Key Points
After treatment with a BiAb and disease relapse, patients with myeloma can be salvaged using sequential T-cell redirection therapy.
Sequential T-cell redirection therapy led to a >80% response rate and a median OS that was not reached at a 30.5-month follow-up.
Abstract
T-cell redirection therapy using chimeric antigen receptor (CAR) T cells and bispecific antibodies (BiAbs) has shown promising efficacy in heavily pretreated patients with relapsed/refractory multiple myeloma (RRMM), leading to the approval of 2 CAR T-cell products and numerous BiAb trials. Data on the outcomes after relapse following BiAbs are urgently required to develop strategies for sequencing salvage therapies. We identified 58 patients progressing after a BiAb trial at Mount Sinai Hospital. Progression-free survival (PFS) to the first salvage (PFS1), second salvage therapy (PFS2), and overall survival (OS) were estimated using the Kaplan-Meier method. The median age of the patients was 67 years, and 78% had high-risk cytogenetics. They had a median of 6 prior therapy lines, 89% were triple-class refractory, and 44% were penta-drug refractory. After the BiAb trial, patients were followed for a median of 30.5 months and received a median of 2 additional salvage therapies (range, 1-9). The most common first salvage was T-cell redirection in 19 patients (10 BiAb and 9 CAR T cells). Ten patients underwent T-cell redirection as a second salvage treatment. T-cell redirection therapy as first or second salvage was feasible and associated with a median PFS1 of 28.9 months, PFS2 of 30.9 months, and an OS of 62% at 2 years. The sequential use of different T-cell redirection therapies is possible and may lead to deep and durable responses following the relapse after BiAb therapy in RRMM.
Introduction
Over the past decade, the clinical outcomes of patients with multiple myeloma (MM) have substantially improved with the introduction of newer generations of immunomodulatory drugs, proteasome inhibitors, monoclonal antibodies, selective nuclear export inhibitors, B-cell maturation antigen (BCMA) antibody-drug conjugates (ADCs), and T-cell redirection therapies, including chimeric antigen receptor (CAR) T cells and bispecific antibodies (BiAbs).1-5
T-cell redirection therapies have gained momentum since the introduction of autologous BCMA-directed CAR T cells, which have shown overall response rates (ORRs) ranging between 73% and 97% in patients with relapsed/refractory MM (RRMM).6,7 However, CAR T cells are limited by the integrity of the patient’s endogenous pool of T cells, extended manufacturing time, and prolonged inpatient monitoring for complications, such as cytokine release syndrome and neurotoxicity. The efficacy of BiAbs in engaging endogenous CD3+ T cells with several different target antigens has been investigated in clinical trials, including BCMA, G protein-coupled receptor class 5 member D (GPRC5D), Fc receptor homolog 5 (FcRH5), and cell-surface glycoprotein CD2 subset 1 (CS1); however, they offer an off-the-shelf option and have also been shown to lead to deep responses in multiple clinical trials, with ORRs ranging between 65% and 79%, with a manageable safety profile.8-14
The clinical outcomes of patients with RRMM who progress after clinical trials with BiAbs remain unknown and whether patients can be salvaged with other BiAbs or CAR T cells remains an important clinical question. Here, we address the growing unmet need to identify the optimal salvage therapies for disease control after disease progression in BiAb trials by retrospectively analyzing the outcomes and treatments of patients seen at our institution.
Methods
Clinical data collection
We retrospectively identified 115 patients with RRMM with disease progression after therapy in a BiAb phase 1 dose escalation or phase 2 clinical trial conducted between January 2018 and January 2021 at the Tisch Cancer Institute, Mount Sinai Hospital, New York, NY. We collected data on patient demographics, disease characteristics, treatment regimen(s), and clinical outcomes up to December 2021. We used the time of starting a new treatment after the clinical trial as the index date for our analysis. This retrospective study was approved by the institutional review board of Mount Sinai Hospital and complied with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice (GCP #11-1433). Disease characterization and responses were assessed by the treating physician according to the International Myeloma Working Group criteria.15-17 Adverse events were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.18
Statistical analysis
Baseline patient and treatment characteristics were summarized for continuous variables as the median (range) and categorical variables as counts (percentages). Group comparisons of continuous variables were performed using Wilcoxon rank-sum tests and categorical variables using the χ2 test or Fisher exact test, as appropriate. The median follow-up time from the start of first salvage therapy (FST) was estimated using the reverse Kaplan-Meier method.19 Distributions of progression-free survival (PFS) to FST (PFS1), PFS2, and overall survival (OS) were estimated using the Kaplan-Meier method with comparisons between patient groups using the log-rank test. Pointwise estimates from PFS1, PFS2, and OS distributions were reported with corresponding confidence intervals (CIs) estimated using Greenwood’s formula. The median OS, PFS1, and PFS2 times were reported with the corresponding CIs constructed using the nonparametric method developed by Brookmeyer and Crowley.
