Key Points
Nilotinib treatment demonstrated sustained long-term efficacy in pediatric patients with CML-CP.
The overall benefit-risk assessment of nilotinib for pediatric patients with CML remains favorable, making it a valuable treatment option.
Abstract
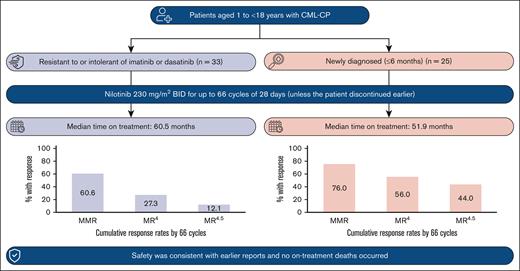
The efficacy and safety of nilotinib in pediatric patients with imatinib/dasatinib resistant/intolerant (R/I) or newly diagnosed (ND) Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia in chronic phase (CML-CP) was demonstrated in the phase 2, open-label DIALOG study. In this final analysis, long-term efficacy and safety are presented for patients who completed 66 cycles (of 28 days) of treatment with nilotinib (230 mg/m2 twice daily) or discontinued early. Overall, 59 patients were enrolled and 58 were treated (R/I, n = 33; ND, n = 25; median time on treatment: 60.5 and 51.9 months, respectively). In the R/I cohort, the cumulative major molecular response (MMR; BCR::ABL1 international scale [IS] ≤ 0.1%) rate was 60.6%, and no patients had a confirmed loss of MMR. Among ND patients, the best overall MMR rate was 76.0%; 3 patients had a confirmed loss of MMR. The cumulative molecular response MR4 (BCR::ABL1IS ≤ 0.01%) and MR4.5 (BCR::ABL1IS ≤ 0.0032%) rates by 66 cycles were 27.3% and 12.1% in the R/I cohort, and 56.0% and 44.0% in the ND cohort, respectively. The safety profile of nilotinib was consistent with those of earlier reports. No on-treatment deaths occurred. These long-term (up to ∼5 years) data support the efficacy and safety of nilotinib in pediatric patients with Ph+ CML-CP. This trial was registered at www.clinicaltrials.gov.uk as #NCT01844765.
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm associated with the activity of the BCR::ABL1 fusion oncoprotein, a product of the Philadelphia chromosome that is formed by a reciprocal translocation between the long arms of chromosomes 9 and 22.1 Incidence of CML among children and adolescents is low, accounting for ∼2% to 3% of all leukemias in children aged <15 years and 9% in adolescents aged 15 to 19 years.2
Multiple tyrosine kinase inhibitors (TKIs), including imatinib,3,4 dasatinib,5,6 and nilotinib,7,8 have been approved for the treatment of pediatric patients with Philadelphia chromosome–positive (Ph+) CML. Currently, bosutinib,9 ponatinib,10 and asciminib11 (a BCR::ABL1 inhibitor that works by specifically targeting the ABL myristoyl pocket)12 are under clinical investigation for children.
Nilotinib (Tasigna, AMN107), a second-generation TKI considered a more potent inhibitor of BCR::ABL1 than imatinib,13 has been approved for the treatment of pediatric patients with newly diagnosed (ND) Ph+ CML in chronic phase (CML-CP) and Ph+ CML-CP that is resistant or intolerant (R/I) to prior therapy.7,8 In the United States, nilotinib has also been approved for patients aged ≥1 years with Ph+ CML in accelerated phase (AP) that is R/I to prior TKI therapy.14
Clinical outcomes in pediatric patients with CML have been improved with TKI treatment. However, similar to adults, not all pediatric patients respond to imatinib or dasatinib, and some may become resistant to these therapies. In a phase 3 pediatric study, imatinib failure was observed in 27% of the patients15 and in a phase 4 study, 30% of the patients discontinued imatinib mainly because of an unsatisfactory response.16 Before nilotinib, there were no approved therapies for pediatric patients with imatinib or dasatinib R/I CML-CP. Children may receive TKI therapy for many years, including during times of active growth and onset of puberty and, therefore, face potential unique side effects of receiving treatment while actively growing.17-20
DIALOG is a phase 2, open-label study (NCT01844765) of nilotinib for pediatric patients with Ph+ CML-CP. The primary efficacy end points from the DIALOG study have been previously reported, and the efficacy and safety of nilotinib in this patient population was demonstrated.21 Analysis of long-term safety data from DIALOG revealed a trend toward growth deceleration over time after ≥36 and ≥48 cycles of treatment.22,23
We present the final analysis of efficacy and safety data from the DIALOG study after the completion of 66 cycles of treatment or until study treatment discontinuation. To our knowledge, this is the longest follow-up of TKI treatment for pediatric patients with Ph+ CML-CP.
Materials and methods
Study design and patient population
DIALOG was a multicenter, open-label, noncontrolled phase 2 study of nilotinib for pediatric patients with Ph+ CML conducted at 36 centers across 13 countries; methods have previously been published.21,23 In brief, eligible patients aged 1 to <18 years were enrolled into 1 of 3 cohorts: (1) patients with Ph+ CML-CP R/I to either imatinib or dasatinib (R/I cohort), (2) patients with Ph+ CML-AP R/I to either imatinib or dasatinib, and (3) patients with ND Ph+ CML-CP (ND cohort).
Nilotinib was administered orally at a dose of 230 mg/m2 twice daily (rounded to the nearest 50 mg to a maximum of 400 mg twice daily)24 in capsule form for a total of 66 cycles of 28 days unless the patient discontinued early. The same nilotinib dose was selected for both ND pediatric patients and those R/I based on pharmacokinetic (PK) data from adult studies, which demonstrated an overlap of PK parameters for 300 and 400 mg doses and the acceptable safety profile of a 400 mg dose. Prior pediatric data have demonstrated the 230 mg/m2 dose to have a safety profile consistent with that observed in adults with PK exposure coverage of both the 300 and 400 mg doses.24
All patients completed a safety follow-up of 30 days after the last dose of study treatment and were followed up for survival every 6 months until study completion (defined as the completion of last patient safety visit). At the end of the planned 66 cycles, patients could continue to receive nilotinib if they continued to demonstrate a clinical benefit by enrollment into a rollover study or via a managed access program, if nilotinib was not available commercially in their country of residence.25 The final analysis was based on the last patient’s last visit on 28 August 2020.
