Key Points
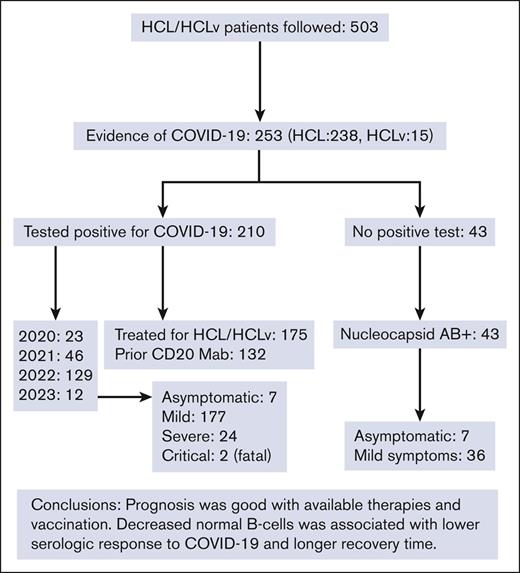
During the COVID-19 pandemic, 253 of 503 patients with HCL or the high-risk variant HCLv had evidence of COVID-19.
The level of normal B cells was associated with the level of COVID-19 antibody production and time to recovery.
Abstract
Hairy cell leukemia (HCL), similar to its variant HCLv, is a B-cell malignancy associated with decreased humoral immunity. We prospectively monitored the largest cohort of patients with HCL/HCLv to date (n = 503) for COVID-19 by symptoms, antibody, and polymerase chain reaction (PCR) and/or antigen positivity. Fifty percent (253 of 503) of the patients with HCL/HCLv (238 HCL and 15 HCLv) had evidence of COVID-19, with 210 (83%) testing positive by PCR or rapid-antigen test. Of the 43 patients without positive tests, all had nucleocapsid antibodies indicating COVID-19 exposure, 7 recalled no symptoms, and 36 had mild symptoms. Of the 210 who tested positive, 23, 46, 129, and 12 cases occurred in 2020, 2021, 2022, and 2023, respectively. Among them, 175 began treatment for HCL/HCLv 0.4 to 429 (median, 66) months before, and 132 had their last dose of anti-CD20 monoclonal antibody 0.2 to 229 (median, 63) months before. Two patients died, including a young woman who began rituximab 2 months after first-line cladribine before vaccine availability. Nearly all patients with HCL/HCLv recovered uneventfully from COVID-19 including those without vaccination or those with significant immunosuppression and recent treatment. However, decreased normal B cells from HCL or treatment was associated with lower spike antibody levels as a response to COVID-19 (P = .0094) and longer recovery time (P = .0036). Thus, in a large cohort of patients with HCL/HCLv and in the first to determine relationships between COVID-19 outcome and immune markers, mortality was relatively low (∼1%), sequelae were uncommon, and recovery from COVID-19 was longer if normal B cells were low after recent treatment. The trials are registered at www.clinicaltrials.gov as #NCT01087333 and #NCT04362865.
Introduction
The COVID-19 pandemic has greatly diminished with respect to incidence and death rates in the past 1 to 2 years, but to a significant extent, it remains an issue in the management of patients with hairy cell leukemia (HCL). This disease is an indolent B-cell malignancy, constituting 2% of all leukemias, which is highly responsive to treatment, but both disease burden and therapy can adversely affect humoral immunity and potentially worsen infections.1-3 A variant of HCL, HCLv, is more aggressive, responds more poorly to treatment,4,5 and lacks the BRAF V600E mutation.6 Two early single-patient case reports of COVID-19 in HCL included a patient with suspected relapse7 and 1 with newly diagnosed HCL,8 both of whom required intubation for severe COVID-19 pneumonia. More recently reported cases included 2 patients diagnosed with HCL after treatment of COVID-19 with remdesivir; of whom, 1 also received dexamethasone9 and the other received levofloxacin and was diagnosed with HCL 4 months later after presenting with Pneumocystis jirovecii.10 Pagano et al included 23 HCL cases in a study of 3801 cases of hematologic malignancies.11 Lamure et al reported 40 patients with HCL and COVID-19 from a database of 2422 lymphoproliferative neoplasms associated with COVID-19 infection, including 7 patients with HCL diagnosed <1 month before COVID-19, 5 after first-line therapy, 11 after achieving remission, 15 with untreated HCL, 1 with refractory HCL, and 1 with unknown status.12 Of the 40 patients, 9 died of COVID-19 and 1 died of unknown cause. Tiacci et al reported 48 patients with HCL infected with COVID-19, including 37 unvaccinated and 11 testing positive after vaccination.13 Five (14%) of the 37 unvaccinated patients died, with advanced age, comorbidities, and active disease under treatment being risk factors. We followed, to our knowledge, the largest HCL/HCLv cohort to date (n = 503) during the COVID-19 pandemic and studied a total of 251 of these patients with evidence of COVID-19 infection.
