Key Points
A small subset of tFL with DLBCL morphology show a PMBL gene expression signature (tFL-PMBLsig-pos).
tFL-PMBLsig-pos cases show immunophenotypic and genetic features of FL/DLBCL and PMBL but lack PMBL-associated 9p gain/amplification.
Abstract
We investigated the clinicopathologic features of 5 follicular lymphomas (FLs) that transformed (tFL) morphologically to diffuse large B-cell lymphomas (DLBCLs) and had a primary mediastinal large B-cell lymphoma (PMBL)–like gene expression profile (tFL-PMBLsig-pos). None of the tFL-PMBLsig-pos cases arose in the mediastinum, all cases tested had a germinal center B-cell phenotype, 20% were CD30+, 60% CD23+, 80% MAL+, 20% CD200+, and 0% CD273/PDL2+. Whole-exome sequencing detected alterations in genes associated with both FL/DLBCL (CREBBP, KMT2C, KMT2D, ARID1A, HIST1 members, and TNFRSF14) and PMBL (JAK-STAT pathway genes, B2M, and CD58). Copy number (CN) analysis detected gains/amplification of REL and STAT6 in 60%, gains of SOCS1 in 40%, and gains of chromosome 16, including IL4R, in 40% of the cases. CN gains/amplification of BCL6 and MYC and loss of TNFRSF14 and TNFAIP3 were identified in 20% of the cases. Three of 5 cases lacked a BCL2 rearrangement. Despite having some features that are less common in DLBCL (MAL and CD23 expression and JAK-STAT activation), these tFL-PMBLsig-pos cases lack the most characteristic CN alteration seen in PMBL (9p24.1 gain/amplification). This cohort expands the biologic heterogeneity of tFL, illustrating a subset with gene expression and some genetic features reminiscent of PMBL, with potential treatment implications that include the use of novel targeted therapies.
Introduction
Follicular lymphoma (FL) is one of the most prevalent lymphomas in western countries.1-3 Newly diagnosed FL generally follows a chronic relapsing clinical course. However, there is about a 3% risk per year of transformation to a more aggressive lymphoma, which is associated with a significantly worse prognosis.4,5 More than 90% of transformed FLs (tFLs) are of diffuse large B-cell lymphoma (DLBCL) morphology, usually of the germinal center B-cell (GCB) type, but transformation to other types of lymphomas, including classic Hodgkin lymphoma, high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements, B-lymphoblastic leukemia/lymphoma, plasmablastic lymphoma, and even histiocytic/dendritic cell tumors, is documented.6,7
Although most DLBCLs are classified into 2 main groups (GCB and activated B-cell types), recent studies have shown more variation than can be described by these 2 categories.8,9 Duns et al recently characterized a set of de novo nonmediastinal DLBCLs that have clinical features of typical DLBCL but share immunophenotypic and genetic features with primary mediastinal large B-cell lymphoma (PMBL).10 Our study furthers this previous observation with the identification of 5 FLs that transformed into morphologic DLBCL and showed a PMBL-like gene expression (GE) signature (tFL-PMBLsig-pos). Immunophenotypic and molecular investigations were performed to determine how similar these tFL-PMBLsig-pos cases were to cases of bona fide PMBL.
Methods
Case selection and Lymph3Cx NanoString assay
The Lymph3Cx assay, which discriminates between DLBCL and PMBL and assigns a cell-of-origin (COO) group on the basis of GE, was performed on 170 tFLs with DLBCL morphology from Mayo Clinic, University of Pittsburgh Medical Center, and British Columbia Cancer (BCC) using protocols as previously described.10-12 This assay identified 5 tFL-PMBLsig-pos cases (3 of 24 cases tested at Mayo Clinic and 2 of 146 cases tested at BCC), which were further studied. All available clinical and laboratory data, diagnostic slides, and formalin-fixed paraffin-embedded tissue blocks or unstained slides were obtained with the approval of the institutional review/ethics boards of Mayo Clinic, BCC, and University of Pittsburgh Medical Center. The study was performed according to the Declaration of Helsinki.
Histology and immunophenotypic review
Conventional immunohistochemically stained slides prepared at the time of diagnosis were reviewed. The details of the immunohistochemical stainings performed on the 5 tFL-PMBLsig-pos cases are described in supplemental Methods.