To account for immortal time bias, the landmark approach20 was used to examine the associations between receipt of T-cell redirection therapy at FST or second salvage therapy (SST) and OS, PFS1, and PFS2. A landmark time of 1 month was selected, as it was the time at which all patients (who eventually received SST at the time of data extraction) received their SST. Patients who died within 1 month of FST initiation were excluded from further analyses. OS was defined as the time from initiation of FST to death owing to any cause. Patients who survived the last follow-up were censored for OS. PFS1 was defined as the time from initiation of FST to progression on FST or death from any cause. Patients who were alive at the last follow-up and did not experience progression to FST were censored at their last assessment for the progression. PFS2 is a concept recommended by the European Medicines Agency to capture the possible negative effects of first-line therapy on next-line therapy.21 PFS2 encompasses the duration from the time of starting the first treatment to the time of progression or death while on second-line treatment (supplemental Figure 1). Thus, PFS2 was defined as the time from initiation of FST to progression on SST or death from any cause. Patients who were alive at the last follow-up and did not experience progression on SST or who did not receive SST at the point of data extraction were censored at their last assessment for progression. Death before the initiation of SST was considered to be a PFS2 event. All analyses were performed using R version 4.1.1 and SAS version 9.4 (SAS Institute Inc, Cary, NC). Hypothesis testing was 2-sided and conducted at the 5% level of significance.
Results
Patient demographics and baseline characteristics
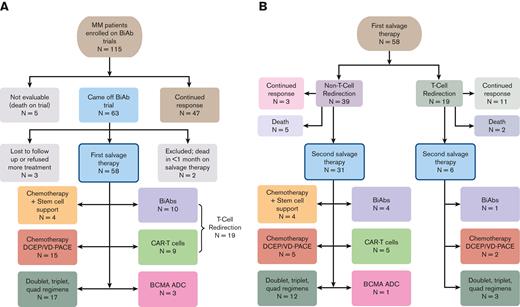
Out of 115 patients who enrolled in different BiAb trials, 5 expired while on a trial, 47 continued to exhibit a response, and 63 patients came off the trials by our data cutoff (Figure 1). Out of the 63 patients, 2 patients were lost to follow-up, 1 patient refused further therapy, and 60 patients went on to receive salvage therapy. Of the patients who received salvage therapy, 2 were excluded from further analyses because they died within 1 month from starting therapy, leaving 58 evaluable patients. The demographic and baseline characteristics of the 58 patients are summarized in Table 1.
Treatment breakdown of the patients with MM cohort. (A) Flowchart showing that the initial retrospective query yielded 115 patients with RRMM enrolled in a BiAb clinical trial at Mount Sinai Hospital. Of these 115 patients, we focused on 58 patients who came off the trials and received salvage therapy, including other T-cell redirection therapies, chemotherapy, and triplet regimens. (B) Clinical outcomes of therapies administered to 58 patients as FST and breakdown of treatments given to 37 patients as SST.
Treatment breakdown of the patients with MM cohort. (A) Flowchart showing that the initial retrospective query yielded 115 patients with RRMM enrolled in a BiAb clinical trial at Mount Sinai Hospital. Of these 115 patients, we focused on 58 patients who came off the trials and received salvage therapy, including other T-cell redirection therapies, chemotherapy, and triplet regimens. (B) Clinical outcomes of therapies administered to 58 patients as FST and breakdown of treatments given to 37 patients as SST.
Patient baseline characteristics
| . | Overall, N = 58 . | T-cell redirection, N = 28 . | Other, N = 30 . | P value . |
|---|---|---|---|---|
| Age at diagnosis, y, median (min-max) | 60 (35-77) | 59 (41-77) | 61 (35-77) | .9256 |
| Age at FST, y, median (min-max) | 67 (41-83) | 67 (47-81) | 67 (41-83) | .6022 |