Patients and methods
Patients
Patients with HCL and HCLv were followed on sample collection protocols for HCL/HCLv in general (#NCT01087333) and/or for patients with COVID-19 (#NCT04362865) with or without hematologic malignancies. All patients signed consents on these protocols that were approved by the National Cancer Institute institutional review board. Patients were usually from the United States and had samples sent in by Fedex. A minority of patients were international including Canada and Europe and required international shipping. Many of the patients were on HCL treatment protocols at National Institutes of Health (NIH), and samples were obtained during patient visits or sent via Fedex from outside the area when they were not at NIH. Other patients who were not on NIH treatment protocols wanted to send in samples to characterize their HCL cells and consented to both the HCL/HCLv and COVID protocols. A minority of patients were from the local area and were drawn at NIH for both protocols. To ensure safety, patients sending samples were sent empty tubes with appropriate containers, with directions to draw a complete blood count–differential at home at the time the tubes were filled. A complete blood count–differential could be run at NIH for those patients without one at home. Assessment dates after infection ranged between September 2020 and April 2023. For patients on NIH treatment protocols, assessments were usually made every 12 weeks, and for many patients who alerted us to their acute COVID-19 infection, samples were often sent 3 to 4 weeks after symptom onset or test positivity.
Assessments
Patients were assessed by (1) quantifying HCL/HCLv cells in the peripheral blood by flow cytometry; (2) quantifying lymphocyte subsets including natural killer cells; (3) measuring antibodies to spike and nucleocapsid regions of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); and (4) quantifying immunoglobulin G (IgG), IgA, and IgM levels. Flow cytometry involved detailed characterization and quantitation of HCL/HCLv cells by CD19, CD22, CD20, CD11c, CD25, CD45, CD103, CD11c, CD123, CD200, and other antigens as described.14 Patients with non-HCL/HCLv populations including chronic lymphocytic leukemia (CLL) and monoclonal B-cell lymphocytosis required the quantitation of those population to determine the number of normal B cells. Two tests were used for measuring spike (S) and nucleocapsid (N) antibodies. At the beginning of the study, the luciferase immunoprecipitation system was used for measuring antibodies against the SARS-CoV-2 spike and nucleocapsid proteins as described.15 This assay was used to quantify N and S antibodies from April 2020 to February 2022. The Elecsys Anti-SARS-CoV-2 S and Elecsys Anti-SARS-CoV-2 double-sandwich enzyme-linked immunosorbent assays were subsequently used to quantify S and detect N antibodies, respectively.16 The Elecsys assays were used to detect N antibodies beginning in December 2020, S antibodies in February 2021, and to quantify S antibodies beginning in July 2021. Statistics used the Wilcoxon rank-order test (Mann-Whitney) as determined by GraphPad Prism version 9.3, and P values were 2-sided.
Results
Patient characteristics
The characteristics shown in Table 1 include 253 patients with HCL/HCLv, of 503 followed, who had evidence of COVID-19. Most of the patients (n = 238) had classic HCL based on phenotype (CD25+ and CD123+), whereas 15 had the variant HCLv (CD25– and CD123–). The male to female ratio was 4:1, as expected for HCL/HCLv. In 210 of the 253 patients, evidence of COVID-19 infection included a positive polymerase chain reaction (PCR) or rapid-antigen test, whereas the other 43 patients lacked either of those tests but had a positive nucleocapsid antibody. Of these 43 patients, 36 had symptoms of COVID-19, whereas 7 were asymptomatic. Most of the patients testing positive (129 of 210) were diagnosed in 2022, well after vaccines became available. Most of the patients who tested positive (175 of 210) had prior treatment, with the most common types of treatments being cladribine and rituximab combination (45 of 175 treated patients). Of the 175 patients testing positive with prior treatment, 132 had some form of monoclonal antibody (mAb) to CD20, usually rituximab. In these 132 patients, the last dose of CD20 mAb was 0.2 to 229 months (median, 62.7) before testing positive. Of the 153 patients whose response status to last HCL/HCLv treatment was known at the time they tested positive, 123 were in complete remission (CR), 18 were in at least partial response, 10 had stable disease, and 2 had progression. Of the 153 in CR, 41 had detectable minimal residual disease (MRD), 79 did not, and MRD was not evaluated in 33. We did not see an obvious effect of COVID-19 on response in HCL/HCLv. With respect to vaccination status in the 253 patients with HCL/HCLv with evidence of COVID-19, 28 were not vaccinated and 225 were vaccinated either with Moderna (n = 77), Pfizer (n = 110), Johnson & Johnson (n = 6), Sinovac (n = 1), or a combination of different types (n = 31). Of the 210 patients with HCL/HCLv who tested positive, 160 were vaccinated 17 to 810 days (median, 452 days) before testing positive, including 56 with Moderna, 77 with Pfizer, 3 with Johnson & Johnson, and 24 with a combination (Table 1).