Whole-exome sequencing (WES) and copy number (CN) analysis
The details of these studies performed on the 5 tFL-PMBLsig-pos cases are described in supplemental Methods.
Results and discussion
Molecular classification of tFL using GE assays
Lymph3Cx analysis of 170 tFLs with a DLBCL morphology identified 5 (3%) tFL-PMBLsig-pos cases. Of the remaining tFLs, 142 (84%) had a DLBCL GE signature; DLBCL-COO-GE (Lymph2Cx) analysis showed that 112 (79%) were GCB type, 20 (14%) were activated B-cell type, and 10 (7%) were unclassified. The remaining 23 (13%) tFLs showed an uncertain PMBL/DLBCL GE signature.
Clinicopathologic features of tFL-PMBLsig-pos cases
The 5 tFL-PMBLsig-pos cases included 4 males and 1 female with a median age of 62 years (range, 51-74 years). Four of the 5 patients had a history of FL diagnosed 1 to 12 years before the observation of tFL-PMBLsig-pos, for which all 4 patients received chemotherapy (Table 1). In 1 patient, the FL was diagnosed concurrently with the tFL-PMBLsig-pos. None of the tFL-PMBLsig-pos cases arose in the mediastinum, and none of the patients had a history of mediastinal disease. All 5 patients were subsequently treated with various regimens, with 4 receiving treatment that included rituximab and 3 receiving radiotherapy. At a median follow-up of 1.7 years (range, 0.7-10.8 years), 1 patient is alive with no evidence of lymphoma, 1 patient achieved a complete response but died of other causes, 1 patient is alive with persistent disease, and 2 patients died with progressive disease.
Clinical features of 5 tFL-PMBLsig-pos cases
| . | 1 . | 2 . | 3 . | 4 . | 5 . |
|---|---|---|---|---|---|
| EX00406 . | EX00408 . | EX004010 . | FL1179T2 . | FL1122T2 . | |
| Age (y) | 61 | 72 | 62 | 74 | 51 |
| Gender | M | F | M | M | M |
| FL histologic grade | 3A and 1-2 | 3A | 1-2 | 1 | 1 |
| Time from FL to tFL-PMBLsig-pos (y) | 7 | 12 | 0 | 6 | 1 |
| Prior treatment | R-CHOP, venetoclax, R-ICE, BR, R-GemOx, axicabtagene ciloleucel | R-CHOP, R-ESHAP, R-ICE, lenalidomide | None | R-CVP | Chlorambucil, ACOP-12 |
| Biopsy site | Paraspinal LN | Inguinal LN | Thyroid | Axillary LN | Liver |
| Mediastinal disease | No | No | No | No | No |
| Ann Arbor stage | III | I | III | NA | NA |
| Treatment of tFL-PMBLsig-pos | R2, Pola-R, RT | Rituximab, BR | R-CHOP | R-CHOP, RT | RT, cyclophosphamide |
| Outcome | DWD | AWD | DNED | ANED | DWD |
| Follow-up time from tFL-PMBLsig-pos diagnosis (y) | 0.7 | 2.9 | 1.7 | 10.8 | 0.7 |
| . | 1 . | 2 . | 3 . | 4 . | 5 . |
|---|---|---|---|---|---|
| EX00406 . | EX00408 . | EX004010 . | FL1179T2 . | FL1122T2 . | |
| Age (y) | 61 | 72 | 62 | 74 | 51 |
| Gender | M | F | M | M | M |
| FL histologic grade | 3A and 1-2 | 3A | 1-2 | 1 | 1 |
| Time from FL to tFL-PMBLsig-pos (y) | 7 | 12 | 0 | 6 | 1 |
| Prior treatment | R-CHOP, venetoclax, R-ICE, BR, R-GemOx, axicabtagene ciloleucel | R-CHOP, R-ESHAP, R-ICE, lenalidomide | None | R-CVP | Chlorambucil, ACOP-12 |
| Biopsy site | Paraspinal LN | Inguinal LN | Thyroid | Axillary LN | Liver |
| Mediastinal disease | No | No | No | No | No |
| Ann Arbor stage | III | I | III | NA | NA |
| Treatment of tFL-PMBLsig-pos | R2, Pola-R, RT | Rituximab, BR | R-CHOP | R-CHOP, RT | RT, cyclophosphamide |
| Outcome | DWD | AWD | DNED | ANED | DWD |
| Follow-up time from tFL-PMBLsig-pos diagnosis (y) | 0.7 | 2.9 | 1.7 | 10.8 | 0.7 |
ACOP-12, doxorubicin, cyclophosphamide, vincristine; AWD, alive with disease; BR, bendamustine and rituximab; DNED, died with no evidence of disease; DWD, died with disease; F, female; LN, lymph node; M, male; NA, not available; Pola-R, polatuzumab vedotin and rituximab; R2, lenalidomide and rituximab; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CVP, rituximab, cyclophosphamide, vincristine, prednisone; R-ESHAP, rituximab, etoposide, methylprednisolone, cytarabine, and cisplatin; R-GemOx, rituximab, gemcitabine, and oxaliplatin; R-ICE, rituximab, ifosfamide, carboplatin, and etoposide; RT, radiation therapy.