| Months from diagnosis to FST, mo, median (min-max) | 82 (15-212) | 86 (46-212) | 69 (15-191) | .0415∗ |
| Gender, n (%) | ||||
| Male | 30 (52) | 14 (50) | 16 (53) | 1.0000 |
| Female | 28 (48) | 14 (50) | 14 (47) | |
| Myeloma subtype, n (%) | ||||
| IgG | 25 (43) | 14 (50) | 11 (37) | .5301 |
| IgA | 18 (31) | 7 (25) | 11 (37) | |
| IgM | 1 (2) | 0 (0) | 1 (3) | |
| IgD | 1 (2) | 1 (4) | 0 (0) | |
| Light chain | 13 (22) | 6 (21) | 7 (23) | |
| Cytogenetics, n (%) | ||||
| High risk | 45 (78) | 19 (68) | 26 (87) | .1187 |
| Standard risk | 13 (22) | 9 (32) | 4 (13) | |
| Number of prior lines of therapy, median (min-max) | 6 (3-17) | 7 (3-16) | 6 (3-17) | .6836 |
| Number of prior autologous stem cell transplants, n (%) | ||||
| 0 | 10 (17) | 5 (18) | 5 (17) | 1.0000† |
| 1 | 34 (59) | 16 (57) | 18 (60) | |
| 2 | 14 (24) | 7 (25) | 7 (23) | |
| Refractoriness, n (%) | ||||
| No | 0 (0) | 0 (0) | 0 (0) | 1.0000 |
| Yes | 58 (100) | 28 (100) | 30 (100) | |
| Triple-class refractory | 51 (88) | 25 (89) | 26 (87) | .7617 |
| Penta-drug refractory | 25 (43) | 13 (46) | 12 (40) | .6243 |
| Thalidomide | 6 (10) | 3 (11) | 3 (10) | 1.0000 |
| Lenalidomide | 44 (76) | 19 (68) | 25 (83) | .2244 |
| Pomalidomide | 49 (84) | 23 (82) | 26 (87) | .7260 |
| Bortezomib | 37 (64) | 19 (68) | 18 (60) | .5925 |
| Ixazomib | 6 (10) | 3 (11) | 3 (10) | 1.0000 |
| Carfilzomib | 48 (83) | 23 (82) | 25 (83) | 1.0000 |
| Elotuzumab | 19 (33) | 6 (21) | 13 (43) | .0973 |
| CD38 | 56 (97) | 28 (100) | 28 (93) | .4918 |
| Selinexor | 12 (21) | 6 (21) | 6 (20) | 1.0000 |
| Alkylator | 32 (55) | 15 (54) | 17 (57) | 1.0000 |
| BiAb target on trial, n (%) | ||||
| BCMAxCD3 | 9 (16) | 5 (18) | 4 (13) | .7260 |
| GPRC5DxCD3 | 49 (84) | 23 (82) | 26 (87) | |
| Extramedullary disease before the trial, n (%) | 19 (33) | 9 (32) | 10 (33) | 1.0000 |
| Extramedullary disease at relapse from BiAb trial, n (%) | 23 (40) | 11 (39) | 12 (40) | 1.0000 |
| Extramedullary disease after first salvage, n (%) | 22 (38) | 8 (29) | 14 (47) | .1844 |
| Eastern Cooperative Oncology Group performance status at FST, n (%) | ||||
| 0 | 24 (41) | 13 (46) | 11 (37) | .3121 |
| 1 | 30 (52) | 15 (54) | 15 (50) | |
| 2 | 3 (5) | 0 (0) | 3 (10) | |
| 3 | 1 (2) | 0 (0) | 1 (33) | |
| Radiation therapy at FST, n (%) | 12 (21) | 5 (18) | 7 (23) | .6069 |
| Prior BCMA ADC, n (%) | 3 (5) | 1 (4) | 3 (7) | 1.0000 |
| Prior CAR T-cell therapy, n (%) | 3 (5) | 0 (0) | 3 (10) | .2377 |
| Hemoglobin at FST, median (min-max) | 10.3 (7.1-14.4) | 11.2 (7.6-14.4) | 9.9 (7.1-12.2) | .0075∗ |
| Platelet count at FST, median (min-max) | 168 (26-749) | 191 (59-456) | 142.5 (26-749) | .0137∗ |
| Absolute neutrophil count at FST, median (min-max) | 3 (0.6-7.4) | 2.8 (0.7-7.4) | 3.1 (0.6-0.1) | .9938 |
| Creatinine level at FST, median (min-max) | 1 (0.4-2.1) | 1.0 (0.5-2.1) | 1.0 (0.4-1.7) | .8586 |
| Calcium level at FST, median (min-max) | 9.1 (7.5-14.8) | 9.2 (8.1-10.5) | 9.1 (7.5-14.8) | .7446 |
| . | Overall, N = 58 . | T-cell redirection, N = 28 . | Other, N = 30 . | P value . |
|---|---|---|---|---|
| Age at diagnosis, y, median (min-max) | 60 (35-77) | 59 (41-77) | 61 (35-77) | .9256 |
| Age at FST, y, median (min-max) | 67 (41-83) | 67 (47-81) | 67 (41-83) | .6022 |
| Months from diagnosis to FST, mo, median (min-max) | 82 (15-212) | 86 (46-212) | 69 (15-191) | .0415∗ |
| Gender, n (%) | ||||
| Male | 30 (52) | 14 (50) | 16 (53) | 1.0000 |
| Female | 28 (48) | 14 (50) | 14 (47) | |
| Myeloma subtype, n (%) | ||||
| IgG | 25 (43) | 14 (50) | 11 (37) | .5301 |
| IgA | 18 (31) | 7 (25) | 11 (37) | |
| IgM | 1 (2) | 0 (0) | 1 (3) | |
| IgD | 1 (2) | 1 (4) | 0 (0) | |
| Light chain | 13 (22) | 6 (21) | 7 (23) | |