Ethics
This study was conducted in accordance with the principles of the Declaration of Helsinki and local laws and regulations. The study protocol and all amendments were approved by the independent ethics committee and/or institutional review board for each study center. Prior written informed consent was provided by the patients’ parents or caregivers and from patients able to provide a signature.
End points and assessments
The objective of this analysis was to evaluate the long-term efficacy and safety (including growth assessments) of nilotinib in study participants. The primary efficacy and safety analysis was reported when all patients had completed 24 cycles of treatment or discontinued earlier,21 and later safety analyses were reported when all patients had completed 36 and 48 cycles of treatment or discontinued earlier.22,23 Secondary efficacy end points included the rate of molecular, cytogenetic, and hematologic response; time to response; duration of response; time to disease progression; event-free survival (EFS); and overall survival (OS) (supplemental Methods).
Safety assessments included adverse events (AEs), which were recorded by preferred term according to Medical Dictionary for Regulatory Activities (version 23.0); severity was assessed according to Common Terminology Criteria for Adverse Events (version 4.03). Serious AEs (SAEs) were defined as AEs that were fatal or life threatening, resulted in persistent or significant disability/incapacity, or required inpatient hospitalization or prolonged existing hospitalization. AEs of special interest (AESIs) were defined based on groups of preferred terms. Routine clinical laboratory evaluations and thyroid function tests were performed. Assessment of growth, development, and sexual maturation were performed as previously reported (supplemental Methods).21,23
Statistical analysis
All analyses were conducted descriptively using the full analysis set (FAS; all patients who received ≥1 dose of study treatment). Efficacy results were based on the final analysis with cumulative data after the last patient’s last visit took place. Response rates according to time points were reported; these were cumulative and indicate the total number of patients who achieved that response level at any time. Confidence intervals (CIs) for response rates were calculated using the Pearson-Clopper method as per the cohort.26 The time to first major molecular response (MMR; BCR::ABL1 international scale [IS] ≤ 0.1%), first complete cytogenetic response (CCyR), first major cytogenetic response (MCyR), and first complete hematologic response (CHR) were summarized for responders. EFS was defined as the time from first study drug intake to the first occurrence of loss of CHR or MCyR, progression to AP or blast crisis (BC), or death from any cause during treatment.27 The time of EFS was censored on the date of last on-treatment assessment for patients without an event. Disease progression was defined as progression to AP/BC or CML-related death. Any value of AP or BC within the first 4 weeks of study treatment was not defined as a progression to AP/BC within the study, unless the patient discontinued study treatment because of progression or the treating physician determined that there was unsatisfactory therapeutic effect with the first 8 weeks. Duration of response and disease progression was estimated by Kaplan-Meier (K-M) methodology. OS was defined as the time from first study drug intake to the date of death due to any cause during the study, including the follow-up period after discontinuation of treatment.
Height and body mass index were summarized at 6-month intervals, using standard deviation scores (SDSs; also known as z score). Height deceleration was quantified based on the number of main percentile lines crossed by patients deviating from the growth curve compared with baseline. Growth data analysis was completed using the FAS and for the prepubescent/pubescent subpopulation at study entry (FAS excluding patients who had completed puberty [defined as Tanner stage 5 attained for both testis and pubic hair for boys and breast development and pubic hair for girls]28,29). As previously described, height was evaluated in a linear mixed-effects model, to assess changes in the slope parameter (FAS only; supplemental Methods).23
Results
Patient disposition
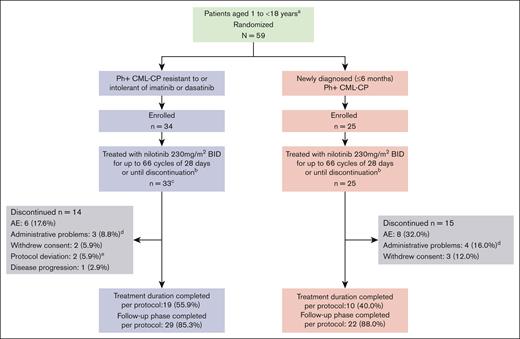
In total, 59 patients were enrolled, of whom 58 were treated (33 of 34 and 25 of 25 patients in the R/I and ND cohorts, respectively; Figure 1). No patient with Ph+ CML-AP was enrolled. Nineteen patients (55.9%) in the R/I cohort and 10 patients (40%) in the ND cohort completed 66 cycles of treatment as per protocol; AE was the most common reason for treatment discontinuation (6 of 34 R/I cohort [17.6%]; 8 of 25 ND cohort [32.0%]).
Study design and patient disposition.aRepresents that the study design includes a third cohort to recruit patients with CML-AP R/I to imatinib or dasatinib. No patients were enrolled in this cohort. bDenotes that nilotinib was administered at a dose of 230 mg/m2 twice daily (rounded to the nearest 50 mg, to a maximum dose of 400 mg) based on the recommended dose for adults of 400 mg twice daily, scaled to body surface area. At any time, discontinuation was allowed based on patient/investigator decision or because of unacceptable toxicities, disease progression, protocol deviations resulting in a significant risk to the patient’s safety, use of prohibited treatments, or pregnancy. Patients who discontinued the treatment early were contacted for study evaluation completion and survival status. cDenotes that 1 enrolled patient did not receive any study medication. dIndicates that administrative problems were 4 cases of “lack of efficacy” (2 in each cohort), 1 case of “lack of study compliance by patient” (R/I imatinib/dasatinib Ph+ CML-CP cohort), and 2 cases of “new cancer therapy” (1 in each cohort; the CRF does not have separate reasons for discontinuation for such cases, the information was thus voluntarily stored under the category “administrative problem”). eIndicates that protocol deviations were 2 cases of noncompliance. CRF, case report form.
