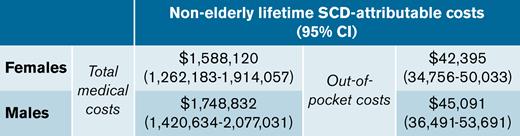
Key Points
The nonelderly lifetime burden of total medical costs attributable to sickle cell disease was $1.7 million.
Patients incurred $44 000 in out-of-pocket costs due to sickle cell disease over their nonelderly lifetimes.
Abstract
Sickle cell disease (SCD) is a severe monogenic disease associated with high morbidity, mortality, and a disproportionate burden on Black and Hispanic communities. Our objective was to estimate the total healthcare costs and out-of-pocket (OOP) costs attributable to SCD among commercially insured individuals over their nonelderly lifetimes (0 to 64 years of age). We constructed a retrospective cohort of individuals with diagnosed SCD using Truven Health Marketscan commercial claims data from 2007 through 2018, compared with matched control subjects from the Medical Expenditure Panel Survey. We estimated Kaplan-Meier sample average costs using previously reported survival curves for SCD and control subjects. Individuals with SCD (20 891) and control subjects (33 588) were included in our analysis. The SCD sample had a mean age of 25.7 (standard deviation, 17.4) years; 58.0% were female. Survival-adjusted costs of SCD peaked at age 13 to 24 years and declined at older ages. There was no significant difference in total medical costs or OOP costs between the sexes. SCD-attributable costs over 0 to 64 years of age were estimated to be $1.6 million (95% confidence interval [CI], $1.3M-$1.9M) and $1.7 million (95% CI, $1.4M-$2.1M) for females and males with SCD, respectively. The corresponding OOP estimates were $42 395 (95% CI, $34 756-$50 033) for females and $45 091 (95% CI, $36 491-$53 691) for males. These represent a 907% and 285% increase in total medical and OOP costs over control subjects, respectively. Although limited to the commercially insured population, these results indicate that the direct economic burden of SCD is substantial and peaks at younger ages, suggesting the need for curative and new medical therapies.
Introduction
Sickle cell disease (SCD) is an inherited blood disorder caused by a mutation in the β-globin gene that affects approximately 100 000 individuals in the United States, primarily of African American descent, and is associated with lifelong morbidity and increased risk of mortality.1 Individuals with SCD experience numerous debilitating and life-threatening complications throughout their lifetimes, including vaso-occlusive pain episodes (VOEs), which occur when the vascular obstruction leads to ischemic tissue damage and are the most common reason for hospital admission.2-4
Due to these complications, individuals with SCD typically incur high healthcare costs, with inpatient care contributing the greatest share.5-7 The total economic burden of inpatient care for individuals with SCD in the United States was $811 million in 2016 alone.2 SCD-related lifetime medical costs were estimated at $1.2 million (2020 US dollars).8,9 Despite being nearly 20 years old and derived from a single state’s Medicaid program, this estimate is widely used to establish the baseline costs of medical care for SCD.9-12 Other studies of the burden of medical costs are limited to descriptive summaries,5-8,13 and none provide clear estimates of the lifetime incremental medical costs paid by health insurers or, importantly, the out-of-pocket (OOP) burden that SCD individuals face compared with those without SCD or related blood disorders.
Understanding the economic burden of SCD on health insurers and patients is important at this time. A number of very costly but potentially curative therapies for SCD are on the horizon,14 with 10 gene therapies currently in the clinical testing stage.15 These therapies have the potential to revolutionize care for SCD. However, with expected prices of nearly $2 million,16,17 issues of affordability and access will be points of contention between patients, payers, and policymakers.9,18
The objectives of this study were to provide age- and sex-specific estimates of total healthcare costs and OOP costs attributable to SCD over the first 64 years of life in a commercially insured population. In addition to providing a better understanding of the individual and health system burden of SCD, our estimates of the overall lifetime burden of SCD in the United States can serve as key reference points for evaluating the economic impact of anticipated treatments for SCD. This work is part of the National Heart, Lung, and Blood Institute Cure Sickle Cell Initiative (https://curesickle.org).
Methods
Data sources
We constructed a retrospective cohort of individuals with SCD using the Truven Health Marketscan Commercial Claims and Encounters Database. The Truven database contains claims data for over 115 million individuals and their dependents from all 50 states with employer-sponsored private health insurance. These data include outpatient and inpatient medical claims, prescription drug claims, health utilization records, payer and individual costs, and demographic characteristics of individuals, including their age at enrollment, sex, geographic region, and insurance plan type. Race was not available in the data.
We constructed a cohort of individuals representing a control population using data from MEPS (Medical Expenditure Panel Survey).19 MEPS is a nationally representative survey of the general population of noninstitutionalized civilians in the United States. We used the Household Component (HC) of MEPS, which contains data on the demographic characteristics of participants, including their age on December 31 of the sampling year, sex, race, and geographic region. These data were supplemented by the Medical Provider Component, which has healthcare utilization, diagnosis codes, charges, and payments from medical providers and pharmacies that provided care to individuals in the HC using follow-back surveys.