Patient characteristics
| Patients with HCL/HCLv and evidence of COVID-19 infection | 253 |
| Type of HCL | |
| Classic HCL | 238 |
| HCLv | 15 |
| Demographics | |
| Males | 202 |
| Females | 51 |
| Ages | 28-86 (median, 59) |
| Evidence for COVID-19 infection | |
| Positive COVID-19 test, either rapid-antigen test or PCR | 210 |
| No positive test, but nucleocapsid positive | 43 |
| COVID-19 symptoms | 36 |
| Asymptomatic | 7 |
| Year that patients tested positive for COVID-19 (n = 209) | |
| 2020 | 23 |
| 2021 | 46 |
| 2022 | 129 |
| 2023 | 12 |
| Patients who were untreated before testing positive for COVID-19 | 35 |
| Patients who were treated before testing positive for COVID-19 | 175 |
| Months from starting last treatment to testing positive for COVID-19 | 0.4-429 |
| Median mo from last treatment to testing positive for COVID-19 | 65.7 |
| Last treatment known | 175 |
| Bendamustine | 1 |
| Bendamustine-Rituximab | 5 |
| Binimetinib | 1 |
| BL22 anti-CD22 recombinant immunotoxin | 5 |
| Cladribine | 22 |
| Cladribine followed by rituximab within 6 mo | 11 |
| Cladribine with rituximab beginning the same wk (CDAR) | 45 |
| Dabrafenib-trametinib | 9 |
| Pentostatin followed by rituximab within 6 mo | 1 |
| Pentostatin-rituximab | 9 |
| Encorafenib-binimetinib | 4 |
| Fludarabine | 1 |
| Ibrutinib | 8 |
| Ibrutinib-rituximab | 1 |
| Interferon | 1 |
| Moxetumomab pasudotox | 15 |
| Moxetumomab pasudotox-obinutuzumab | 1 |
| Moxetumomab pasudotox-rituximab | 6 |
| RCHOP | 1 |
| Rituximab | 26 |
| Vemurafenib | 1 |
| Vemurafenib-obinutuzumab | 1 |
| Patients who received CD20 mAb before testing positive for COVID-19 | 132 |
| Months from last dose of CD20 mAb to COVID-19+ | 0.2-229 |
| Median months from last dose CD20 mAb to COVID-19+ | 62.7 |
| Vaccination status known | 253 |
| Not vaccinated | 28 |
| Vaccinated | 225 |
| Moderna | 77 |
| Pfizer | 110 |
| Johnson & Johnson | 6 |
| Sinovac | 1 |
| Combination | 31 |
| Of 210 HCL/HCLv patients who tested positive | |
| Vaccinated prior to testing positive | 160 |
| Days from first vaccination to testing positive: 17-810 (median 452) | |
| Moderna vaccine before testing positive | 56 |
| Pfizer vaccine before testing positive | 77 |
| Johnson & Johnson before testing positive | 3 |
| Combination before testing positive | 24 |
| Received a recognized specific treatment for COVID-19 | 70 |
| Convalescent plasma | 1 |
| Convalescent plasma and remdesivir | 3 |
| Bamlanivimab-etesevimab | 2 |
| Bebtelovimab | 2 |
| Casirivimab-imdevimab | 12 |
| Casirivimab-imdevimab, remdesivir, bebtelovimab | 1 |
| Sotrovimab | 4 |
| Tixagevimab-cilgavimab | 2 |
| Unknown mAb | 1 |
| Molnupiravir | 2 |
| Paxlovid | 39 |
| Remdesivir | 1 |
| No recognized specific treatment for COVID-19 | 140 |
| Disease severity in 210 HCL/HCLv patients testing positive | |
| Asymptomatic | 7 |
| Specific treatment | |
| None | 6 |
| Bamlanivimab-Etesevimab | 1 |
| Mild | 177 |
| Severe | 24 |
| Specific treatment | |
| None | 12 |
| Tixagevimab-cilgavimab | 1 |
| Unknown mAb | 1 |
| Paxlovid | 3 |
| Convalescent plasma | 1 |
| Casirivimab-imdevimab | 3 |
| Casirivimab-imdevimab, remdesivir, bebtelovimab | 1 |
| Convalescent plasma and remdesivir | 2 |
| Critical | 2 |
| Specific treatment: none | 2 |