| Cytogenetics, n (%) | ||||
| High risk | 45 (78) | 19 (68) | 26 (87) | .1187 |
| Standard risk | 13 (22) | 9 (32) | 4 (13) | |
| Number of prior lines of therapy, median (min-max) | 6 (3-17) | 7 (3-16) | 6 (3-17) | .6836 |
| Number of prior autologous stem cell transplants, n (%) | ||||
| 0 | 10 (17) | 5 (18) | 5 (17) | 1.0000† |
| 1 | 34 (59) | 16 (57) | 18 (60) | |
| 2 | 14 (24) | 7 (25) | 7 (23) | |
| Refractoriness, n (%) | ||||
| No | 0 (0) | 0 (0) | 0 (0) | 1.0000 |
| Yes | 58 (100) | 28 (100) | 30 (100) | |
| Triple-class refractory | 51 (88) | 25 (89) | 26 (87) | .7617 |
| Penta-drug refractory | 25 (43) | 13 (46) | 12 (40) | .6243 |
| Thalidomide | 6 (10) | 3 (11) | 3 (10) | 1.0000 |
| Lenalidomide | 44 (76) | 19 (68) | 25 (83) | .2244 |
| Pomalidomide | 49 (84) | 23 (82) | 26 (87) | .7260 |
| Bortezomib | 37 (64) | 19 (68) | 18 (60) | .5925 |
| Ixazomib | 6 (10) | 3 (11) | 3 (10) | 1.0000 |
| Carfilzomib | 48 (83) | 23 (82) | 25 (83) | 1.0000 |
| Elotuzumab | 19 (33) | 6 (21) | 13 (43) | .0973 |
| CD38 | 56 (97) | 28 (100) | 28 (93) | .4918 |
| Selinexor | 12 (21) | 6 (21) | 6 (20) | 1.0000 |
| Alkylator | 32 (55) | 15 (54) | 17 (57) | 1.0000 |
| BiAb target on trial, n (%) | ||||
| BCMAxCD3 | 9 (16) | 5 (18) | 4 (13) | .7260 |
| GPRC5DxCD3 | 49 (84) | 23 (82) | 26 (87) | |
| Extramedullary disease before the trial, n (%) | 19 (33) | 9 (32) | 10 (33) | 1.0000 |
| Extramedullary disease at relapse from BiAb trial, n (%) | 23 (40) | 11 (39) | 12 (40) | 1.0000 |
| Extramedullary disease after first salvage, n (%) | 22 (38) | 8 (29) | 14 (47) | .1844 |
| Eastern Cooperative Oncology Group performance status at FST, n (%) | ||||
| 0 | 24 (41) | 13 (46) | 11 (37) | .3121 |
| 1 | 30 (52) | 15 (54) | 15 (50) | |
| 2 | 3 (5) | 0 (0) | 3 (10) | |
| 3 | 1 (2) | 0 (0) | 1 (33) | |
| Radiation therapy at FST, n (%) | 12 (21) | 5 (18) | 7 (23) | .6069 |
| Prior BCMA ADC, n (%) | 3 (5) | 1 (4) | 3 (7) | 1.0000 |
| Prior CAR T-cell therapy, n (%) | 3 (5) | 0 (0) | 3 (10) | .2377 |
| Hemoglobin at FST, median (min-max) | 10.3 (7.1-14.4) | 11.2 (7.6-14.4) | 9.9 (7.1-12.2) | .0075∗ |
| Platelet count at FST, median (min-max) | 168 (26-749) | 191 (59-456) | 142.5 (26-749) | .0137∗ |
| Absolute neutrophil count at FST, median (min-max) | 3 (0.6-7.4) | 2.8 (0.7-7.4) | 3.1 (0.6-0.1) | .9938 |
| Creatinine level at FST, median (min-max) | 1 (0.4-2.1) | 1.0 (0.5-2.1) | 1.0 (0.4-1.7) | .8586 |
| Calcium level at FST, median (min-max) | 9.1 (7.5-14.8) | 9.2 (8.1-10.5) | 9.1 (7.5-14.8) | .7446 |
IgG, immunoglobulin G.
P value < .05.
Among patients that underwent autologous stem cell transplant.
The 58 patients who underwent salvage therapy after the BiAb trial had a median age of 67 years (range, 41-83) at the time of receiving FST, and 52% of those patients were male. Patients underwent FST after discontinuing a BiAb trial at a median of 82 months (range, 15-212) from the time of diagnosis of MM. Myeloma subtypes comprised 78% intact immunoglobulin and 22% light chain only. Cytogenetics by fluorescence in situ hybridization were available for all 58 patients, of which 45 patients (78%) had high-risk cytogenetics, including gain 1q21, deletion 17p, t(4;14), t(14;16), and t(14;20).22 Most patients were heavily pretreated with a median of 6 prior lines of therapy (range, 3-17), and 83% had received at least 1 autologous stem cell transplant. Furthermore, all patients were triple-class exposed, 88% were triple-class refractory, and 43% were penta-drug refractory at the time of trial enrollment. Moreover, 6 patients were previously exposed to anti-BCMA therapy (3 CAR T-cell therapy and 3 ADC). Finally, 40% of the patients had extramedullary disease at the time of coming off the BiAb trial.