Study population
We created a retrospective longitudinal cohort of individuals from the Truven database who fulfilled a previously validated case definition for SCD at any point between 2007 and 2018.20 Individuals had ≥1 inpatient claim or 2 outpatient or emergency department claims for SCD in any position using the International Classification of Disease, 9th and 10th edition codes (ICD-9: 282.41, 282.42, and 282.6x; ICD-10: D57.0x, D57.1x, D57.2x, D57.4x, and D57.8x, excluding 282.5 and D57.3; sickle cell trait). This criterion has >90% sensitivity and specificity in identifying pediatric SCD and has been widely applied to populations of all ages; however, its performance in older adults has not been specifically evaluated.20,21 Individuals were followed from the start of their first claim encounter (irrespective of when SCD was diagnosed) from January 1, 2007, until December 31, 2018, or any period of continuous enrollment exceeding 1 year therein. We excluded all observations of individuals ≥65 years to eliminate periods in which all patients were eligible for Medicare. Because only the year of birth was available, we assumed an individual’s day and month of birth coincided with their date of enrollment.
We selected control individuals from MEPS who were surveyed between 2007 and 2018. We restricted this sample to those who reported their primary race as Black since the majority of individuals with SCD are Black,1 and without race/ethnicity information from the Truven database, we were unable to match more granularly. Individuals selected from MEPS also had private insurance covering hospital and physician services and did not have claims for blood disorders (ICD-9: 280 to 289; ICD-10: D50 to D89) during the study period. We refer to this cohort as “matched controls” to reflect the absence of blood disorders, including SCD.
Direct medical costs
We assessed all-cause total medical costs and OOP costs by year of individual age. Total costs indicate gross payments to a provider for a service. OOP costs were the sum of deductible, copayment, and coinsurance paid by individuals in both the Truven and MEPS data. We included costs from physician and nonphysician office visits, urgent care, ambulance, emergency department, inpatient, end-stage renal disease facility visits, prescription medications, and dental and vision services. MEPS additionally included the costs of home healthcare services and medical equipment, which, when included in the total costs of matched controls, provided us with a conservative estimate of SCD-attributable costs, which were calculated as the difference between costs in individuals with SCD and matched controls. All costs were converted to 2020 US dollars using the Personal Consumption Expenditure Price Index for total costs and the US Consumer Price Index for all urban consumers for OOP costs.22,23
Statistical analysis
All analyses were conducted in STATA V16.1 (StataCorp, College Station, TX). We estimated annual total medical costs and OOP costs among survivors in both the SCD and matched control populations. In the SCD population, we applied flexible link and variance function generalized linear models for total and OOP costs separately, with individual sex, age, age2, age categories (0 to 5; 6 to 12; 13 to 18; 19 to 24; 25 to 34; 35 to 54; and 55 to 64 years), geographic region, insurance plan type, calendar year, and the number of months observed per year of individual age (to account for variation in enrollment length) included as covariates.24,25 Age was centered around its mean to improve estimation. Standard errors were obtained using 1000 individual-level clustered bootstrapped replicates.
We used weighted generalized linear regression models (γ distribution, log link) with individual sex, age, geographic region, insurance plan type, and calendar year included as covariates for matched controls. We applied a logistic regression-based propensity score analysis to generate weights for each individual in the MEPS sample based on their sex, age, the interaction between sex and age, geographic region, insurance plan type, and calendar year. Cost estimates were weighted by the odds of the propensity scores to ensure the matched controls were similar to our SCD sample, with higher odds representing a higher probability of belonging to the SCD sample. We compared differences in healthcare utilization and medical costs between surviving SCD individuals and matched controls using 2-sample independent t tests.
We applied a Kaplan-Meier sample average (KMSA) estimator to calculate the age-specific and nonelderly lifetime burden of SCD-attributable total costs and OOP costs. We multiplied the probability of surviving to each age for individuals with SCD and matched controls with the average total costs or OOP costs among individuals who survived to that age.26 The difference between the sum of these products over 0 to 64 years of age gave the nonelderly lifetime burden of SCD-attributable costs. Estimates of age- and sex-specific survival probabilities for individuals with SCD were derived from digitized survival curves presented by Lubeck and colleagues.27 We conducted a bootstrap analysis of these digitized data to obtain standard errors. For matched controls, we applied 2017 general population life tables for Black males and females (separately).28 These estimates were assumed to be deterministic. Further details on the KMSA calculations can be found in the Appendix.
Two components contribute to the burden of SCD-attributable costs: the higher intensity of medical care among survivors at a certain age and the difference in the probability of surviving to a certain age. We decomposed SCD-attributable total and OOP costs into the component “intensity effect” (the KMSA estimator of costs evaluated with the survival probabilities of SCD individuals applied to the difference in survivor costs between SCD individuals and matched controls) and the “mortality effect” (the KMSA estimator of costs evaluated with survivor costs for matched controls applied to the difference in survival probabilities between SCD individuals and matched controls). The justification for this decomposition is shown in the Appendix.
This study used retrospective data with no identifiable information and did not meet the definition of human subjects research by the University of Washington Institutional Review Board. MarketScan Research Databases are proprietary and are available through application to IBM.
Results
The cohort selection procedure is shown in Figure 1. A total of 20 891 individuals who fulfilled the case definition of SCD were included in our study cohort. The mean age at enrollment was 25.7 years (standard deviation, 17.4, 5.5% with age 0, and 40.7% <18 years of age), 58.0% were females, and the majority were from the South region (Table 1). The matched control sample consisted of 33 588 individuals who were sampled in MEPS between 2007 and 2018. After weighting based on the estimated propensity score, the control sample exhibited similar demographic characteristics to the SCD sample (Table 1).