Study design and patient disposition.aRepresents that the study design includes a third cohort to recruit patients with CML-AP R/I to imatinib or dasatinib. No patients were enrolled in this cohort. bDenotes that nilotinib was administered at a dose of 230 mg/m2 twice daily (rounded to the nearest 50 mg, to a maximum dose of 400 mg) based on the recommended dose for adults of 400 mg twice daily, scaled to body surface area. At any time, discontinuation was allowed based on patient/investigator decision or because of unacceptable toxicities, disease progression, protocol deviations resulting in a significant risk to the patient’s safety, use of prohibited treatments, or pregnancy. Patients who discontinued the treatment early were contacted for study evaluation completion and survival status. cDenotes that 1 enrolled patient did not receive any study medication. dIndicates that administrative problems were 4 cases of “lack of efficacy” (2 in each cohort), 1 case of “lack of study compliance by patient” (R/I imatinib/dasatinib Ph+ CML-CP cohort), and 2 cases of “new cancer therapy” (1 in each cohort; the CRF does not have separate reasons for discontinuation for such cases, the information was thus voluntarily stored under the category “administrative problem”). eIndicates that protocol deviations were 2 cases of noncompliance. CRF, case report form.
Patient characteristics
In both cohorts, the median age was 13 years, and most patients were male (Table 1). In the R/I cohort, 31 (93.9%) patients had previously received imatinib and 2 (6.1%) patients had previously received dasatinib; 3 (9.1%) patients were intolerant of imatinib; 25 (75.8%) were resistant to imatinib; 3 (9.1%) patients were intolerant of and resistant to imatinib; and 2 (6.1%) were resistant to dasatinib. Of the 29 patients in the R/I cohort with evaluable mutational analyses at baseline, 3 had BCR::ABL1 baseline mutations, and none of the 25 patients in the ND cohort had BCR::ABL1 baseline mutations. At baseline, 2 patients in the R/I cohort and 3 patients in the ND cohort had additional chromosomal abnormalities in Ph+ metaphases, and 2 patients in the R/I cohort had additional chromosomal abnormalities in Ph− metaphases.
Baseline characteristics
| . | R/I cohort∗ n = 33 . | ND cohort n = 25 . |
|---|---|---|
| Median age (range), y | 13 (2-17) | 13 (10-16) |
| Patients aged 1 to <12 y, n (%) | 12 (36.4) | 6 (24.0) |
| Patients aged 12 to <18 y, n (%) | 21 (63.6) | 19 (76.0) |
| Female, n (%) | 12 (36.4) | 12 (48.0) |
| Male, n (%) | 21 (63.6) | 13 (52.0) |
| Median BMI SDS, (range) | −0.57 (−3.4 to 3.4) | −0.09 (−2.8 to 2.1) |
| Median height SDS, (range) | −0.56 (−4.3 to 1.2) | 0.06 (−0.9 to 1.7) |
| Pubertal status, n (%) | ||
| Tanner stage 1 (prepubertal) | 9 (27.3) | 4 (16.0) |
| Tanner stage 2-4 | 15 (45.5) | 17 (68.0) |
| Tanner stage 5† | 8 (24.2) | 4 (16.0) |
| Missing, n (%) | 1 (3.0) | 0 |
| Prior antineoplastic TKI therapies, n (%) | ||
| Imatinib | 31 (93.9) | N/A |
| Dasatinib | 2 (6.1) | N/A |
| Intolerant of imatinib/dasatinib, n (%)‡ | 6 (18.2)/0 | N/A |
| Resistant to imatinib/dasatinib, n (%)‡ | 28 (84.8)/2 (6.1) | N/A |
| BCR::ABL1IS, n (%) | ||
| ≤0.0032 | 1 (3.0) | 0 |
| >0.0032 to ≤0.01 | 1 (3.0) | 0 |
| >0.01 to ≤0.1 | 5 (15.2) | 0 |
| CCyR at baseline, n (%) | 14 (42.4) | 0 |
| Known BCR::ABL1 mutation at baseline, n/m§ | 3/29 | 0/25 |
| Presence of other chromosomal abnormalities in Ph+ metaphases, n (%) | 2 (6.1)‖ | 3 (12.0)¶ |
| Presence of chromosomal abnormalities in Ph− metaphases, n (%) | 2 (6.1)# | 0 |
| . | R/I cohort∗ n = 33 . | ND cohort n = 25 . |
|---|---|---|
| Median age (range), y | 13 (2-17) | 13 (10-16) |
| Patients aged 1 to <12 y, n (%) | 12 (36.4) | 6 (24.0) |
| Patients aged 12 to <18 y, n (%) | 21 (63.6) | 19 (76.0) |
| Female, n (%) | 12 (36.4) | 12 (48.0) |
| Male, n (%) | 21 (63.6) | 13 (52.0) |
| Median BMI SDS, (range) | −0.57 (−3.4 to 3.4) | −0.09 (−2.8 to 2.1) |
| Median height SDS, (range) | −0.56 (−4.3 to 1.2) | 0.06 (−0.9 to 1.7) |
| Pubertal status, n (%) | ||
| Tanner stage 1 (prepubertal) | 9 (27.3) | 4 (16.0) |
| Tanner stage 2-4 | 15 (45.5) | 17 (68.0) |
| Tanner stage 5† | 8 (24.2) | 4 (16.0) |
| Missing, n (%) | 1 (3.0) | 0 |
| Prior antineoplastic TKI therapies, n (%) | ||
| Imatinib | 31 (93.9) | N/A |
| Dasatinib | 2 (6.1) | N/A |
| Intolerant of imatinib/dasatinib, n (%)‡ | 6 (18.2)/0 | N/A |
| Resistant to imatinib/dasatinib, n (%)‡ | 28 (84.8)/2 (6.1) | N/A |
| BCR::ABL1IS, n (%) | ||
| ≤0.0032 | 1 (3.0) | 0 |
| >0.0032 to ≤0.01 | 1 (3.0) | 0 |
| >0.01 to ≤0.1 | 5 (15.2) | 0 |
| CCyR at baseline, n (%) | 14 (42.4) | 0 |
| Known BCR::ABL1 mutation at baseline, n/m§ | 3/29 | 0/25 |
| Presence of other chromosomal abnormalities in Ph+ metaphases, n (%) | 2 (6.1)‖ | 3 (12.0)¶ |
| Presence of chromosomal abnormalities in Ph− metaphases, n (%) | 2 (6.1)# | 0 |
BMI, body mass index; N/A, not applicable.
Patients were resistant to and/or intolerant of 1 prior TKI, either imatinib or dasatinib.