Treatments after a BiAb trial consisted of other BiAbs, CAR T cells, or conventional salvage regimens
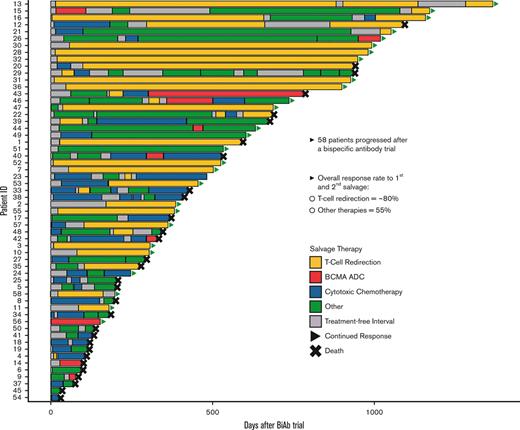
The 58 patients who relapsed from the BiAb trial (49 GPRC5D and 9 BCMA) were followed up for a median of 30.5 months from the time they came off from the BiAb trial (range, 3.2-45 months). Patients managed to receive a median of 2 lines of salvage therapy after coming off the trial, ranging between 1 and 9 lines of therapy. The most common initial salvage therapy included T-cell redirecting therapy in 19 patients, which included 10 patients who received a second BiAb and 9 who received BCMA-directed CAR T cells (Figure 1A). Moreover, 19 patients received chemotherapy, including 4 patients who received stem cell support after melphalan ± carmustine. Fifteen patients received combination chemotherapy regimens such as DCEP, VDCEP, and VD-PACE (A, doxorubicin; D, dexamethasone; C, cyclophosphamide; E, etoposide; P, cisplatin; V, bortezomib). In addition, combination therapies (doublet, triplet, and quadruple therapies) were utilized in another 17 patients and those included anti-CD38–, selinexor-, and venetoclax-based combinations. Finally, a BCMA ADC was administered to 3 patients.
Most of the patients received >1 salvage therapy after the BiAb trial, whereby 37 out of 58 patients eventually received SST (Figure 1B). Among the 19 patients who received T-cell redirection therapy as FST, 11 (58%) continued to respond, whereas 6 (32%) moved on to an SST. In contrast, out of 39 patients who received non–T-cell redirection therapy, only 3 (8%) continued to exhibit a response, whereas 31 (79%) moved on to an SST. Notably, 10 patients received a T-cell redirecting therapy as SST after the initial salvage. The choice of using T-cell redirection as a second salvage was predicated on individual needs for rapid cytoreduction or the availability of a trial opportunity at the time of relapse following a BiAb. Notably, 1 patient received T-cell redirection as the FST and SST. This led to a total of 28 individual patients receiving T-cell redirection therapy as either a FST or SST after coming off a BiAb trial, the outcomes of which are described below.
Sequential T-cell redirection is feasible in patients progressing after BiAb therapy
The best response to FST following the BiAb trial varied widely and included 13 complete responses, 4 very good partial responses (VGPRs), 18 partial responses, 2 minimal responses, 9 stable diseases, and 12 patients with disease progression for an ORR of 60% (Table 2).
Patient responses to FST
| . | Overall, N = 58 . | FST . | P value . | |
|---|---|---|---|---|
| T-cell redirection, N = 19 . | Other, N = 39 . | |||
| Response to FST, n (%) | ||||
| Stringent complete response | 4 (7) | 4 (21) | 0 (0) | <.0001∗ |
| Complete response | 9 (15.5) | 8 (42) | 1 (3) | |
| VGPR | 4 (7) | 0 (0) | 4 (10) | |
| Partial response | 18 (31) | 4 (21) | 14 (36) | |
| Minimal response | 2 (3) | 0 (0) | 2 (5) | |
| Stable disease | 9 (15.5) | 1 (5) | 8 (20) | |
| Progressive disease | 12 (21) | 2 (11) | 10 (26) | |
| ORR on FST, n (%) | 35 (60) | 16 (84) | 19 (49) | .0095∗ |
| ORR on FST, 95% CI | 47-73 | 60-97 | 32-65 | |
| Clinical benefit rate on FST, n (%) | 37 (64) | 16 (84) | 21 (54) | .0239∗ |
| Clinical benefit rate on FST, 95% CI | 50-76 | 60-97 | 37-70 | |
| . | Overall, N = 58 . | FST . | P value . | |
|---|---|---|---|---|
| T-cell redirection, N = 19 . | Other, N = 39 . | |||
| Response to FST, n (%) | ||||
| Stringent complete response | 4 (7) | 4 (21) | 0 (0) | <.0001∗ |
| Complete response | 9 (15.5) | 8 (42) | 1 (3) | |
| VGPR | 4 (7) | 0 (0) | 4 (10) | |
| Partial response | 18 (31) | 4 (21) | 14 (36) | |
| Minimal response | 2 (3) | 0 (0) | 2 (5) | |
| Stable disease | 9 (15.5) | 1 (5) | 8 (20) | |
| Progressive disease | 12 (21) | 2 (11) | 10 (26) | |
| ORR on FST, n (%) | 35 (60) | 16 (84) | 19 (49) | .0095∗ |
| ORR on FST, 95% CI | 47-73 | 60-97 | 32-65 | |
| Clinical benefit rate on FST, n (%) | 37 (64) | 16 (84) | 21 (54) | .0239∗ |
| Clinical benefit rate on FST, 95% CI | 50-76 | 60-97 | 37-70 | |
P value < .05.
