical trials were not designed to address the mechanistic basis of the observed outcomes. In the absence of such data, possible explanations for the lack of efficacy in the HALT-IT trial have been proposed. These include delayed recognition of GI bleeding and thus delayed initiation of definitive therapies and/or administration of TXA, inherent demographic differences in an older population with significantly more comorbidities, and less activation of fibrinolysis in GI bleeding states, perhaps combined with a preexisting inherent hypofibrinolytic state.28 One could add the possibility of patient-to-patient variation in plasma TXA levels or half-life, although given the excellent bioavailability of oral TXA combined with the fact that it is a potent inhibitor of fibrinolysis across a relatively wide dosing range,29 this possibility seems less likely. However, it was not specifically addressed. Because TXA was administered over multiple days, we focused on steady-state trough levels and did not assay peak concentrations. To our knowledge, no previous study has quantified trough TXA levels in a multidose protocol to correlate them with bleeding outcomes. Despite the patient-to-patient variability in TXA levels, we did not observe any correlation with grade 2+ bleeding.
The laboratory assessment of systemic fibrinolysis has been a longstanding challenge that has impeded the ability to evaluate its contribution to bleeding in inherited or acquired disorders. Fibrinolysis in plasma is strongly inhibited by the large molar excess of antifibrinolytic regulatory molecules, particularly PAI-1. Therefore, to interrogate fibrinolytic activity, it is usually necessary to either add an exogenous plasminogen activator (such as tPA) or deplete endogenous levels of antifibrinolytic regulatory molecules.17,30 The most commonly used method for the assessment of fibrinolysis in clinical practice has been the whole-blood viscoelastic assays, thromboelastography (TEG) or rotational thromboelastometry.31,32 This approach has been used widely in trauma settings.32-34 However, some authors have questioned the sensitivity and specificity of TEG in the identification of endogenous fibrinolysis.33,34 We used the ECLT as a global assay to assess fibrinolysis at presentation in plasma samples from 171 patients with trauma. ECLT values ≤ 2.5th percentile of the reference range were present in 83 (48.5%) patients with trauma, indicating that hyperfibrinolysis has a significantly higher prevalence than can generally be appreciated by TEG.15 In that study, we also reported that a higher incidence of massive transfusion was noted in the ECLT-defined hyperfibrinolytic group than in those with a normal ECLT profile.15 Further studies are needed to determine whether hyperfibrinolysis defined by the ECLT is predictive of bleeding-related outcomes in trauma.
The absence of any evidence of systemic activation of fibrinolysis defined by the ECLT in the FEAT study samples is thus in marked contrast to trauma and leads to the hypothesis that this finding may explain the absence of hemostatic efficacy of TXA in A-TREAT. Given that platelets are a rich source of PAI-1, an a priori hypothesis in the A-TREAT trial was that local PAI-1 levels (both on the surface of activated platelets and in the surrounding milieu of a hemostatic plug) would be significantly reduced in the presence of severe thrombocytopenia, thereby promoting local fibrinolysis-related bleeding. Indeed, the concept of local hyperfibrinolysis as a contributor to bleeding in the absence of systemic hyperfibrinolysis has precedent in clinical medicine. For example, in women with heavy menstrual bleeding (whether associated with a known hemostatic defect such as von Willebrand disease or not), TXA is widely used. Several studies have shown that menstrual fluid from women with heavy menstrual bleeding has elevated fibrinolytic activity compared with control subjects.35,36 Although these patients do not have any evidence of associated systemic fibrinolysis,37 they respond well to TXA with quantitative bleeding reduction of 34% to 59%.35,38 Clearly, assessment of local hyperfibrinolysis in subjects with thrombocytopenia requires a different approach, but the absence of any hemostatic benefit of TXA in A-TREAT suggests that this is unlikely to be a major contributor to bleeding in these patients. However, it should be noted that subjects with acute promyelocytic leukemia, a distinct subtype of acute myeloid leukemia (AML M3) that is commonly associated with hyperfibrinolytic disseminated intravascular coagulation and associated bleeding,39,40 were excluded from participation in A-TREAT. In aggregate, these data suggest that a greater understanding of the underlying mechanisms that affect hemostasis may help to select the optimal clinical situations for the use of TXA.
A limitation of this study is that FEAT enrollment was not initiated concurrently with A-TREAT. Differences between the subjects in FEAT and the other A-TREAT subjects were observed in BMI, primary diagnosis, and mix of the 3 treatment categories. Nonetheless, despite these demographic differences, the proportion of FEAT and non-FEAT subjects who experienced grade 2+ bleeding was identical. Arguably, the lack of any correlative fibrinolysis studies using a viscoelastic whole blood approach, such as TEG, could also be construed as a weakness of the FEAT study. However, it is not clear that TEG is the established gold standard assay for the assessment of fibrinolysis in this patient population. Additionally, because of the rarity of severe WHO grade 3 or 4 bleeding in the A-TREAT study, we cannot state with certainty that systemic fibrinolytic activation, and thus possibly also efficacy of TXA, is not present in the most severe types of hemorrhage occurring in patients with severe thrombocytopenia related to myelosuppressive therapies.
Finally, an interesting observation from the FEAT trial is that trough TXA concentrations varied from 0.7 to 10 μg/mL in patients receiving the same dosing schedule. This was not explained on the basis of BMI or route of TXA administration (oral or IV) but was associated with serum creatinine. The global assays that we used as pharmacodynamic readouts, namely tPA-CLT and PG, both correlated well with TXA plasma concentrations, and thus may be used to estimate the extent of fibrinolytic inhibition in patients treated with TXA. Based on our observation, we suggest that additional correlative studies of TXA pharmacokinetics and pharmacodynamics should be incorporated into future studies examining the efficacy and safety of TXA in hemorrhagic disorders.
Acknowledgments
The authors acknowledge the patients who graciously agreed to participate in this study, as well as the clinical coordinators at the 3 study sites.
This study was supported by National Institutes of Health grants RO1HL146226, UO1HL122894, and UO1HL143403.
The content of this work is solely the responsibility of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the funder (National Heart, Lung, and Blood Institute).
Authorship
Contribution: N.S.K. and S.M. obtained funding for this study; A.I. and N.S.K. designed the study and wrote the initial draft of the manuscript; A.I., A.T.L., and L.A.H. performed all the plasma-based assays and were supervised by N.S.K. and A.S.W.; B.d.L. contributed essential reagents; S.M., S.P.B., and H.H. had access to all the study data and performed the statistical analysis; T.B.G., D.J.T., and N.S.K. were responsible for enrolling study patients at their respective sites; and all authors reviewed and contributed to later versions and revision of the manuscript.
Conflict-of-interest disclosure: B.d.L is employed by Synapse Research Institute, a not-for-profit member of the STAGO Diagnostic group that holds a patent on calibrated plasmin generation measurements. T.B.G. is a consultant for Cellphire Corporation. The remaining authors declare no competing financial interests.
Correspondence: Nigel S. Key, University of North Carolina School of Medicine, 8008B Mary Ellen Jones Building, CB# 7035, 116 Manning Dr, Chapel Hill, NC 27599; e-mail: nigel_key@med.unc.edu.
References
Author notes
Data are available on request from the corresponding author, Nigel S. Key (nigel_key@med.unc.edu).
The full-text version of this article contains a data supplement.