Patients had completed puberty before study enrollment.
Three patients were both intolerant of and resistant to imatinib and are counted once as resistant and once as intolerant.
Numerator (n) is the number of patients with known baseline mutations. Denominator (m) is the number of patients with an evaluable baseline mutational assessment. Mutations detected at baseline were E255K and E255V in 1 patient, G250E and E255K in 1 patient, and L387M in 1 patient.
Data were available for 30 patients with R/I CML-CP. The abnormalities in Ph+ metaphases were 47,XX, t(9,22)(Q34;Q11.2), +der(22)t(9;22)[18]/46, XX[2] in 1 patient and a 3-way translocation between chromosome 4, 9, and 22 in 1 patient.
The abnormalities in Ph+ metaphases were chromosome-3 abnormality in 1 patient, 46,IDEM,add(14)(Q22) OR inv(14)(Q22Q32)[14] in 1 patient, and trisomy-19 in 1 patient.
Data were available for 30 patients with R/I CML-CP. The abnormalities in Ph− metaphases were 46, XX, del(17)(p?) and t(11;17) (1 patient each).
Baseline median height SDSs were −0.56 and 0.06 in the R/I and ND cohorts, respectively. At baseline, 8 of 33 (24.2%) and 4 of 25 (16.0%) patients in the R/I and ND cohorts, respectively, had completed pubertal development.
Nilotinib exposure
Median time on treatment was 60.5 months (range, 0.7-63.5 months) and 51.9 months (range, 1.4-61.2 months) in the R/I and ND cohorts, respectively. The median actual dose intensity in the R/I cohort was 436.9 mg/m2 per day (range, 196.0-493.0 mg/m2 per day), representing a median relative dose intensity of 95% (range, 43%-107%) compared with the planned dose of 230 mg/m2 twice daily; actual and relative dose intensity in the ND cohort were 377.0 mg/m2 per day (range, 149.0-468.0 mg/m2 per day) and 82% (range, 32%-102%), respectively. Fifteen (45.5%) and 8 (32.0%) patients in the R/I and ND cohorts, respectively, were exposed to nilotinib for ≥60 months.
Efficacy
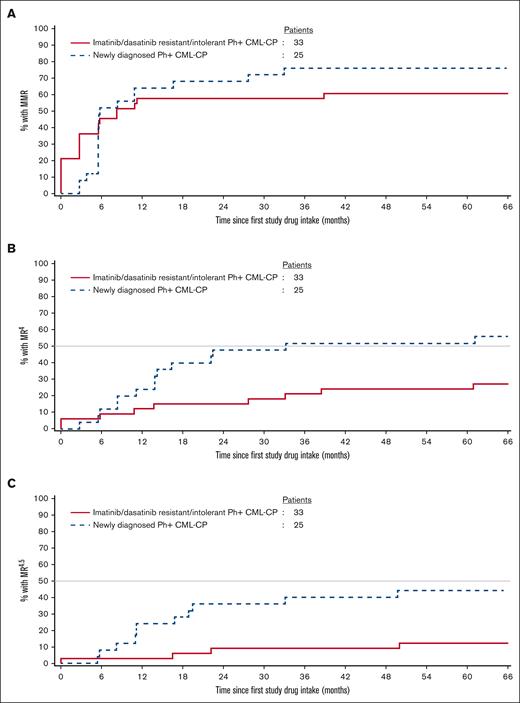
At study conclusion, 20 patients (60.6%) in the R/I cohort were in MMR at least once (including 7 patients who were in MMR at baseline), with a median time to first MMR of 2.8 months (95% CI, 0.0-5.8); with the exclusion of patients in MMR at baseline, the median time to first MMR was 5.6 months (95% CI, 2.8-10.9). Similarly, 19 patients (76.0%) in the ND cohort achieved MMR, with a median time to first MMR of 5.6 months (95% CI, 5.5-10.8). None of the 20 R/I cohort patients who achieved MMR had a confirmed loss of MMR by study completion; 3 of 19 ND cohort patients (15.8%) had a confirmed loss of MMR. Cumulative MMR rates are shown in Figure 2. In a post hoc analysis, the cumulative molecular response MR4 (BCR::ABL1IS ≤ 0.01%) and MR4.5 (BCR::ABL1IS ≤ 0.0032%) rates by 66 cycles were 27.3% (9 of 33) and 12.1% (4 of 33; 2 patients were in MR4 [1 of whom was in MR4.5] at baseline), respectively, in the R/I cohort and 56.0% (14 of 25) and 44.0% (11 of 25), respectively, in the ND cohort (Figure 2).
Cumulative molecular response rates. Cumulative incidence of (A) MMR, (B) MR4, and (C) MR4.5 as per the cohort. In the R/I cohort at baseline, 7 patients were already in MMR, 2 patients were already in MR4, and 1 patient was already in MR4.5; hence, the K-M curves start at 21%, 6%, and 3% from day 0, respectively.
Cumulative molecular response rates. Cumulative incidence of (A) MMR, (B) MR4, and (C) MR4.5 as per the cohort. In the R/I cohort at baseline, 7 patients were already in MMR, 2 patients were already in MR4, and 1 patient was already in MR4.5; hence, the K-M curves start at 21%, 6%, and 3% from day 0, respectively.
At baseline, 21 (63.6%) and 14 (42.4%) R/I cohort patients were in MCyR and CCyR, respectively. Twenty-eight patients (84.8%) were in MCyR by cycle 36, and 27 patients (81.8%) were in CCyR by cycle 48; rates remained the same until study conclusion. One R/I cohort patient progressed to BC after 10.1 months on treatment,21 after which no additional events of loss of CHR or CCyR, progression to AP/BC, or on-treatment death occurred. The K-M estimated rate of EFS was 96.3% (95% CI, 76.5-99.5) at 12 months and remained unchanged until study completion.