| Patients with HCL/HCLv and evidence of COVID-19 infection | 253 |
| Type of HCL | |
| Classic HCL | 238 |
| HCLv | 15 |
| Demographics | |
| Males | 202 |
| Females | 51 |
| Ages | 28-86 (median, 59) |
| Evidence for COVID-19 infection | |
| Positive COVID-19 test, either rapid-antigen test or PCR | 210 |
| No positive test, but nucleocapsid positive | 43 |
| COVID-19 symptoms | 36 |
| Asymptomatic | 7 |
| Year that patients tested positive for COVID-19 (n = 209) | |
| 2020 | 23 |
| 2021 | 46 |
| 2022 | 129 |
| 2023 | 12 |
| Patients who were untreated before testing positive for COVID-19 | 35 |
| Patients who were treated before testing positive for COVID-19 | 175 |
| Months from starting last treatment to testing positive for COVID-19 | 0.4-429 |
| Median mo from last treatment to testing positive for COVID-19 | 65.7 |
| Last treatment known | 175 |
| Bendamustine | 1 |
| Bendamustine-Rituximab | 5 |
| Binimetinib | 1 |
| BL22 anti-CD22 recombinant immunotoxin | 5 |
| Cladribine | 22 |
| Cladribine followed by rituximab within 6 mo | 11 |
| Cladribine with rituximab beginning the same wk (CDAR) | 45 |
| Dabrafenib-trametinib | 9 |
| Pentostatin followed by rituximab within 6 mo | 1 |
| Pentostatin-rituximab | 9 |
| Encorafenib-binimetinib | 4 |
| Fludarabine | 1 |
| Ibrutinib | 8 |
| Ibrutinib-rituximab | 1 |
| Interferon | 1 |
| Moxetumomab pasudotox | 15 |
| Moxetumomab pasudotox-obinutuzumab | 1 |
| Moxetumomab pasudotox-rituximab | 6 |
| RCHOP | 1 |
| Rituximab | 26 |
| Vemurafenib | 1 |
| Vemurafenib-obinutuzumab | 1 |
| Patients who received CD20 mAb before testing positive for COVID-19 | 132 |
| Months from last dose of CD20 mAb to COVID-19+ | 0.2-229 |
| Median months from last dose CD20 mAb to COVID-19+ | 62.7 |
| Vaccination status known | 253 |
| Not vaccinated | 28 |
| Vaccinated | 225 |
| Moderna | 77 |
| Pfizer | 110 |
| Johnson & Johnson | 6 |
| Sinovac | 1 |
| Combination | 31 |
| Of 210 HCL/HCLv patients who tested positive | |
| Vaccinated prior to testing positive | 160 |
| Days from first vaccination to testing positive: 17-810 (median 452) | |
| Moderna vaccine before testing positive | 56 |
| Pfizer vaccine before testing positive | 77 |
| Johnson & Johnson before testing positive | 3 |
| Combination before testing positive | 24 |
| Received a recognized specific treatment for COVID-19 | 70 |
| Convalescent plasma | 1 |
| Convalescent plasma and remdesivir | 3 |
| Bamlanivimab-etesevimab | 2 |
| Bebtelovimab | 2 |
| Casirivimab-imdevimab | 12 |
| Casirivimab-imdevimab, remdesivir, bebtelovimab | 1 |
| Sotrovimab | 4 |
| Tixagevimab-cilgavimab | 2 |
| Unknown mAb | 1 |
| Molnupiravir | 2 |
| Paxlovid | 39 |
| Remdesivir | 1 |
| No recognized specific treatment for COVID-19 | 140 |
| Disease severity in 210 HCL/HCLv patients testing positive | |
| Asymptomatic | 7 |
| Specific treatment | |
| None | 6 |
| Bamlanivimab-Etesevimab | 1 |
| Mild | 177 |
| Severe | 24 |
| Specific treatment | |
| None | 12 |
| Tixagevimab-cilgavimab | 1 |
| Unknown mAb | 1 |
| Paxlovid | 3 |
| Convalescent plasma | 1 |
| Casirivimab-imdevimab | 3 |
| Casirivimab-imdevimab, remdesivir, bebtelovimab | 1 |
| Convalescent plasma and remdesivir | 2 |
| Critical | 2 |
| Specific treatment: none | 2 |
CDAR, cladribine with immediate rituximab.