Because the patients were treated in phase 1/2 trials, their specific dose strata of BiAbs affected our findings; 10 out of 15 patients (67%) who received the recommended phase 2 dose (RP2D) when treated with a second BiAb, such as FST or SST, had an ORR of 80% compared with 60% in those who did not receive the RP2D of the BiAb. The median time on treatment was shorter in patients receiving dosages lower than the RP2D (2.5 months vs 7.5 months).
Interestingly, 2 patients were sequentially treated with agents targeting the same antigen, an anti-BCMA BiAb and anti-BCMA CAR T-cell therapy and showed good responses to both. The first patient had a VGPR to a BiAb on the clinical trial before the study index date, followed by a partial response when treated with CAR T cells as FST. On the contrary, the second patient achieved a complete response to CAR T-cell therapy as FST, and VGPR to the BiAb as SST. None of the 4 patients who received an anti-BCMA ADC as FST or SST received prior BCMA-directed therapy.
Notably, the depth and duration of the response to the first BiAb did not predict the response to the second T-cell redirection therapy. Furthermore, there was no difference in the outcomes when switching from GPRC5D to BCMA vs transitioning from BCMA- to GPRC5D-targeted therapy.
Sequential T-cell redirection is associated with better responses, PFS, and OS
A formal comparison of outcomes between patients receiving T-cell redirection therapy as the FST or SST and those receiving other therapies is not possible because of confounding by important clinical prognostic factors that cannot be adequately controlled in this small study. However, for reference purposes, we analyzed patients who received conventional salvage therapy. In general, there was no significant difference in the majority of baseline characteristics between patients who received T-cell redirection therapy and those who received conventional salvage therapy. However, patients who received T-cell redirection therapy had a higher median hemoglobin level (P = .0075) and platelet count (P = .0137) at the time of FST and were given their first salvage at a median of 86 months from the time of MM diagnosis compared with 69 months for patients who received all other therapies (P = .0415).
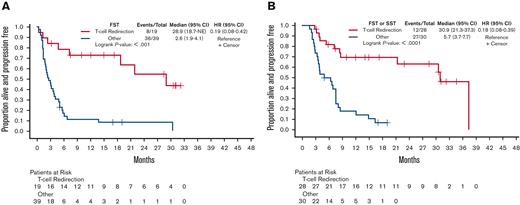
The ORR of the 19 patients who directly transitioned from a BiAb to another T-cell redirection therapy (10 BiAb and 9 CAR T-cell therapy) was significantly higher (84%) than that of the ORR of the 39 patients (49%) who received other types of therapies (P < .01). The median PFS1 of those 19 patients who received T-cell redirection therapy as FST was 28.9 months (95% CI, 18.7-NE) compared with 2.6 months (95% CI, 1.9-4.1) in the 39 patients who received all other types of therapies as their first salvage (Figure 2A). More specifically, the 10 patients who received a BiAb as their FST had a median PFS of 18.7 months (95% CI, 2.3-18.7), whereas the 9 patients who got CAR T-cell therapy did not reach their median PFS1 (95% CI, 28.9-NE), but this was not statistically significant (P = .1054) (supplemental Figure 2). Furthermore, because 9 additional patients received T-cell redirection therapy as part of their SST, we also evaluated PFS2. Consequently, the median PFS2 of the 28 patients who received T-cell redirection therapy was 30.9 months (95% CI, 21.3-37.3) vs 5.7 months (95% CI, 3.7-7.7) for the 30 patients who did not receive T-cell redirection therapy as FST or SST (Figure 2B).
Salvage therapy with T-cell redirection improves PFS. (A) PFS1 of 19 patients receiving T-cell redirection as the FST compared with 39 patients receiving all other types of treatments. (B) PFS2 of 28 patients receiving T-cell redirection as FST or SST compared with 30 patients receiving all other types of treatments. HR, hazard ratio.
Salvage therapy with T-cell redirection improves PFS. (A) PFS1 of 19 patients receiving T-cell redirection as the FST compared with 39 patients receiving all other types of treatments. (B) PFS2 of 28 patients receiving T-cell redirection as FST or SST compared with 30 patients receiving all other types of treatments. HR, hazard ratio.