In the ND cohort, 21 patients (84.0%) were in CCyR by cycle 12 and until study completion; median time to first CCyR was 5.6 months (95% CI, 5.5-5.6). In the ND cohort, 1 patient had a confirmed loss of CCyR; 1 patient had confirmed loss of MCyR; and 1 patient temporarily matched the technical definition criteria for progression to AP/BC (ie, the progression was not considered to be clinically notable based on clinical review because of temporary interruption of nilotinib treatment for 13 days during the first 28-day cycle owing to a prolonged QT event) solely on account of increased basophil cell count, 1 month after the start of nilotinib, but the patient continued treatment and was in CCyR by cycle 6. This patient discontinued nilotinib treatment because of hyperbilirubinemia after 13.8 months on treatment without losing CCyR. The K-M estimated rates of EFS at 12 months was 91.2% (95% CI, 69.0-97.7) for the ND cohort and remained unchanged until study completion.
While on treatment, no patients had emerging BCR::ABL1 mutations. The median time to disease progression was not reached in either of the cohorts. The K-M estimated rates of disease progression on nilotinib at 12 months for the R/I and ND cohorts were 3.7% (95% CI, 0.5-23.5) and 8.4% (95% CI, 2.2-29.6), respectively, and remained so until the study completion. The median OS was not estimable. The K-M estimated rate of OS at 24 months was 96.9% (95% CI, 79.8-99.6) for patients in the R/I cohort and remained unchanged until study completion. The K-M estimated rate of OS for patients in the ND cohort up to 24 months was 100% (95% CI, 100-100), 95.5% (95% CI, 71.9-99.3) at 48 months, and 81.6% (95% CI, 50.4-94.2) at 66 months.
Safety
All patients in both cohorts experienced at least 1 AE (any grade). Grade 3/4 AEs were observed in 20 of 33 (60.6%) and 18 of 25 (72.0%) patients in the R/I and ND cohorts, respectively (Table 2). The most common AEs were increased blood bilirubin levels (R/I cohort: 48.5% [16 of 33]; ND cohort: 64.0% [16 of 25]) and headache (R/I cohort: 39.4% [13 of 33]; ND cohort: 60.0% [15 of 25]; supplemental Table 1). The most frequently reported (≥5%) grade 3/4 nonhematologic AEs were increased blood bilirubin level (R/I cohort: 9.1%; ND cohort: 16.0%), increased alanine aminotransferase level (ALT; R/I cohort: 12.1%; ND cohort: 12.0%), and rash (R/I cohort: 9.1%; ND cohort: 8.0%; supplemental Table 1). The most common grade 3/4 hematologic AEs reported in ≥5% of patients included anemia (6.1%) in the R/I cohort and decreased neutrophil count (12.0%), decreased platelet count (8.0%), and neutropenia (8.0%) in the ND cohort.
Safety summary
| AE, n (%) . | R/I cohort n = 33 . | ND cohort n = 25 . | All patients N = 58 . | |||
|---|---|---|---|---|---|---|
| All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | |
| Any AE | 33 (100) | 20 (60.6) | 25 (100) | 18 (72.0) | 58 (100) | 38 (65.5) |
| AEs suspected to be treatment related | 29 (87.9) | 16 (48.5) | 24 (96.0) | 17 (68.0) | 53 (91.4) | 33 (56.9) |
| AEs leading to discontinuation | 6 (18.2) | 2 (6.1) | 8 (32.0) | 4 (16.0) | 14 (24.1) | 6 (10.3) |
| AEs leading to dose adjustment or interruption∗ | 22 (66.7) | 15 (45.5) | 18 (72.0) | 16 (64.0) | 40 (69.0) | 31 (53.4) |
| SAEs | 11 (33.3) | 7 (21.2) | 4 (16.0) | 3 (12.0) | 15 (25.9) | 10 (17.2) |
| SAEs suspected to be treatment related | 1 (3.0)† | 0 | 2 (8.0)‡ | 1 (4.0) | 3 (5.2) | 1 (1.7) |
| All deaths§ | 1 (3.0)‖ | — | 3 (12.0)¶ | — | 4 (6.9) | — |
| AE, n (%) . | R/I cohort n = 33 . | ND cohort n = 25 . | All patients N = 58 . | |||
|---|---|---|---|---|---|---|
| All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | |
| Any AE | 33 (100) | 20 (60.6) | 25 (100) | 18 (72.0) | 58 (100) | 38 (65.5) |
| AEs suspected to be treatment related | 29 (87.9) | 16 (48.5) | 24 (96.0) | 17 (68.0) | 53 (91.4) | 33 (56.9) |
| AEs leading to discontinuation | 6 (18.2) | 2 (6.1) | 8 (32.0) | 4 (16.0) | 14 (24.1) | 6 (10.3) |
| AEs leading to dose adjustment or interruption∗ | 22 (66.7) | 15 (45.5) | 18 (72.0) | 16 (64.0) | 40 (69.0) | 31 (53.4) |
| SAEs | 11 (33.3) | 7 (21.2) | 4 (16.0) | 3 (12.0) | 15 (25.9) | 10 (17.2) |
| SAEs suspected to be treatment related | 1 (3.0)† | 0 | 2 (8.0)‡ | 1 (4.0) | 3 (5.2) | 1 (1.7) |
| All deaths§ | 1 (3.0)‖ | — | 3 (12.0)¶ | — | 4 (6.9) | — |
A patient with multiple reasons for dose reduction or interruption is only counted once.
One patient had a SAE of grade 1 growth hormone deficiency.
One patient had diarrhea, abdominal pain, and rash (all grade 1), and 1 patient had grade 1 QT prolongation and grade 3 hyperbilirubinemia.
Includes deaths that occurred during the treatment and survival follow-up periods; no deaths occurred during nilotinib treatment.
Reason for death was CML, which occurred during the follow-up period.
Reasons for death were CML, respiratory failure, and after transplantation of lymphoproliferative disorder (1 patient each), all of which occurred during the follow-up period.
AEs suspected to be related to study drug occurred in 29 of 33 (87.9%) and 24 of 25 (96.0%) patients in the R/I and ND cohorts, respectively. The most frequently reported AEs suspected to be treatment related were increased blood bilirubin (48.5% [9.1% were grade 3/4] and 60.0% [16.0% were grade 3/4] in the R/I and ND cohorts, respectively) and increased ALT levels (30.3% [12.1% were grade 3/4] and 40.0% [12.0% were grade 3/4] in the R/I and ND cohorts, respectively; supplemental Table 2).