All tFL-PMBLsig-pos cases had typical centroblastic DLBCL cytology as well as fine sclerosis that is typical of PMBL (Figure 1 and Table 2). All 4 tested cases exhibited CD10 and/or BCL6 expression. Additional PMBL-associated markers evaluated showed that 1 of 5 cases were CD30+, 3 of 5 CD23+, 4 of 5 MAL+, 1 of 5 CD200+, and 0 of 5 CD273/PDL2+. BCL2 rearrangements were identified in 2 of 5 cases, BCL6 rearrangements in 2 of 5 cases, and MYC rearrangements in 0 of 5 cases. DLBCL-COO-GE (Lymph2Cx) analysis showed that 4 cases were GCB, and 1 was unclassified.
Morphology and immunophenotype of tFL-PMBLsig-pos cases. (A) The tFL-PMBLsig-pos (case 2) is composed of sheets of large B cells with predominantly centroblastic cytology that coexpress CD20 and CD10 (not shown). (B) CD23 is diffusely positive. (C) CD30 shows prominent background nonspecific staining with only rare strongly positive cells. (D) A large subset of the neoplastic B cells expresses MAL and (E) CD200 but is negative for (F) CD273/PDL2. Original magnifications ×500 (A) and ×200 (B-F); A, hematoxylin and eosin stain and B-F, immunohistochemical stain with hematoxylin counterstain.
Morphology and immunophenotype of tFL-PMBLsig-pos cases. (A) The tFL-PMBLsig-pos (case 2) is composed of sheets of large B cells with predominantly centroblastic cytology that coexpress CD20 and CD10 (not shown). (B) CD23 is diffusely positive. (C) CD30 shows prominent background nonspecific staining with only rare strongly positive cells. (D) A large subset of the neoplastic B cells expresses MAL and (E) CD200 but is negative for (F) CD273/PDL2. Original magnifications ×500 (A) and ×200 (B-F); A, hematoxylin and eosin stain and B-F, immunohistochemical stain with hematoxylin counterstain.