Demographic characteristics and healthcare services use of surviving individuals with SCD and matched controls
| . | Individuals with SCD . | Matched Controls . | ||||
|---|---|---|---|---|---|---|
| n | 20 891 | 33 588 | ||||
| Follow-up, yr | 4.4 (SD 2.8) | NA | ||||
| Age (yr),∗ % | 25.7 (SD 17.4) | 24.7 (17.0) | ||||
| Male | 42.0 | 42.8 | ||||
| Region, % | ||||||
| Northeast | 19.2 | 21.2 | ||||
| Northcentral | 15.2 | 15.8† | ||||
| South | 55.6 | 56.2 | ||||
| West | 8.3 | 6.7 | ||||
| Unknown | 1.7 | — | ||||
| Calendar year,∗ % | ||||||
| 2007-2009 | 51.0 | 46.5 | ||||
| 2010-2012 | 21.6 | 20.5 | ||||
| 2013-2015 | 19.0 | 22.9 | ||||
| 2016-2018 | 8.5 | 10.1 | ||||
| Plan type, % | ||||||
| HMO | 14.6 | 17.8 | ||||
| Other‡ | 85.4 | 78.6 | ||||
| Unknown | — | 3.6 | ||||
| Healthcare Services Use (Mean [SE] Visits/Yr) | ||||||
| Age at enrollment | 0-18 (n = 8 512) | 19-30 (n = 4 156) | 31-64 (n = 8 223) | 0-18 (n = 8 871) | 19-30 (n = 6 103) | 31-64 (n = 18 614) |
| Office visits§ | 4.55 (0.03) | 4.51 (0.04) | 6.49 (0.04) | 2.42 (0.11) | 2.39 (0.15) | 3.72 (0.12) |
| Outpatient & urgent care visitsǁ | 4.86 (0.04) | 4.73 (0.08) | 4.77 (0.05) | 0.08 (0.01) | 0.15 (0.05) | 0.32 (0.02) |
| Ambulance use | 0.05 (0.003) | 0.09 (0.005) | 0.12 (0.007) | 0.002 (0.001) | 0.005 (0.001) | 0.004 (0.001) |
| Emergency department visits | 1.32 (0.01) | 1.90 (0.04) | 1.12 (0.02) | 0.14 (0.009) | 0.20 (0.01) | 0.18 (0.007) |
| Inpatient admissions | 0.57 (0.007) | 0.69 (0.013) | 0.46 (0.006) | 0.04 (0.008) | 0.07 (0.01) | 0.07 (0.004) |
| Prescription fills | 8.02 (0.07) | 9.77 (0.13) | 15.94 (0.12) | 1.57 (0.09) | 2.38 (0.13) | 8.45 (0.25) |
| . | Individuals with SCD . | Matched Controls . | ||||
|---|---|---|---|---|---|---|
| n | 20 891 | 33 588 | ||||
| Follow-up, yr | 4.4 (SD 2.8) | NA | ||||
| Age (yr),∗ % | 25.7 (SD 17.4) | 24.7 (17.0) | ||||
| Male | 42.0 | 42.8 | ||||
| Region, % | ||||||
| Northeast | 19.2 | 21.2 | ||||
| Northcentral | 15.2 | 15.8† | ||||
| South | 55.6 | 56.2 | ||||
| West | 8.3 | 6.7 | ||||
| Unknown | 1.7 | — | ||||
| Calendar year,∗ % | ||||||
| 2007-2009 | 51.0 | 46.5 | ||||
| 2010-2012 | 21.6 | 20.5 | ||||
| 2013-2015 | 19.0 | 22.9 | ||||
| 2016-2018 | 8.5 | 10.1 | ||||
| Plan type, % | ||||||
| HMO | 14.6 | 17.8 | ||||
| Other‡ | 85.4 | 78.6 | ||||
| Unknown | — | 3.6 | ||||
| Healthcare Services Use (Mean [SE] Visits/Yr) | ||||||
| Age at enrollment | 0-18 (n = 8 512) | 19-30 (n = 4 156) | 31-64 (n = 8 223) | 0-18 (n = 8 871) | 19-30 (n = 6 103) | 31-64 (n = 18 614) |
| Office visits§ | 4.55 (0.03) | 4.51 (0.04) | 6.49 (0.04) | 2.42 (0.11) | 2.39 (0.15) | 3.72 (0.12) |
| Outpatient & urgent care visitsǁ | 4.86 (0.04) | 4.73 (0.08) | 4.77 (0.05) | 0.08 (0.01) | 0.15 (0.05) | 0.32 (0.02) |
| Ambulance use | 0.05 (0.003) | 0.09 (0.005) | 0.12 (0.007) | 0.002 (0.001) | 0.005 (0.001) | 0.004 (0.001) |
| Emergency department visits | 1.32 (0.01) | 1.90 (0.04) | 1.12 (0.02) | 0.14 (0.009) | 0.20 (0.01) | 0.18 (0.007) |
| Inpatient admissions | 0.57 (0.007) | 0.69 (0.013) | 0.46 (0.006) | 0.04 (0.008) | 0.07 (0.01) | 0.07 (0.004) |
| Prescription fills | 8.02 (0.07) | 9.77 (0.13) | 15.94 (0.12) | 1.57 (0.09) | 2.38 (0.13) | 8.45 (0.25) |
HMO, health maintenance organization; EPO, exclusive provider organization; POS, point-of-service; CDHP, consumer directed health plan; HDHP, high deductible health plan.