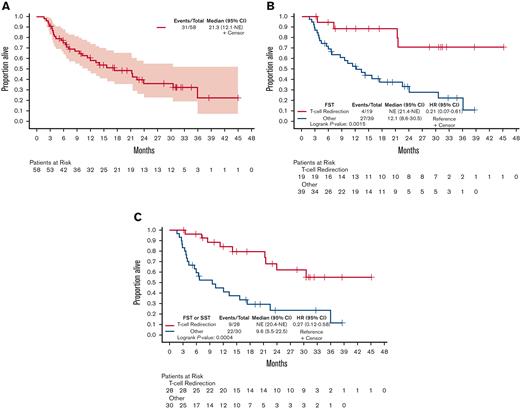
The OS of the cohort was 21.3 months (95% CI, 12.1-NE) (Figure 3A). The median OS of 19 patients who received T-cell redirection therapy as FST was not reached (95% CI, 21.4-NE) compared with 12.1 months (95% CI, 8.6-30.5) in the 39 patients not receiving T-cell redirection therapy as FST (Figure 3B). The median OS of the 10 patients who received a BiAb as FST was 21.4 months (95% CI, 21.3-NE) whereas that of the 9 patients who received CAR T-cell therapy as FST was not reached; however, this was not statistically significant (P = .1730) (supplemental Figure 3). In the 28 patients who received T-cell redirection therapy as FST or SST, the median OS was also not reached (95% CI, 24.0-NE) and was 62% (95% CI, 44-88) at 2 years compared with a median of 9.6 months (95% CI, 5.5-22.5) and 24% (95% CI, 11-49) at 2 years, respectively, for the remaining 30 patients (Figure 3C).
Salvage therapy with T-cell redirection enhances OS. (A) OS of the full cohort of 58 patients. (B) OS of 19 patients receiving T-cell redirection as the FST compared with 39 patients receiving all other types of treatments. (C) OS of 28 patients receiving T-cell redirection as FST or SST compared with 30 patients receiving all other types of treatments.
Salvage therapy with T-cell redirection enhances OS. (A) OS of the full cohort of 58 patients. (B) OS of 19 patients receiving T-cell redirection as the FST compared with 39 patients receiving all other types of treatments. (C) OS of 28 patients receiving T-cell redirection as FST or SST compared with 30 patients receiving all other types of treatments.
Discussion
BiAbs targeting CD3 along with 1 of several antigens expressed on myeloma cells (GPRC5D, BCMA, FcRH5, and CS1) are currently going through clinical trials with promising response rates and manageable toxicity profiles. Meanwhile, 2 anti-BCMA CAR T cells have been approved for the treatment of relapsed myeloma. Relapses are seen after both CAR T-cell therapy and BiAb treatments. Patients progressing to BiAb therapy may have exhausted T cells, increased regulatory T cells or antigen down-modulation by the tumor cells or other mechanisms of immune evasion. Given the expanding clinical utility and development of T-cell redirection therapy, there is an unmet need for understanding whether a patient failing 1 T-cell redirection therapy would respond to another T-cell redirecting therapy, and in the long-term, how best to sequence these therapies. In this manuscript, we report for the first time the feasibility of sequencing T-cell redirection therapies from a single institutional experience.
Our study had several limitations, including its retrospective nature and the fact that it is a single-center experience. All patients had previously satisfied entry into a clinical trial of a BiAb before progressing and requiring salvage therapy. However, at the time of relapse, some patients were not considered clinical trial candidates because of cytopenias or the rapid progression of the disease. Furthermore, the availability of clinical trial slots was random, and patients had to satisfy prespecified criteria to be eligible for treatment, such as not being previously treated with a therapy targeting the same myeloma protein. Moreover, the availability of stem cells for rescue, provided some patients with another window of opportunity for salvage, which many patients did not have.
Our data suggest that after treatment with a BiAb, this high-risk patient population can still exhibit favorable outcomes when exposed again to T-cell redirection therapeutics such as other BiAbs and CAR T-cell therapy. Although conventional salvage therapy had a relatively good ORR of approximately 50%, it did not lead to durable responses, which translated to significantly lower PFS and OS rates. On the contrary, transitioning from a BiAb to another T-cell redirection therapy yielded an ORR of >80%, with a median PFS1 of 28.9 months and a median OS that was not reached. Furthermore, 58% of those patients continued to exhibit a response to T-cell redirection at the time of analysis compared with only 8% in patients who received conventional therapy as the FST. Unfortunately, some patients could not directly transition to another T-cell redirection therapy on a trial. However, these patients were still salvaged when treated with T-cell redirection such as SST and still exhibited deep and durable responses, leading to a median PFS2 of 30.9 months and a median OS that was not reached.
Interestingly, there was no statistically significant difference in PFS1 and OS between patients receiving a BiAb or CAR T-cell therapy as FST, indicating that both CAR T cells and BiAbs had excellent outcomes. However, further studies are required to provide a basis for choosing 1 over another and for determining the appropriate sequence. In addition, we have seen that it is possible to use both approaches in the same patient to target the same myeloma antigen and still elicit an excellent response to both the BiAb and CAR T cells. Thus, even though switching the targets of T-cell redirection therapies can explain the responses observed in sequential treatments, it seems that the method of redirection and the immune microenvironment may also play a role.