Pathologic features of 5 tFL-PMBLsig-pos cases
| . | 1 . | 2 . | 3 . | 4 . | 5 . |
|---|---|---|---|---|---|
| EX00406 . | EX00408 . | EX004010 . | FL1179T2 . | FL1122T2 . | |
| Histology | Centroblasts | Centroblasts | Centroblasts | Centroblasts | Centroblasts |
| Fine sclerosis | Yes | Yes | Yes | Yes | Yes |
| Concurrent FL (histologic grade) | No | Yes (3A) | Yes (1-2) | No | No |
| CD10 | + | + | − | − | NA |
| BCL6 | + | + | + | + | NA |
| IRF4/MUM1 | + | − | − | NA | NA |
| CD23 | − | + | + | + | − |
| CD30 | − | − | + | − | − |
| CD200 | − | + | − | − | − |
| CD273/PDL2 | − | − | − | − | − |
| MAL | + | + | − | + | + |
| Ki-67 | 80% | 50% | NA | 70% | NA |
| EBV-ISH (EBER) | − | − | NA | NA | NA |
| MYC rearrangement∗ | − | − | − | − | − |
| BCL2 rearrangement∗ | + | − | − | − | + |
| BCL6 rearrangement∗ | + | − | + | − | − |
| COO (Lymph2Cx) | GCB | GCB | UNC | GCB | GCB |
| . | 1 . | 2 . | 3 . | 4 . | 5 . |
|---|---|---|---|---|---|
| EX00406 . | EX00408 . | EX004010 . | FL1179T2 . | FL1122T2 . | |
| Histology | Centroblasts | Centroblasts | Centroblasts | Centroblasts | Centroblasts |
| Fine sclerosis | Yes | Yes | Yes | Yes | Yes |
| Concurrent FL (histologic grade) | No | Yes (3A) | Yes (1-2) | No | No |
| CD10 | + | + | − | − | NA |
| BCL6 | + | + | + | + | NA |
| IRF4/MUM1 | + | − | − | NA | NA |
| CD23 | − | + | + | + | − |
| CD30 | − | − | + | − | − |
| CD200 | − | + | − | − | − |
| CD273/PDL2 | − | − | − | − | − |
| MAL | + | + | − | + | + |
| Ki-67 | 80% | 50% | NA | 70% | NA |
| EBV-ISH (EBER) | − | − | NA | NA | NA |
| MYC rearrangement∗ | − | − | − | − | − |
| BCL2 rearrangement∗ | + | − | − | − | + |
| BCL6 rearrangement∗ | + | − | + | − | − |
| COO (Lymph2Cx) | GCB | GCB | UNC | GCB | GCB |
The symbol + indicates positive and − indicates negative.
NA, not available; UNC, unclassified.
MYC, BCL2, and BCL6 rearrangements detected by fluorescence in situ hybridization.
Mutations and CN alterations in tFL-PMBLsig-pos cases
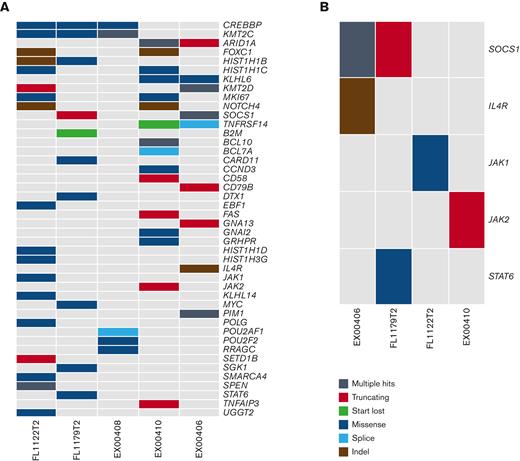
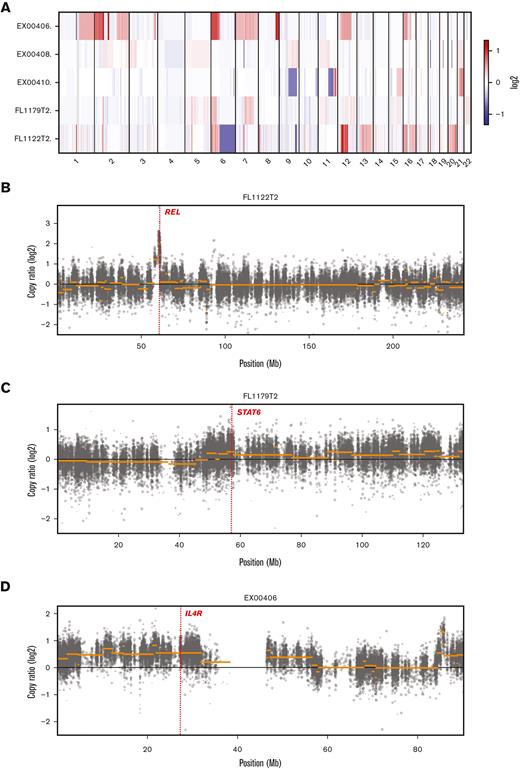
WES of the 5 tFL-PMBLsig-pos cases demonstrated sequence variants in genes associated with both FL/DLBCL9,13,14 (CREBBP [3 of 5], KMT2C [3 of 5], HIST1 members [3 of 5], KMT2D [2 of 5], ARID1A [2 of 5], and TNFRSF14 [2 of 5]) and PMBL15 (JAK-STAT pathway genes [4 of 5], B2M [1 of 5], and CD58 [1 of 5]) (Figure 2). Two of the mutations identified in the tFL-PMBLsig-pos cases are known to result in JAK-STAT activation (STAT6 p.E372K and SOCS1 p.F101L, each identified in 1 of 5 cases).16,17 CN analysis identified gains/amplification of REL (2p16.1) and STAT6 (12q13.3) in 3 of 5 cases; gains of large sections of chromosome 16, including the IL4R gene region, in 2 of 5; gains of SOCS1 (16p13.13) in 2 of 5; gains/amplification of BCL6 (3q27.3) and MYC (8q24.21) in 1 of 5; and both deletions and gains of 11q in 1 of 5 (Figure 3). CN losses of TNFRSF14 (1p36.32) and TNFAIP3 (6q23.3) gene regions were identified in 1 of 5 cases.