Determined at enrollment.
Midwest region.
Other plan types are comprehensive, EPO, POS, POS with capitation, CDHP, and HDHP.
Visits to physicians’ offices outside of hospitals.
Visits to outpatient facilities adjoining hospital facilities.
All types of healthcare utilization were higher in surviving individuals with SCD than in matched controls for any age group (Table 1). For SCD individuals, the annual number of office visits (visits to physicians’ offices outside hospitals) and the combined number of outpatient visits (facilities adjoining hospitals) and urgent care visits averaged across all ages was 5.4 (standard error [SE], 0.02) and 4.8 (SE, 0.03), respectively. Individuals with SCD incurred an excess burden of 2.46 (SE, 0.05) office visits, 4.61 (SE, 0.05) urgent care visits, 1.17 (SE, 0.02) emergency department visits, 0.49 (SE, 0.01) inpatient visits, and 7.37 (SE, 0.13) prescription fills per year averaged across all ages, relative to matched controls (all P < .01). Annual medical costs varied widely between individuals with SCD (total costs: median, $9607; interquartile range [IQR], $1927-$34 265; OOP costs: median, $547; IQR, $109-$1803), but mean costs increased with the number of months per year of follow-up with a VOE (0 months: $26 134; SE, $488; 1 month: $37 196; SE, $695; 2+ months: $81 741; SE, $1387).
The estimated annual total costs among surviving individuals with SCD and matched controls are presented in Table 2. More than 87% of total costs could be attributed to SCD for all age groups in males and females. Mean annual total costs among survivors were $36 128 (95% CI, $33 904-$38 352) for females and $38 445 (95% CI, $36 292-$40 598) for males. SCD-attributable costs were $32 311 (95% CI, $28 533-$36 089) and $35 203 (95% CI, $31 812-$38 594), respectively. Total costs were not significantly different between males and females for any age group (all P > .10). Costs increased with age among survivors. A smoothed age profile of total costs is shown in the supplemental Figure 2.
Estimated total annual medical costs and OOP costs (95% CI) among surviving individuals with SCD and matched controls, by age group and sex
| Age (yr) . | Individuals with SCD . | Matched Controls . | SCD-attributable Costs∗ . | P value† . |
|---|---|---|---|---|
| Total medical costs, $ | ||||
| Females, yr | ||||
| 0-5 | 26 835 (16 400-37 271) | 1 722 (1 405-2 040) | 25 113 (3 661-46 565) | .01 |
| 6-12 | 28 575 (20 001-37 149) | 2 006 (1 678-2 334) | 26 569 (10 521-42 617) | <.01 |
| 13-18 | 37 283 (34 637-39 930) | 2 316 (1 986-2 647) | 34 967 (30 039-39 895) | <.01 |
| 19-24 | 41 020 (36 422-45 618) | 2 631 (2 291-2 971) | 38 389 (30 020-46 758) | <.01 |
| 25-34 | 34 105 (30 478-37 732) | 3 162 (2 789-3 536) | 30 943 (24 600-37 286) | <.01 |
| 35-54 | 36 574 (34 132-39 017) | 4 520 (3 956-5 084) | 32 054 (28 119-35 989) | <.01 |
| 55-64 | 47 780 (37 100-58 460) | 6 230 (5 148-7 313) | 41 550 (26 767-56 333) | <.01 |
| Males, yr | ||||
| 0-5 | 29 327 (20 927-37 681) | 1 546 (1 166-1 926) | 27 781 (10 327-45 235) | <.01 |
| 6-12 | 31 504 (25 376-37 632) | 1 767 (1 368-2 167) | 29 737 (18 040-41 434) | <.01 |
| 13-18 | 40 306 (36 909-43 703) | 2 038 (1 629-2 447) | 38 268 (32 216-44 320) | <.01 |
| 19-24 | 44 228 (37 015-51 442) | 2 338 (1 910-2 767) | 41 890 (30 415-53 365) | <.01 |
| 25-34 | 36 691 (34 288-39 093) | 2 774 (2 311-3 237) | 33 917 (30 367-37 467) | <.01 |
| 35-54 | 39 968 (36 783-43 154) | 4 013 (3 352-4 675) | 35 955 (31 461-40 449) | <.01 |
| 55-64 | 50 981 (37 542-64 420) | 5 439 (4 419-6 459) | 45 542 (29 305-61 779) | <.01 |
| OOP COSTS, $ | ||||
| Females, yr | ||||