Fatal COVID-19 in HCL
Two of the 253 patients with HCL/HCLv died after infection with COVID-19; of whom, the first was more related to COVID-19 than the second. A woman aged 37 years with newly diagnosed HCL and baseline white blood cell (WBC) count 0.82 × 109/L, absolute neutrophil count (ANC) 0.33 × 109/L, hemoglobin (Hgb) 7.2 g/dL, and platelets (Plt) 50 × 109/L began 5 doses of single-agent cladribine in September 2020. After 2 months, the blood counts improved to WBC 1.02 × 109/L, ANC 0.73 × 109/L, Hgb 9.3 g/dL and Plt 81 × 109/L, and the patient began (outside NIH) 8 weekly doses of rituximab. In 2 months, the counts improved further to WBC 4.02, ANC 354, Hgb 9.2, and Plt 193, but she was admitted 10 days later with COVID-19 pneumonia. Her pulmonary function deteriorated, causing atrial fibrillation, and she spent several months on high flow oxygen and bilevel positive airway pressure. At 3 months after admission (7 months after cladribine), blood counts remained stable with no HCL or normal B cells detected in blood, CD4 count 80/mm3, and IgG 348 mg/dL. Eight months after cladribine was initiated, while still admitted, she required intubation and died of respiratory failure 3 weeks later. The second patient was an 83-year-old-male with classic HCL first diagnosed in 2017, treated with cladribine with 2-year remission, and 8 weekly doses of rituximab were used in 2021, followed by 4 cycles of moxetumomab pasudotox. The patient received 3 doses of Pfizer COVID-19 vaccine in 2021, with the last 1 in October. In November, he received radiation therapy for prostate cancer with baseline WBC 1.12 × 109/L, ANC 0.57 × 109/L, Hgb 8.7 mg/dL, and Plt 42 × 109/L. In February 2022, he presented with fever and fatigue and had a positive PCR test for COVID-19. Vemurafenib was begun to improve cytopenias, and he required admission mainly because of skin toxicities of vemurafenib. His pneumonia required an assisted oxygen concentrator, and he died of pneumonia 1 month after testing positive.
Immune response to COVID-19 in the absence of vaccine
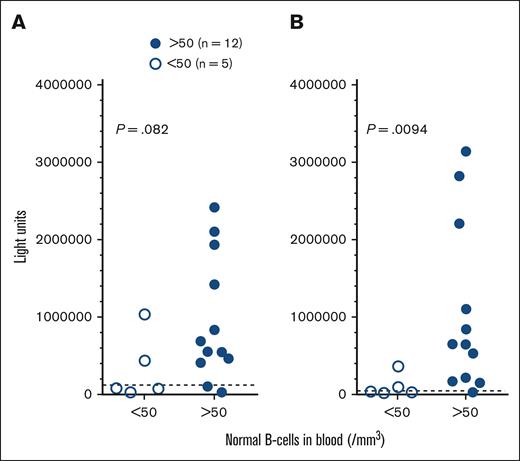
As shown in Table 1, because most of the patients with HCL/HCLv were diagnosed with COVID-19 after the availability of vaccines, only a minority of patients could be assessed for immune response to COVID-19 alone. As shown in Figure 1, 5 patients with normal B cells <50/mm3 had significantly lower S antibodies (P = .0094) and trended to have lower N antibodies (P = .082) than patients with normal B cells >50/mm3. None of these patients were vaccinated before COVID-19 infection. Assessing blood for antibody levels, the patient with lowest antibody levels (nucleocapsid 34276 and spike 29006 LU), despite normal B cells of >50/mm3, had normal B cells 72/mm3, compared with a median of 118 for patients with B cells >50/mm3. There was no significant relationship between CD4 and IgG level and antibody response to COVID-19 (data not shown).
COVID-19 antibodies in infected patients. Levels of nucleocapsid (A) and spike (B) antibodies in HCL/HCLv patients after COVID-19 infection. Comparing those who had <50 (□) vs >50 (●) normal B cells/mm3. Most samples were taken before vaccine availability and no patients received COVID-19 vaccine. The positive value cutoffs (dashed lines) were 125 000 (A) and 45 000 (B) light units.
COVID-19 antibodies in infected patients. Levels of nucleocapsid (A) and spike (B) antibodies in HCL/HCLv patients after COVID-19 infection. Comparing those who had <50 (□) vs >50 (●) normal B cells/mm3. Most samples were taken before vaccine availability and no patients received COVID-19 vaccine. The positive value cutoffs (dashed lines) were 125 000 (A) and 45 000 (B) light units.