SAEs were reported in 11 R/I cohort patients (33.3%) and 4 ND cohort patients (16.0%). One R/I cohort patient (grade 1 growth hormone deficiency) and 2 ND cohort patients (1 patient experienced diarrhea, abdominal pain, and rash [all grade 1], and 1 patient who experienced QT prolongation [grade 1] and hyperbilirubinemia [grade 3]) had SAEs suspected to be study drug related.
AESI are presented in Table 3, and Medical Dictionary for Regulatory Activities preferred terms reported in ≥1 patient within each group term are shown in supplemental Table 3. Details of AESIs that occurred up to 48 cycles have previously been reported, including 3 growth-related AEs.23 After the 48-cycle analysis, 3 additional ND cohort patients reported AESIs of increased blood cholesterol, increased blood glucose, and fluid retention (1 patient each).
AESIs
| AE, n (%) . | R/I cohort n = 33 . | ND cohort n = 25 . | All patients N = 58 . | |||
|---|---|---|---|---|---|---|
| All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | |
| Increased blood cholesterol | 3 (9.1) | 0 | 3 (12.0) | 1 (4.0) | 6 (10.3) | 1 (1.7) |
| Increased blood glucose | 2 (6.1) | 0 | 1 (4.0) | 0 | 3 (5.2) | 0 |
| Fluid retention | 2 (6.1) | 0 | 5 (20.0) | 1 (4.0) | 7 (12.1) | 1 (1.7) |
| Edema and other fluid retention | 2 (6.1) | 0 | 5 (20.0) | 1 (4.0) | 7 (12.1) | 1 (1.7) |
| Growth retardation | 2 (6.1) | 0 | 1 (4.0) | 0 | 3 (5.2) | 0 |
| Hepatotoxicity | 19 (57.6) | 9 (27.3) | 17 (68.0) | 7 (28.0) | 36 (62.1) | 16 (27.6) |
| Drug-induced liver injury | 1 (3.0) | 1 (3.0) | 0 | 0 | 1 (1.7) | 1 (1.7) |
| Hepatic transamine and bilirubin level elevations | 19 (57.6) | 8 (24.2) | 17 (68.0) | 7 (28.0) | 36 (62.1) | 15 (25.9) |
| Myelosuppression (thrombocytopenia) | 1 (3.0) | 0 | 8 (32.0) | 3 (12.0) | 9 (15.5) | 3 (5.2) |
| QT prolongation∗ | 5 (15.2) | 0 | 3 (12.0) | 0 | 8 (13.8) | 0 |
| Rash | 16 (48.5) | 5 (15.2) | 15 (60.0) | 3 (12.0) | 31 (53.4) | 8 (13.8) |
| AE, n (%) . | R/I cohort n = 33 . | ND cohort n = 25 . | All patients N = 58 . | |||
|---|---|---|---|---|---|---|
| All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | |
| Increased blood cholesterol | 3 (9.1) | 0 | 3 (12.0) | 1 (4.0) | 6 (10.3) | 1 (1.7) |
| Increased blood glucose | 2 (6.1) | 0 | 1 (4.0) | 0 | 3 (5.2) | 0 |
| Fluid retention | 2 (6.1) | 0 | 5 (20.0) | 1 (4.0) | 7 (12.1) | 1 (1.7) |
| Edema and other fluid retention | 2 (6.1) | 0 | 5 (20.0) | 1 (4.0) | 7 (12.1) | 1 (1.7) |
| Growth retardation | 2 (6.1) | 0 | 1 (4.0) | 0 | 3 (5.2) | 0 |
| Hepatotoxicity | 19 (57.6) | 9 (27.3) | 17 (68.0) | 7 (28.0) | 36 (62.1) | 16 (27.6) |
| Drug-induced liver injury | 1 (3.0) | 1 (3.0) | 0 | 0 | 1 (1.7) | 1 (1.7) |
| Hepatic transamine and bilirubin level elevations | 19 (57.6) | 8 (24.2) | 17 (68.0) | 7 (28.0) | 36 (62.1) | 15 (25.9) |
| Myelosuppression (thrombocytopenia) | 1 (3.0) | 0 | 8 (32.0) | 3 (12.0) | 9 (15.5) | 3 (5.2) |
| QT prolongation∗ | 5 (15.2) | 0 | 3 (12.0) | 0 | 8 (13.8) | 0 |
| Rash | 16 (48.5) | 5 (15.2) | 15 (60.0) | 3 (12.0) | 31 (53.4) | 8 (13.8) |
A patient with multiple severity grades for an AE was counted only under the maximum grade. No events were reported for the following AESI group terms: cardiovascular event (including ischemic cerebrovascular events, ischemic heart disease, peripheral arterial occlusive disease, and other cardiovascular events); cardiac failure; pancreatitis, lipase, and amylase elevations; renal events; or significant bleeding (including central nervous system or gastrointestinal hemorrhage).
Medical Dictionary for Regulatory Activities preferred terms included syncope for 1 patient and QT prolonged for 7 patients (reported in Hijiya et al21).
No patient died during the study while on treatment. Four patients died during the survival follow-up phase of the study. One R/I cohort patient discontinued the study because of progression to BC and subsequently died of lymphoid BC 8 months after the end of nilotinib treatment. Three ND cohort patients died; causes of deaths were CML, respiratory failure, and posttransplantation lymphoproliferative disorder (1 patient each); all deaths occurred ≥1 year after the discontinuation of nilotinib, and all had subsequently received other antineoplastic therapy.
Nilotinib dose reductions and discontinuations
Twenty-one R/I cohort patients (63.6%) and 16 ND cohort patients (64.0%) required ≥1 dose reduction; and 21 R/I cohort patients (63.6%) and 20 ND cohort patients (80.0%) required ≥1 dose interruption. Twenty-two R/I cohort patients (66.7%) and 18 ND cohort patients (72.0%) required a dose reduction or interruption because of an AE. The most frequent (≥10% of patients) AEs requiring dose adjustment or interruption were increased blood bilirubin level (24.2%), increased ALT level (12.1%), and rash (12.1%) in the R/I cohort and increased blood bilirubin (44.0%), aspartate aminotransferase (16%), and ALT levels (16%) and vomiting (12.0%) in the ND cohort.