| 0-5 | 1 232 (1 123-1 341) | 195 (167-223) | 1 037 (813-1 261) | <.01 |
| 6-12 | 1 152 (975-1 328) | 224 (197-252) | 928 (597-1 259) | <.01 |
| 13-18 | 1 236 (1 129-1 342) | 257 (229-284) | 979 (781-1 177) | <.01 |
| 19-24 | 1 379 (1 285-1 473) | 287 (256-318) | 1 092 (920-1 264) | <.01 |
| 25-34 | 1 399 (1 303-1 496) | 349 (313-386) | 1 050 (881-1 219) | <.01 |
| 35-54 | 1 324 (1 261-1 387) | 495 (429-561) | 829 (719-939) | <.01 |
| 55-64 | 1 445 (1 305-1 584) | 673 (539-806) | 772 (556-988) | <.01 |
| Males | ||||
| 0-5 | 1 223 (1 102-1 344) | 192 (149-234) | 1 031 (777-1 285) | <.01 |
| 6-12 | 1 181 (1 025-1 336) | 219 (174-264) | 962 (665-1 259) | <.01 |
| 13-18 | 1 271 (1 184-1 358) | 250 (203-298) | 1 021 (864-1 178) | <.01 |
| 19-24 | 1 399 (1 294-1 504) | 279 (225-332) | 1 120 (949-1 291) | <.01 |
| 25-34 | 1 434 (1 314-1 554) | 333 (277-388) | 1 101 (920-1 282) | <.01 |
| 35-54 | 1 394 (1 301-1 486) | 484 (401-567) | 910 (767-1 053) | <.01 |
| 55-64 | 1 463 (1 310-1 616) | 648 (512-785) | 815 (598-1 032) | <.01 |
| Age (yr) . | Individuals with SCD . | Matched Controls . | SCD-attributable Costs∗ . | P value† . |
|---|---|---|---|---|
| Total medical costs, $ | ||||
| Females, yr | ||||
| 0-5 | 26 835 (16 400-37 271) | 1 722 (1 405-2 040) | 25 113 (3 661-46 565) | .01 |
| 6-12 | 28 575 (20 001-37 149) | 2 006 (1 678-2 334) | 26 569 (10 521-42 617) | <.01 |
| 13-18 | 37 283 (34 637-39 930) | 2 316 (1 986-2 647) | 34 967 (30 039-39 895) | <.01 |
| 19-24 | 41 020 (36 422-45 618) | 2 631 (2 291-2 971) | 38 389 (30 020-46 758) | <.01 |
| 25-34 | 34 105 (30 478-37 732) | 3 162 (2 789-3 536) | 30 943 (24 600-37 286) | <.01 |
| 35-54 | 36 574 (34 132-39 017) | 4 520 (3 956-5 084) | 32 054 (28 119-35 989) | <.01 |
| 55-64 | 47 780 (37 100-58 460) | 6 230 (5 148-7 313) | 41 550 (26 767-56 333) | <.01 |
| Males, yr | ||||
| 0-5 | 29 327 (20 927-37 681) | 1 546 (1 166-1 926) | 27 781 (10 327-45 235) | <.01 |
| 6-12 | 31 504 (25 376-37 632) | 1 767 (1 368-2 167) | 29 737 (18 040-41 434) | <.01 |
| 13-18 | 40 306 (36 909-43 703) | 2 038 (1 629-2 447) | 38 268 (32 216-44 320) | <.01 |
| 19-24 | 44 228 (37 015-51 442) | 2 338 (1 910-2 767) | 41 890 (30 415-53 365) | <.01 |
| 25-34 | 36 691 (34 288-39 093) | 2 774 (2 311-3 237) | 33 917 (30 367-37 467) | <.01 |
| 35-54 | 39 968 (36 783-43 154) | 4 013 (3 352-4 675) | 35 955 (31 461-40 449) | <.01 |
| 55-64 | 50 981 (37 542-64 420) | 5 439 (4 419-6 459) | 45 542 (29 305-61 779) | <.01 |
| OOP COSTS, $ | ||||
| Females, yr | ||||
| 0-5 | 1 232 (1 123-1 341) | 195 (167-223) | 1 037 (813-1 261) | <.01 |
| 6-12 | 1 152 (975-1 328) | 224 (197-252) | 928 (597-1 259) | <.01 |
| 13-18 | 1 236 (1 129-1 342) | 257 (229-284) | 979 (781-1 177) | <.01 |
| 19-24 | 1 379 (1 285-1 473) | 287 (256-318) | 1 092 (920-1 264) | <.01 |
| 25-34 | 1 399 (1 303-1 496) | 349 (313-386) | 1 050 (881-1 219) | <.01 |
| 35-54 | 1 324 (1 261-1 387) | 495 (429-561) | 829 (719-939) | <.01 |
| 55-64 | 1 445 (1 305-1 584) | 673 (539-806) | 772 (556-988) | <.01 |
| Males | ||||
| 0-5 | 1 223 (1 102-1 344) | 192 (149-234) | 1 031 (777-1 285) | <.01 |
| 6-12 | 1 181 (1 025-1 336) | 219 (174-264) | 962 (665-1 259) | <.01 |
| 13-18 | 1 271 (1 184-1 358) | 250 (203-298) | 1 021 (864-1 178) | <.01 |
| 19-24 | 1 399 (1 294-1 504) | 279 (225-332) | 1 120 (949-1 291) | <.01 |
| 25-34 | 1 434 (1 314-1 554) | 333 (277-388) | 1 101 (920-1 282) | <.01 |
| 35-54 | 1 394 (1 301-1 486) | 484 (401-567) | 910 (767-1 053) | <.01 |
| 55-64 | 1 463 (1 310-1 616) | 648 (512-785) | 815 (598-1 032) | <.01 |
All costs are in 2020 USD. Raw costs (not model-estimated) are shown in supplemental Table 1 for comparison.