Recovery from COVID-19 with respect to normal B cells
To determine whether the level of normal B cells affected the time of recovery from COVID-19, we focused on the 210 patients who tested positive, and compared time from onset of symptoms (or testing positive if asymptomatic) to symptom resolution with respect to normal B-cell level. As shown in Figure 2, there was a significant difference between days for recovery comparing patients with 0 to 25 (n = 45) vs ≥100 (n = 90) normal B cells/mm3 (P = .0036), but there was a significant overlap and very wide variation within each group. The median days to recovery for these 2 groups were quite close (15 vs 10 days). In each group, there was a mixture of patients with and without prior immunization, probably accounting for the variability, and there were insufficient patients for multivariable analysis.
Time to recovery from COVID-19. Defined as time from beginning to end of symptoms, comparing patients with HCL/HCLv with normal B cells 0-25/mm3 (n = 45, □) vs ≥100 cells/mm3 (n = 90, ●).
Time to recovery from COVID-19. Defined as time from beginning to end of symptoms, comparing patients with HCL/HCLv with normal B cells 0-25/mm3 (n = 45, □) vs ≥100 cells/mm3 (n = 90, ●).
Normal B-cell level with respect to last dose of CD20 mAb
To determine the time course of normal B-cell recovery in patients with HCL/HCLv exposed to COVID-19 after anti-CD20 mAb, we measured normal B cells and found significant differences at <1 year (n = 17), 1 to 1.5 years (n = 11), and >1.5 years (n = 131) after the last dose of anti-CD20 mAb, with P <.0001 comparing the first 2 groups and P = .0024 between the last 2 groups (Figure 3). The median normal B-cell counts were 0, 64, and 162 cells/mm3, respectively. Normal B-cell counts were all zero from 2.3 to 10.7 months after the last dose of anti-CD20 mAb, and in the first group, 1 patient had 37 normal B cells/mm3 at 11.6 months. Thus, in patients with HCL, similar to those with other B-cell malignancies, normal B cells usually remain undetectable longer than 6 months after the last dose of anti-CD20 mAb, begin to recover 1 to 1.5 years after anti-CD20 mAb, and normalize only after 1.5 years. Moreover, there is considerable variability after 1.5 years, with 20 patients having <50 normal B cells/mm3 at 23 to 176 months (median, 81 months) after the last dose of anti-CD20 mAb. Thus, normal B cells recover slowly after anti-CD20 mAb in patients who were infected with COVID-19, with some patients remaining with impaired humoral immunity for many years. Nevertheless, the outcomes in these patients after COVID-19 infection remained generally favorable.
Time course of normal B-cell recovery after last anti-CD20 Mab in patients testing positive for COVID-19. Comparing <1 year (n = 17, □), 1-1.5 years (n = 11, ●), and >1.5 years (n = 131, Δ).
Time course of normal B-cell recovery after last anti-CD20 Mab in patients testing positive for COVID-19. Comparing <1 year (n = 17, □), 1-1.5 years (n = 11, ●), and >1.5 years (n = 131, Δ).
Recovery from COVID-19 with respect to vaccination status
To determine whether vaccination against COVID-19 was helpful before infection, we compared time of recovery in patients with or without vaccination before testing positive for COVID-19. As shown in Figure 4, there was significant variability in time of recovery of COVID-19, with a trend (P = .088) toward shorter recovery in patients who were vaccinated (median 9.0 days) vs not vaccinated (median 14.5 days). Patients were considered vaccinated if they had received at least 1 dose of vaccine. As shown in Table 1, most of these patients were infected with COVID-19 in 2022 and therefore had access to COVID-19 vaccines for >1 year. As mentioned above and as listed in Table 1, 160 patients had been vaccinated before testing positive. Of these 160 patients at that time, 5 had just 1, 35 had 2, 61 had 3, 47 had 4, 12 had 5, and 1 had >5 vaccine doses before testing positive for COVID-19. Figure 4 shows that even patients with no prior vaccination usually managed COVID-19 well but that there may have been some benefit for vaccination.
Time to recovery from COVID-19 vs vaccination status. Comparing patients who were vaccinated (n = 157, □) vs unvaccinated (n = 52, ●).
Time to recovery from COVID-19 vs vaccination status. Comparing patients who were vaccinated (n = 157, □) vs unvaccinated (n = 52, ●).