WES analysis of 5 tFL-PMBLsig-pos cases. (A) Alterations found in a curated list of PMBL and FL/DLBCL-related genes across all 5 tFL-PMBLsig-pos cases. (B) Alterations in the JAK-STAT pathway found among 4 of 5 tFL-PMBLsig-pos cases.
WES analysis of 5 tFL-PMBLsig-pos cases. (A) Alterations found in a curated list of PMBL and FL/DLBCL-related genes across all 5 tFL-PMBLsig-pos cases. (B) Alterations in the JAK-STAT pathway found among 4 of 5 tFL-PMBLsig-pos cases.
CN analysis of 5 tFL-PMBLsig-pos cases. (A) Heatmap showing CN changes by chromosomal location for each tFL-PMBLsig-pos case. (B-D) Graphs showing more detailed CN changes by location in chromosomes 2 (B), 12 (C), and 16 (D). Genes of note are labeled and highlighted with a dotted red line and include REL on chromosome 2 (gains in 3 of 5 cases; case 5/FL1122T2 illustrated), STAT6 on chromosome 12 (gains/amplification in 3 of 5 cases; case 4/FL1179T2 illustrated), and IL4R on chromosome 16 (gains in 2 of 5 cases; case 1/EX00406 illustrated).
CN analysis of 5 tFL-PMBLsig-pos cases. (A) Heatmap showing CN changes by chromosomal location for each tFL-PMBLsig-pos case. (B-D) Graphs showing more detailed CN changes by location in chromosomes 2 (B), 12 (C), and 16 (D). Genes of note are labeled and highlighted with a dotted red line and include REL on chromosome 2 (gains in 3 of 5 cases; case 5/FL1122T2 illustrated), STAT6 on chromosome 12 (gains/amplification in 3 of 5 cases; case 4/FL1179T2 illustrated), and IL4R on chromosome 16 (gains in 2 of 5 cases; case 1/EX00406 illustrated).
COO analysis revealed that the majority of tFLs can be assigned to the GCB-DLBCL subtype, which is in concordance with previously published data.18 In addition, we show that 3% of tFL cases express a PMBL-like GE signature, as identified using the Lymph3Cx assay. The discovery of tFL cases with features of both PMBL and DLBCL is not surprising considering previous descriptions in the literature.10,19,20 Prior studies of DLBCLs with CN alterations characteristic of PMBL (9p24.1 amplification) have shown that such cases have GE and mutational profiles similar to PMBL.19 Likewise, pediatric DLBCL with a PMBL-like GE profile may also harbor mutational features of PMBL.20 Here, we sought to further characterize 5 tFL-PMBLsig-pos cases to extend recent observations in de novo DLBCL.10
Similar to the previous study by Duns et al,10 our tFL-PMBLsig-pos cohort showed morphologic and immunophenotypic features that had some overlap with typical PMBL, including fine sclerosis and frequent expression of CD23 and/or MAL by immunohistochemistry. However, all of the cases in our cohort and most of the de novo DLBCL cases in the Duns et al10 study lacked staining for CD273/PDL2, which is expressed by approximately 70% of bona fide PMBLs.21,22 Similarly, only 20% of tFL-PMBLsig-pos cases showed staining for CD30 or CD200, which are expressed in >60% and >80% of PMBLs, respectively.23 Although combinations of these markers are reported to have a high specificity for PMBL over DLBCL, it is well recognized that they are not 100% sensitive and PMBLs are not infrequently negative for 1 or more of these markers.21-23