SCD-attributable costs are the difference between individuals with SCD and matched controls.
Two-sample independent t test.
OOP costs were fourfold higher for surviving individuals with SCD than for matched controls (Table 2). Overall, individuals with SCD paid 3% to 5% of their total healthcare costs OOP, while controls paid 11% to 12%. Mean annual OOP costs were similar between males ($1333; 95% CI, $1274-$1393) and females ($1317; 95% CI, $1261-$1373). SCD-attributable costs were $942 (95% CI, $840-$1044) and $899 (95% CI, $799-$999), respectively. OOP costs were relatively similar across all ages.
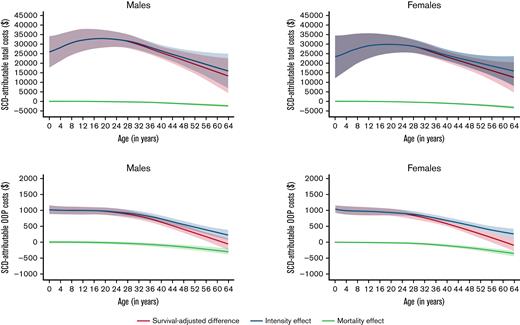
The average nonelderly (0 to 64 year) life expectancy in individuals with SCD was 51.2 years for females and 50.5 for males with SCD, and 62.3 years and 60.3 years in matched controls, respectively (Kaplan-Meier survival curves are shown in the supplemental Figure 3). KMSA estimated annual total costs and SCD-attributable costs peaked between 13 and 24 years; KMSA estimated OOP costs were relatively stable before declining in individuals >40 years with SCD (Figure 2). Costs increased continuously with age among the male and female control cohorts. Over 97% of the SCD-attributable total costs were generated through the intensity of utilization, which also peaked in young adulthood (Figure 3). Earlier mortality in individuals with SCD resulted in only a small decrease in SCD-attributable total costs, even at older ages when survival differences with matched controls were highest. The mortality effect was more influential for OOP costs at older ages, which resulted in higher KMSA estimated OOP costs in controls than individuals with SCD over 61 years.
KMSA estimates of total medical costs and OOP costs among individuals with SCD and matched controls by sex, smoothed over age. All estimates are smoothed and survival-adjusted.
KMSA estimates of total medical costs and OOP costs among individuals with SCD and matched controls by sex, smoothed over age. All estimates are smoothed and survival-adjusted.
Total medical costs and OOP costs attributable to SCD and their decomposition into intensity effects and mortality effects. All estimates are smoothed and survival-adjusted.
Total medical costs and OOP costs attributable to SCD and their decomposition into intensity effects and mortality effects. All estimates are smoothed and survival-adjusted.
Lifetime total costs attributable to SCD for ages 0 to 64 years were $1.6 million (95% CI, $1.3M-$1.9M) for females and $1.7 million (95% CI, $1.4M-$2.1M) for males (Table 3). SCD-attributable nonelderly lifetime OOP costs were $42 395 (95% CI, $34 756-$50 033) for females and $45 091 (95% CI, $36 491-$53 691) for males, representing 2.6% and 2.7% of total costs, respectively. Lifetime total medical costs and OOP costs were 907% and 285% higher for individuals with SCD than for matched controls, respectively. When the mortality effect was removed (ie, the negative costs due to increased mortality among SCD individuals), the SCD-attributable costs were reflected in the intensity effects, which were slightly higher than the original estimates.
Nonelderly (0-64 yr of age) lifetime total costs and OOP costs attributable to SCD and their decomposition into costs due to the intensity and mortality effects
| Sex . | LE (0-64), yr (SCD vs Matched Controls) . | Survival-adjusted Costs for SCD Individuals (95% CI) . | Survival-adjusted Costs for Matched Controls (95% CI) . | SCD-attributable Costs (95% CI) . | SCD-attributable Intensity Effect (95% CI) . | SCD-attributable Mortality Effect (95% CI) . |
|---|---|---|---|---|---|---|
| Total Costs, $ | ||||||
| Females | 51.2 vs 62.3 | 1 812 743 (1 489 450 to 2 136 036) | 224 623 (191 989 to 257 256) | 1 588 120 (1 262 183 to 1 914 057) | 1 644 900 (1 319 718 to 1 970 082) | −56 780 (−85 270 to −28 290) |
| Males | 50.5 vs 60.3 | 1 937 966 (1 612 327 to 2 263 605) | 189 133 (154 174 to 224 093) | 1 748 832 (1 420 634 to 2 077 031) | 1 792 774 (1 465 038 to 2 120 511) | −43 942 (−75 860 to −12 023) |
| OOP Costs, $ | ||||||
| Females | 51.2 vs 62.3 | 67 003 (60 597 to 73 409) | 24 609 (21 084 to 28 134) | 42 395 (34 756 to 50 033) | 48 562 (41 264 to 55 861) | −6 168 (−9 198 to −3 138) |
| Males | 50.5 vs 60.3 | 67 897 (60 696 to 75 098) | 22 806 (18 479 to 27 132) | 45 091 (36 491 to 53 691) | 50 346 (42 005 to 58 686) | −5 254 (−9 188 to −1 320) |
| Sex . | LE (0-64), yr (SCD vs Matched Controls) . | Survival-adjusted Costs for SCD Individuals (95% CI) . | Survival-adjusted Costs for Matched Controls (95% CI) . | SCD-attributable Costs (95% CI) . | SCD-attributable Intensity Effect (95% CI) . | SCD-attributable Mortality Effect (95% CI) . |
|---|---|---|---|---|---|---|
| Total Costs, $ | ||||||
| Females | 51.2 vs 62.3 | 1 812 743 (1 489 450 to 2 136 036) | 224 623 (191 989 to 257 256) | 1 588 120 (1 262 183 to 1 914 057) | 1 644 900 (1 319 718 to 1 970 082) | −56 780 (−85 270 to −28 290) |
| Males | 50.5 vs 60.3 | 1 937 966 (1 612 327 to 2 263 605) | 189 133 (154 174 to 224 093) | 1 748 832 (1 420 634 to 2 077 031) | 1 792 774 (1 465 038 to 2 120 511) | −43 942 (−75 860 to −12 023) |
| OOP Costs, $ | ||||||
| Females | 51.2 vs 62.3 | 67 003 (60 597 to 73 409) | 24 609 (21 084 to 28 134) | 42 395 (34 756 to 50 033) | 48 562 (41 264 to 55 861) | −6 168 (−9 198 to −3 138) |
| Males | 50.5 vs 60.3 | 67 897 (60 696 to 75 098) | 22 806 (18 479 to 27 132) | 45 091 (36 491 to 53 691) | 50 346 (42 005 to 58 686) | −5 254 (−9 188 to −1 320) |
LE, life expectancy.