Treatment for COVID-19
As shown in Table 1, of the 210 patients who tested positive, 70 received specific treatment for COVID-19, including 1 with convalescent plasma, 3 with convalescent plasma and remdesivir, 2 with bamlanivimab-etesevimab, 2 with bebtelovimab, 12 with casirivimab-imdevimab, 1 with casirivimab-imdevimab, remdesivir, and bebtelovimab, 4 with sotrovimab, 2 with tixagevimab-cilgavimab, 1 with an unknown mAb, 2 with molnupiravir, 39 with paxlovid, and 1 of with remdesivir. The remaining 140 patients who tested positive for COVID-19 had no recognized specific treatment for COVID-19. Recovery time was compared between patients with and those without specific treatment for COVID-19, and recovery time was slightly longer with median 12 days in the 70 patients with COVID-19 treatment compared with 9 days in the 140 without COVID-19 treatment (P = .053). Thus, median recovery time was not shorter in patients who were treated for COVID-19 and might have been longer because patients usually deferred treatment if they were low risk or felt well with minor symptoms.
Specific treatment vs disease severity
As shown in Table 1, of the 210 patients who tested positive, disease severity was asymptomatic in 7, mild in 177, severe in 24 (10 of whom had pneumonia), and critical (fatal) in 2. Specific COVID-19 treatments are indicated for each of the severity groups other than the 177 in the mild category. Neither of the 2 critical cases were treated; in 1 case because it occurred early before the development of specific treatments, and in the other case because of other complications occurring in the patient. Half of the patients with HCL/HCLv with severe COVID-19 received specific treatment, including convalescent plasma in 3, Remdesivir in 3, Paxlovid in 3, and mAb treatments in 6, with some patients receiving multiple treatments. The frequent use of specific treatments in the severe group was possibly associated with absent mortality in this group, along with the relatively lower mortality, in general, of the Omicron strains.
Patients with HCL/HCLv with prolonged recovery to COVID-19
Several patients had particularly prolonged recovery. A 64-year-old male with HCLv and prior cladribine, rituximab, splenectomy, rituximab, pentostatin-rituximab, bendamustine-rituximab, and ibrutinib, was on binimetinib with normal B cells 5/mm3 and CD4 count 153/mm3. Three COVID-19 vaccine doses were received in 2021 and a fourth in April 2022. By August 2022, normal B cells were 5/mm3, CD4 count 153/mm3, N antibodies negative, and S antibodies too high to measure (>24 999 U/mL). That month, he presented with herpes zoster virus infection, and 1 week later developed upper respiratory symptoms and tested positive for COVID-19. He received paxlovid, experienced worsening of herpes zoster symptoms after 1 week, later tested negative for COVID-19 after 2 weeks, but had continued upper respiratory symptoms. By October 2022, he received pentostatin-rituximab for increasing adenopathy and tested COVID-19 positive again with recurrent symptoms, normal B cells 1/mm3, CD4 count 68/mm3, negative N antibodies, and S antibodies >24 999 U/mL. He received remdesivir and dexamethasone and finally tested negative 1 week later and recovered 70 days after symptom onset. A 55-year-old female with HCLv received cladribine in November 2019 followed by rituximab, and by September 2020, she developed COVID-19 pneumonia, with symptoms resolving 71 days after onset. A 55-year-old male with HCL who received cladribine in March 2020 had COVID-19 vaccine doses in April and May 2021. COVID-19 pneumonia was diagnosed in August 2021, requiring home-oxygen after discharge. Normal B cells were 2/mm3, CD4 count 140/mm3, N antibodies positive, and S antibodies >24 999 U/mL. Symptoms resolved 72 days after testing positive. A 46-year-old man with prior cladribine, rituximab, and cladribine with immediate rituximab, received moxetumomab pasudotox, and rituximab from August 2020 to February 2021 achieving MRD-negative CR. In August 2021, he was still in MDR-negative CR with absent normal B cells and CD4 count 454/mm3. In November 2021, he developed COVID-19 with URI symptoms treated with bamlanivimab-etesevimab, tested COVID-19-negative despite worsening cough, and then presented with COVID-19 pneumonia in late December 2021, which was treated with remdesivir. Upper respiratory symptoms remained and in May 2022, with normal B cells 23/mm3 and CD4 count 458/mm3, he received bebtelovimab with improvement, having symptoms resolved 113 days after onset. A 59-year-old-male with untreated HCL received COVID-19 vaccine doses in March and April of 2021 and by March 2022 had normal B cells 24/mm3, CD4 count 554/mm3, negative N antibodies, and S antibodies low-positive at 95 U/mL. He developed COVID-19 with upper respiratory symptoms in May 2022, received paxlovid in June 2022, and 1 week later progressed to pneumonia. Symptoms resolved 123 days after onset, with normal B cells 11/mm3, CD4 count 298/mm3, positive N antibodies, and S antibodies >24 999 U/mL. A 71-year-old female