AEs led to treatment discontinuation in 6 R/I cohort patients (18.2%), 2 of whom had grade 3/4 AEs. Since the 48-cycle analysis, 1 additional patient in the R/I cohort had a treatment-related AE that led to study discontinuation (grade 1 bone pain). AEs led to treatment discontinuation in 8 ND cohort patients (32.0%; 4 had grade 3/4 treatment-related AEs); all occurred before cycle 48. Increased blood bilirubin was the most common AE associated with discontinuation (supplemental Table 4).
Effect on growth and development
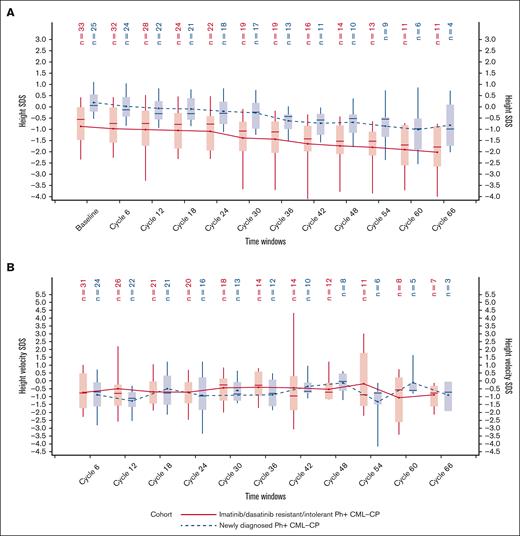
In both cohorts, there was a negative slope of height SDS, indicating a deceleration of growth over time compared with baseline (Figure 3A); the same trend was observed in the prepubescent/pubescent subpopulation (supplemental Figure 1). At baseline, R/I cohort patients were relatively shorter than ND cohort patients, and this remained so throughout the study period. Median height SDSs at baseline and at 66 cycles were −0.56 (range, −4.3 to 1.2) and −1.8 (range, −5.2 to 0.2), respectively, in the R/I cohort; and 0.06 (range, −0.9 to 1.7) and −1.0 (range, −2.0 to 0.7), respectively, in the ND cohort. Overall median change from baseline in height SDS at 66 cycles was −0.7 SDS (range, −2.0 to 0.4) and −0.7 SDS (range, −1.7 to −0.6) in the R/I and ND cohorts, respectively. The estimated average loss of height SDS for each subsequent visit was 6% (6% of height SDS per 6 months) for patients in the R/I cohort and 9% for patients in the ND cohort (P < .0001 for both); corresponding values for the prepubescent/pubescent subpopulation were 7% and 9%, respectively. Height velocity SDS was relatively constant until end of treatment (cycle 66), and the median was always below the 50th percentile of the reference population (SDS of 0) for both cohorts (Figure 3B); the same trend was observed for the prepubescent/pubescent subpopulation (supplemental Figure 1).
Height SDS over time by cohort. Height SDS (A) and height velocity SDS (B) with respect to time as per the cohort (FAS). Plot shows boxes (25th to 75th percentiles) with median as horizontal line. The dots in the boxes and joining lines represent mean values. Whiskers extend to 10th and 90th percentiles. Values outside this range are not displayed.
Height SDS over time by cohort. Height SDS (A) and height velocity SDS (B) with respect to time as per the cohort (FAS). Plot shows boxes (25th to 75th percentiles) with median as horizontal line. The dots in the boxes and joining lines represent mean values. Whiskers extend to 10th and 90th percentiles. Values outside this range are not displayed.
Eight prepubescent/pubescent patients had a height SDS drop that crossed ≥2 main percentile lines (supplemental Table 5). Three patients had growth deceleration AEs suspected to be related to nilotinib exposure, all of which were reported at the cycle 48 analysis.23 Two patients with height decreases of ≥1 main percentile lines experienced AEs, which were ongoing at the last assessment date; 1 ND cohort patient had a grade 2 AE of growth retardation, and 1 R/I cohort patient had grade 1 AEs of growth hormone deficiency and body height below normal. One R/I cohort patient who did not have a height decrease that crossed a main percentile line had an AE of grade 1 growth retardation; height SDS had improved by the end of the study compared with baseline.
In a model-based analysis, changes in height were evaluated considering the covariates of age, pubertal status (as a baseline and time-dependent variable), and sex (supplemental Table 6). In both cohorts, the estimated average loss of height SDS was statistically significantly higher in younger patients (aged <12 years vs ≥12 years). There was no difference in the estimated average loss of height SDS as per the baseline pubertal status (prepubertal vs postpubertal), but as a time-dependent variable, the loss of height SDS was higher during the prepubertal vs postpubertal period in the ND cohort but not in the R/I cohort. This finding should be interpreted with caution, considering that only 4 patients in the ND cohort were prepubertal at baseline. The estimated average loss of height SDS was statistically significantly higher among female patients of the R/I cohort but not of the ND cohort.
No clear trend was observed for body mass index SDS (supplemental Figure 2), bone age SDS (supplemental Table 7), bone density (supplemental Figure 3), or thyroid function. Among patients at risk of delayed puberty at treatment initiation, no patients experienced delayed puberty.
Discussion
The final analysis of the DIALOG study demonstrated long-term (up to 66 cycles) efficacy and safety of nilotinib in pediatric patients with R/I or ND Ph+ CML-CP. The clinical activity and safety of nilotinib 230 mg/m2 twice daily for pediatric patients with R/I or ND Ph+ CML-CP has previously been reported up to cycle 24,21 and further safety data were reported after ≥36 and ≥48 cycles.22,23 To our knowledge, this final analysis is the longest follow-up of pediatric patients with CML-CP receiving treatment with a TKI.