Discussion
We conducted a retrospective cohort study using an employer-sponsored commercial claims database to understand the lifetime burden of SCD on health systems and individuals. Individuals with SCD were compared with an age- and sex-matched sample of the general population with no blood disorders to determine the costs attributed to SCD. After adjusting for differences in survival, nonelderly lifetime (≤64 years) SCD-attributable total medical costs and OOP costs were $1.7 million and $44 000, respectively. The burden of SCD-attributable total costs peaked in young adulthood when the intensity of costs was greatest, and the difference in expected mortality between individuals with SCD and matched controls was small, although this latter factor was much less influential. Surviving individuals with SCD paid approximately $1324 in annual OOP costs, with 69% of these costs attributable to SCD. The substantial burden of total medical costs and OOP costs due to SCD highlights its impact on payers, patients, and caregivers.
Surviving individuals incurred an average of $34 477 per year in SCD-attributable medical costs; payers bore 96% of these costs, and 4% were paid by patients OOP. Our estimates are slightly higher than Salcedo and colleagues,29 who reported excess costs of $31 045 (2020 USD) relative to non-SCD controls in a commercially insured population. However, because controls were not matched to individuals with SCD on race, their sample is likely to include a disproportionate number of Whites, who typically have higher healthcare costs than the Black population, leading to lower attributable cost estimates.30 We also accounted for differential survival between individuals with SCD and matched controls using KMSA mean costs, a key gap in the previous literature. The lifetime burden of total medical costs in individuals with SCD is substantially higher than previously reported ($1.2M in 2020 USD). However, this estimate used data that is nearly 20 years old and only included individuals surviving to 45 years.8 Our analysis took the perspective of a commercial payer responsible for lifetime medical costs up to the point of Medicare eligibility (65 years). Older adults are a critical population to include in assessments of the economic burden of SCD, as survivors incur the highest medical costs of any age group, and increased mortality has only a small impact on expected lifetime total medical costs.
There were significant age-related trends in total medical costs for individuals with SCD. Among survivors, the highest total annual medical costs and OOP costs were in individuals aged 55 to 64 years, reflecting an increasing burden of medical costs with age, which may be attributed to accumulated organ damage.31 However, the average life expectancy for an individual with SCD was 51 years, meaning most patients do not survive long enough to incur the high costs of older adulthood. Survival-adjusted costs, calculated using KMSA estimates, peaked in adolescence and early adulthood due to the high intensity of medical costs and low mortality rates during this period. Medical costs were particularly high during the transition from pediatric to adult care when multiple barriers to receiving optimal and equitable treatment have been identified. These include a lack of providers with expertise in SCD, difficulties transitioning from pediatric to adult facilities, bias against individuals with SCD and frequent or chronic pain, and institutional racism.32-36 These factors may have contributed to the high rates of acute care visits we observed in young adulthood, which is in line with the previous observations.5,13 The decline in survival-adjusted total costs at older ages was primarily driven by a decrease in the excess burden of medical costs relative to the general population, in which total medical costs increased monotonically with age. Lower life expectancy in individuals with SCD contributed a comparatively smaller fraction to this decline.
The burden of medical costs borne directly by individuals with SCD has not previously been examined. Nonelderly lifetime OOP costs were nearly 4 times greater than matched controls and averaged $1324 annually among surviving individuals with SCD. Thus, having SCD accounted for the vast majority of OOP medical costs regardless of age or sex and despite upper limits on OOP costs set by insurance plans. It is worth considering these costs in the context of the population affected by SCD. The annual OOP burden of SCD is >3% of the median household income of Black Americans, whose income and assets are already far below those of White Americans.37 High OOP costs can contribute to documented problems where those with SCD do not seek care for comorbidities, especially acute pain episodes.36 Further, the OOP costs reported here are for privately insured beneficiaries and are limited to healthcare costs. Thus they are likely to be a gross underestimate of the economic impact of SCD on families, as many are not insured, and the full burden of indirect costs such as productivity loss is not reflected.