WES and CN analysis of the tFL-PMBLsig-pos cases likewise showed features of FL/DLBCL and some alterations also commonly seen in PMBL. Although all samples showed alterations affecting known FL/DLBCL driver genes,9,13,14 JAK-STAT pathway–associated alterations were identified in 80% and established JAK-STAT activating mutations demonstrated in 40% of the cases. The frequency of JAK-STAT pathway mutations appears higher in tFL-PMBLsig-pos cases than in typical FL/DLBCL9,13,14 and is similar to that found in bona fide PMBL,15 although admittedly the small size of our tFL-PMBLsig-pos cohort makes these comparisons difficult. Mutations of B2M and CD58, common in bona fide PMBL as part of an immune escape phenotype,15 were identified in 40% of tFL-PMBLsig-pos cases. Acquired mutations and/or deletions of these genes have also been previously recognized in tFL (B2M in 13% and CD58 in 5%),13 but it is uncertain whether such cases might be associated with a PMBL GE signature. Gains/amplification of REL, present in approximately 50% of bona fide PMBLs and 30% of tFLs,3,9,15,24 were identified in 60% of the tFL-PMBLsig-pos cases. However, the most common CN alteration seen in >70% of PMBLs (9p24.1 gain/amplification involving the JAK2/PDL1/PDL2 locus)3,15 was not identified in any of the tFL-PMBLsig-pos cases. Likewise, CDKN2A/B and TP53 alterations, which are enriched in typical tFLs,13 were not identified in the tFL-PMBLsig-pos cohort. Interestingly, 1 tFL-PMBLsig-pos case showed a chromosome 11q aberration with both deletions and gains, a CN pattern more commonly associated with a subset of aggressive lymphomas with Burkitt-like features than with PMBL.25 Although BCL2 rearrangements are found in approximately 80% of tFLs,13,18 only 2 of 5 tFL-PMBLsig-pos cases harbored this abnormality. It is unclear whether this difference reflects the underlying biology of these cases or a sampling bias. It should be noted that the genes evaluated by the Lymph3Cx assay (6 DLBCL characteristic genes and 24 PMBL characteristic genes) are not centered on BCL2 or the apoptotic pathway.11
In conclusion, this study adds to the growing literature defining lymphomas with overlapping DLBCL and PMBL features, extending previous observations to now include such cases arising from FL, and increasing our knowledge of the biology underlying FL transformations. It remains unclear from this limited series which genetic, microenvironmental, or therapeutic differences may predispose FLs to this PMBL-like transformation, although it is notable that some, but not all, of the patients were heavily pretreated before this transformation. Further study is also warranted to determine if the use of novel therapies, such as those targeting the JAK-STAT pathway, might be advantageous in this subset of tFL.
Acknowledgment
Research reported in this publication was supported by National Cancer Institute grant U01CA57581 (L.M.R.).
Authorship
Contribution: T.L. analyzed and interpreted the data and wrote the manuscript; K.L.R. and J.R.C. performed and analyzed immunohistochemistry and edited the manuscript; R.S.R., C.A.R., T.K.Y., and C.L.M. performed and analyzed gene expression data and edited the manuscript; A.C.R., S.H.S., and S.B. provided specimens and edited the manuscript; G.D., C.S., L.M.R., and S.E.G. conceived and designed the study; G.D., C.S., L.M.R., and S.E.G. acquired, analyzed, and interpreted the data; G.D., C.S., L.M.R., and S.E.G. wrote and edited the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: L.M.R. and C.S. are named inventors on a patent filed by the National Cancer Institute (“Methods for determining lymphoma type”). The remaining authors declare no competing financial interests.
Correspondence: Sarah E. Gibson, Mayo Clinic Arizona, 5777 East Mayo Blvd, Phoenix, AZ 85054; e-mail: gibson.sarah@mayo.edu.
References
Author notes
All sequencing data are deposited in a public repository, the European Genome-Phenome Archive (accession number EGAS00001005870; https://ega-archive.org/studies/EGAS00001005870).
Original data are available on request from the corresponding author, Sarah E. Gibson (gibson.sarah@mayo.edu).
The full-text version of this article contains a data supplement.