Previous efficacy analyses reported that MMR was achieved by 39.4% of R/I patients at cycle 6 (R/I cohort primary end point) and the cumulative MMR rate by cycle 24 was 57.6%; among patients who were ND, the cumulative MMR rates were 64.0% and 68.0% by cycles 12 (ND cohort primary end point) and 24, respectively.21 In this final analysis with a median treatment duration of ∼5 years, the MMR rate was sustained with best MMR rates of 60.6% and 76.0% in the R/I and ND cohorts, respectively; no patients in the R/I cohort, and only 3 patients in the ND cohort lost MMR during the study, confirming the sustained efficacy of nilotinib in these patient populations. In addition, by study conclusion, MR4 and MR4.5 had been achieved by 27.3% and 12.1% of patients, respectively, in the R/I cohort, and 56.0% and 44.0% of patients, respectively, in the ND cohorts. Furthermore, secondary end points of MCyR, CCyR, and EFS remained consistent from the previous analysis,21 supporting the sustained efficacious benefit of nilotinib. Moreover, the efficacy of nilotinib observed in pediatric patients with ND CML-CP after ∼5 years of treatment is similar to what has been observed in adult patients.30
The outcomes of the DIALOG study for patients receiving first-line nilotinib are comparable with those of pediatric patients receiving first-line imatinib and dasatinib. The ND cohort in this study had a CCyR rate of 84% by month 12 and an MMR rate of 76% by month 36; both rates were maintained until the end of the study. CCyR and MMR rates at 12 months in a French National phase 4 trial of imatinib-treated pediatric patients with ND CML-CP were 61% and 31%, respectively, with an estimated MMR rate at 36 months of 71%.16 Between 25% and 29% of pediatric patients with CML treated with imatinib will discontinue therapy because of a poor response or toxicity,16,31 and current alternative TKI therapy for these patients include dasatinib and nilotinib. In a phase 2, open-label, nonrandomized prospective trial of pediatric patients receiving dasatinib, CCyR and MMR rates by 12 months in the ND cohort were 92% and 52%, respectively.32 In the R/I cohort of the DIALOG study, the cumulative MMR rate by 12 months was 57.6%, and 60.6% of patients were in MMR at least once during the study. These rates are comparable with those observed in a phase 2 prospective trial evaluating dasatinib treatment in pediatric patients, in which 41% and 55% of patients with imatinib resistant/intolerant CML treated with dasatinib achieved MMR by 12 and 24 months, respectively.32 Compared with previous studies of TKI treatment for pediatric patients with CML-CP, the DIALOG data presented are from a longer observation period; it is important to note that there were no patients with emergent mutations.
In this final analysis, the safety profile of nilotinib was consistent with those in previous reports.21,23 No new safety signals were reported. Adult patients treated with nilotinib have a higher risk of elevated glucose and cardiovascular events compared with imatinib-treated patients.33 In this pediatric study, no cardiovascular events were observed and no grade 3/4 events of increased blood glucose were reported. The DIALOG study protocol included a glucose/hemoglobin A1c increase and cardiovascular events risk management plan while the study was ongoing; however, no risk management plan for after study completion was provided. As noted in the previous safety analysis,23 AESIs of elevated bilirubin and liver enzymes were more common among pediatric patients compared with adults. Overall, in the DIALOG study, 14 patients discontinued treatment because of an AE, 11 of whom had discontinued by 24-cycles21 and 3 patients discontinued after, suggesting that the majority of AEs leading to treatment discontinuation appear early during treatment with nilotinib.
Previously, a growth deceleration trend was noted in the DIALOG study,22,23 and although the proportion of patients with reported growth retardation AEs was low, data continue to show a negative slope in height SDS for both cohorts. Similar studies of pediatric patients treated with imatinib or dasatinib support this observation.18,20 Earlier pediatric studies of patients with CML treated with a TKI have reported the impact of pubertal age on growth impairment with conflicting results.17-19 In a subpopulation analysis of patients considered to be at increased risk of growth impact (prepubescent or pubescent patients) in the DIALOG study, height velocity SDS were relatively constant until the end of the study, and 8 patients experienced a decrease in growth of 2 main percentile lines. As noted previously, growth deceleration might have already occurred in R/I cohort patients before enrollment in the study, and a high proportion of ND cohort patients were pubertal.23
In conclusion, this final analysis of the DIALOG study of nilotinib treatment of up to 66 cycles demonstrated sustained long-term efficacy in pediatric patients with CML-CP in both the R/I and ND patient cohorts. The safety profile in both cohorts was consistent with previously reported safety results for nilotinib in pediatric patients. The trend toward growth deceleration, which was first observed during the 36-cycle analysis22 and confirmed at the 48-cycle analysis,23 is further supported by this long-term analysis. The overall benefit-risk assessment of nilotinib use for pediatric patients with ND Ph+ CML-CP or with Ph+ CML-CP R/I to either imatinib or dasatinib remains favorable, and nilotinib is an additional valuable treatment option for pediatric patients with CML-CP.
Acknowledgments
The authors thank the patients and families who participated in the trial and the investigators involved. The authors thank Helen Findlow, of Novartis Pharmaceuticals UK Ltd, London, United Kingdom, for providing medical writing support, which was funded by Novartis Pharmaceuticals Corporation in accordance with the Good Publication Practice (2022) guidelines.
This study was sponsored and funded by Novartis Pharmaceuticals Corporation.
Authorship
Contribution: N.H. designed the study, and all authors contributed to data acquisition and interpretation, writing the manuscript, and review and approval of the final manuscript.
Conflict-of-interest disclosure: N.H. has received research funding from Novartis and Pfizer; served on the data monitoring committee for Incyte and Novartis; participated on an advisory board for Stemline Therapeutics; and has been a consultant for Novartis. A.M. reports receiving lecture fees from Novartis. H.G. reports receiving lecture fees from Novartis. Z.K. reports receiving grants from Novartis. C.M.Z. reports serving as a consultant to Novartis and has obtained institutional funding from Bristol Myers Squibb and Pfizer for the development of other CML therapies. J.S. reports serving as a consultant to Novartis, Bristol Myers Squibb, and Tolmar Pharmaceuticals, and has received speaker fees from Tolmar Pharmaceuticals. M.I. and S.L. report employment at and ownership of stock in Novartis. K.T. reports employment at Novartis. The remaining authors declare no competing financial interests.
Correspondence: Nobuko Hijiya, Division of Pediatric Hematology/Oncology/Stem Cell Transplantation, Columbia University Irving Medical Center, 161 Fort Washington Ave, HIP7, New York, NY 10032; e-mail: nh2636@cumc.columbia.edu.
References
Author notes
Novartis is committed to sharing with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. Trial data availability is according to the criteria and process described in the data request platform at www.clinicalstudydatarequest.com.
The full-text version of this article contains a data supplement.