Our estimates of the nonelderly lifetime costs attributable to SCD highlight the potential for curative gene therapies or novel disease-modifying therapies to reduce the burden of medical costs on patients and payers. Recently approved treatments such as l-glutamine and crizanlizumab have been shown to reduce acute complications of SCD, and numerous gene therapies with the potential for highly durable clinical benefits are being developed.38-40 However, these therapies impose high costs. Interestingly, the proposed treatment costs with gene therapy are in line with the lifetime total medical costs reported here.16,17 However, one-time interventions with substantial upfront costs and benefits accumulated over many years downstream impose unique payment challenges. This is especially the case for gene therapies, in which the costs of providing treatment to all Medicaid-insured individuals with severe SCD in 10 states are projected at $5.5 billion.9 This presents serious barriers to affordability, and unique payment structures will be required to avoid restricting access.41 There are additional challenges for commercial payers, given that many patients change insurance providers throughout their lifetimes.42 However, payers should consider the costs of medical care for SCD accumulated over a patient’s lifetime, as well as the wider societal benefits of curative therapies. The highest intensity of medical costs occurs among young adults when improved educational attainment could have a substantial impact on lifetime productivity and income.43 In contrast, patients receiving treatment with gene therapies may still incur medical costs from downstream complications.44 Economic analyses will ultimately be required to determine the value of gene therapies and disease-modifying treatments to patients, payers, and society.
Our study has several strengths. Our control population was comprised of individuals from the privately insured general Black population, with observations weighted by demographic and insurance plan characteristics to ensure representativeness to individuals with SCD. Most previous estimates of medical costs are descriptive and do not compare costs to matched controls to determine SCD-attributable costs. In addition, we used generally accepted methods to account for the impact of mortality on lifetime costs.45 Accounting for these factors is necessary to determine the incremental economic burden of SCD from the payer perspective. We also incorporated the individual perspective by assessing OOP costs, which indicate the direct financial burden of medical care for SCD. This patient perspective is crucial for understanding the true disease burden.
Our study has several limitations. We did not match individuals with SCD to controls within the same database due to the lack of data on individual race in the private claims database, which is necessary to compare populations. We believe that matching with external nationally representative data provides more accurate estimates of attributable costs compared with not matching alone or matching within the database without the race variable. Cost estimates for matched controls were based on self-reported data, which has been shown to understate costs among high expenditure cases.46 Although the MEPS database provides nationally representative costs, a comparison of MEPS and Marketscan has revealed that MEPS estimates of expenditures are about 10% lower on average than Marketscan estimates.47 This would mean that our control costs estimates could be higher by about $20 000 to $25 000. However, this indicates a <1.5% impact on our estimates of attributable costs. We also did not have information on individual mortality, which limited us to assessing costs among survivors. Although we adjusted our final estimates for average annual mortality by age and sex from an independent source, medical costs and mortality can be correlated through other factors that could make our SCD-attributable estimates conservative, as patients with less severe SCD are likely to survive longer. In addition, our source of mortality rates was based on a simulated cohort with death rates determined from population registry data.27 Mortality rates in the commercially insured population may be different, which could impact the accuracy of our estimates. Our estimates of burden also do not account for the indirect costs of SCD, including income and productivity loss for both individuals and their caregivers.27,48,49 Lifetime income loss due to SCD has been estimated at $700 000 compared with a matched individual without SCD.27 Future work should focus on combining direct and indirect costs of SCD in a single estimate of the economic burden. Finally, only one-third of the population with SCD is commercially insured, meaning our conclusions may not be representative of the entire population with SCD. Individuals with commercial insurance have been shown to experience fewer acute complications than those insured through Medicaid or Medicare, suggesting there may have been a selection for individuals with milder disease.50 In addition, our cohort was limited to patients <65 years of age regardless of their eligibility for public insurance. Finally, the matched control cohort was restricted to Black individuals, whereas individuals with SCD could have been of any race.
Conclusions
Sickle cell disease imposes a substantial burden of medical costs on patients and commercial payers. Emerging curative therapies may provide critical opportunities to reduce the lifetime medical costs attributed to this disease.51
Acknowledgments
The authors acknowledge the National Heart, Lung, and Blood Institute, participants from Emmes, the CureSC Expert Panel, and the CureSC Initiative. All errors and opinions are ours.
This research was supported by the National Heart, Lung, and Blood Institute, Cure Sickle Cell initiative. The opinions expressed are those of the authors only. This research was funded in part by the National Institutes of Health (NIH) Agreements OT3HL152448 and OT3HL151434. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the NIH.
Authorship
Contribution: A.B., S.D.R., B.D., and M.A.B. conceived the study; K.M.J., A.B., and B.J. conducted the analyses; K.M.J. and A.B. wrote the first draft of the manuscript; and all authors critically commented on the manuscript and approved the final version. A.B. is the guarantor of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anirban Basu, School of Pharmacy, University of Washington, 1959 NE Pacific St, H375Q Box 357631, Seattle, WA 98195; e-mail: basua@uw.edu.
References
Author notes
MarketScan Research Databases are proprietary and are available through application to IBM. Please direct other inquiries to the corresponding author: basua@uw.edu.