Notably, it is likely that the clinical response and survival observed in this retrospective study were affected by several variables, making the statistical comparisons between the 2 treatment groups difficult to interpret. For example, the better performance status and organ function, which are prerequisites for trial enrollment, may contribute to the significantly better survival observed with T-cell redirection therapy than with other salvage therapies. Furthermore, the 58 patients we studied here were all progressing on their first BiAb trial and may have been enriched for worse immune responses relative to the rest of the patients who continued to respond to the first BiAb trial. It is conceivable that patients who are still in remission for several years on their first BiAb may have even better outcomes than those observed here if they relapse and receive sequential T-cell redirection therapy. Moreover, many patients included in this study did not receive RP2D of the BiAb, which could have affected the response depth and durability. This was apparent in the higher ORR observed among patients receiving RP2D than in those who received lower doses.
Given that there are currently several approved and ongoing therapeutic strategies for T-cell redirection in MM, understanding the immune microenvironment before treatment and at relapse would provide insights into the rational sequencing of these treatments. For example, it has been previously shown that older age (>67 years), having a higher frequency of PD-1 positive T cells, HLA-DR-positiveactivated T cells, and regulatory T cells, as well as having a lower effector T cells to myeloma target cell ratio are all associated with decreased efficacy of anti-GPRC5D BiAb.23 Thus, longitudinal assessment of immune compartment changes throughout BiAb treatment and at the time of disease progression and subsequent therapies is urgently needed to understand the biological underpinning of the promising responses observed in our single institution study.
Our findings indicate that progression to BiAb does not preclude salvage therapy with subsequent T-cell redirecting therapies. Future studies with larger numbers of patients treated with therapeutic RP2Ds of BiAbs are needed to account for potential confounding variables, with longer follow-up to help design and benchmark salvage strategies after BiAbs. Better biological insight is also warranted to characterize tumor evolution and microenvironmental changes and to complement the clinical data for optimal sequence and targets of T-cell redirecting therapies.
Acknowledgments
The authors thank all our patients and their caregivers. They also acknowledge the clinical and research staff and the philanthropic support from the Center of Excellence for Multiple Myeloma at Mount Sinai.
This work was supported by grants from the National Cancer Institute R01 (grants CA244899 and CA252222) (S.P.).
Authorship
Contribution: T.H.M., A.C., S.J., and S.P. conceptualized the study; J.L. and E.M. performed the methodology; T.H.M., O.V.O., D.T.M., Y.G.-P., G.L., S.T., D.P., S. Rajeeve, S.A., and A.A. performed the investigation; T.H.M. wrote the manuscript; S.P. provided the resources for the study; A.C., S.J., and S.P. supervised the study; and all authors were responsible for writing, reviewing, and editing the manuscript.
Conflict-of-interest disclosure: T.H.M. received advisory board fees from Legend Biotech. G.L. received advisory board fees from Janssen. L.S. received consulting fees from Takeda. S. Richard received honoraria from Karyopharm and Janssen, advisory board fees from Karyopharm and Celgene/Bristol Myers Squibb, and research support from Janssen, Celgene/Bristol Myers Squibb, and C4 Therapeutics. A.R. received advisory board fees from Celgene/Bristol Myers Squibb, Janssen, Sanofi, and GlaxoSmithKline. J.R. received speaking fees from Celgene/Bristol Myers Squibb, Sanofi, and Janssen, advisory board fees from Celgene/Bristol Myers Squibb, Janssen, Karyopharm, Sanofi, X4 Pharmaceuticals, Oncopeptides, Adaptive Biotechnologies, Secura Bio, AstraZeneca, and Takeda, and consulting fees from Celgene/Bristol Myers Squibb, Secura Bio, and Oncopeptides. H.J.C. is an employee of the Multiple Myeloma Research Foundation and received research funding from Celgene/Bristol Myers Squibb and Takeda. C.R. received consulting fees from Janssen, Artica, Takeda, Amgen, Karyopham, and Caelum Biosciences. A.C. received consulting fees from Amgen, Celgene/Bristol Myers Squibb, Janssen, Karyopharm, and Takeda, advisory board fees from Amgen, Celgene/Bristol Myers Squibb, Janssen, Karyopharm, Takeda, Sanofi, and Seattle Genetics, and research support from Amgen, Array Biopharma, Celgene/Bristol Myers Squibb, GlaxoSmithKline, Janssen, Takeda, Novartis, Oncoceutics, Pharmacyclics, and Seattle Genetics. S.J. received advisory board fees and consulting fees from Celgene/Bristol Myers Squibb, Janssen, Legend Biotech, Karyopharm, Sanofi, and Takeda. S.P. received advisory board fees from GRAIL and research support from Celgene/Bristol Myers Squibb, Amgen, and Karyopharm. The remaining authors declare no competing financial interests.
Correspondence: Samir Parekh, Hess Center for Science and Medicine, Icahn School of Medicine at Mount Sinai, 1470 Madison Ave, Box 1079, New York, NY 10029; e-mail: samir.parekh@mssm.edu.
References
Author notes
Data are available on request from the corresponding author, Samir Parekh (samir.parekh@mssm.edu).
The full-text version of this article contains a data supplement.