with multiply relapsed HCL received 4 COVID-19 vaccine doses in 2021 and an Omicron booster in September 2022, but by November 2022, the spike antibody remained low at 1635 U/mL, N antibodies negative, and normal B cells 52/mm3. Because of progression from vemurafenib, she began moxetumomab pasudotox and obinutuzumab in November 2022, and by December 2022 and January 2023 normal B cells were 5/mm3 and then 0/mm3. N antibodies became positive without recent COVID-19 symptoms, and the S antibodies fell to 1077 U/mL and 402 U/mL, respectively. In February 2023, she developed COVID-19 symptoms and tested positive by rapid-antigen test. Imaging showed small ground glass abnormalities, and cough remained mild, so moxetumomab-obinutuzumab was continued, achieving MRD-negative CR. By March and May 2023, PCR was still positive for COVID-19, and despite remdesivir, mild cough continued. With PCR still positive in June 2023, remdesivir was given again leading to resolution of cough and PCR negativity, finally, 144 days after symptom onset. A 45-year-old female received a COVID-19 vaccine in April 2021 and by October 2021 developed COVID-19 with multifocal pneumonia treated with tixagevimab-cilgavimab. She had persistent vocal cord dysfunction, arthralgias, hand/foot paresthesias, wheezing, dyspnea, fatigue, poor memory with “brain fog,” intermittent hives, dizziness, and headache. She was diagnosed with HCL and treated with cladribine in June 2022 with baseline ANC 1.15 × 109/L and Plt 99 × 109/L. Six weeks later, she had resolved ANC and Plt, undetectable circulating HCL cells, normal B cells 2/mm3, CD4 count 357/mm3, positive N antibodies, and S antibodies >24 999 U/mL. Nevertheless, her symptoms attributed to COVID-19 persisted >600 days after onset.
Discussion
Our goal was to study COVID-19 in patients with HCL/HCLv. We found that out of 503 patients followed during the pandemic, 253 had evidence of COVID-19, with 210 of them testing positive and the others with positive N antibody and usually COVID-19 symptoms. Only 1 of these patients died with clear relationship to COVID-19, 1 died with COVID-19 complications along with other comorbidities, 1 developed chronic symptoms, and the remaining patients recovered without sequelae. We found that low levels of normal B cells, which were reduced usually by anti-CD20 mAbs such as rituximab, were associated with lower antibody levels after COVID-19 infection and slightly longer median time to recovery. We found only a trend to shorter recovery times in patients who were vaccinated before exposure to COVID-19, although most patients had access to recognized COVID-19 treatments if needed.
Avoiding fatal COVID-19
Unlike previously reported studies of COVID-19 in HCL, which included patients reported up until 26 January 202212 and up until April 2022,13 most of our patients tested positive in 2022 (Table 1) when COVID-19 was more treatable, less lethal, and vaccines were more readily available. As mentioned above, we did report 1 young patient with HCL who was doing well after first-line treatment with cladribine and unfortunately was infected with fatal COVID-19 after receiving rituximab 2 months after cladribine and just before the availability of COVID-19 vaccines. Consensus guidelines from HCL experts published in 2021 after this case occurred recommended avoiding rituximab or other treatments likely to harm immunity until patients could achieve acceptable immunity through vaccination.3 Some untreated patients with HCL have absent or low levels of normal B cells because of the disease and cannot be expected to respond to vaccination. For such patients, passive immunization with mAb may be useful, assuming SARS-CoV-2 variants are susceptible to them. For these patients, small studies have suggested the benefit of nonimmunosupressive treatments such as vemurafenib, which induce major remissions or at least correct cytopenias, and allow patients to respond to vaccination17 or recover from severe infection.18,19 With the advent of COVID-19 vaccines and effective drug treatments and lower rates of mortality and morbidity to COVID-19, first-line therapy for HCL is now with much lower risk than what it was earlier in the pandemic. Nevertheless, it is still prudent to assess and optimize vaccination status, if possible, before beginning immunosupressive treatment for HCL/HCLv.
Heterogeneity of HCL/HCLv patients studied
Although this study, to our knowledge, is by far the largest collection of COVID-19 cases and HCL/HCLv, and the first to be able to determine relationships between COVID-19 outcome and immune markers such as normal B cells, it was limited by the heterogeneity of the population. The wide variation in antibody response to COVID-19 with respect to normal or reduced normal B cells (Figure 1) may be related to other factors in patients, such as comorbidities, virulence of the particular COVID-19 strain, and the HCL/HCLv disease burden. Moreover, time of recovery of COVID-19 symptoms, measured in days from onset to cessation of symptoms, is a soft and subjective end point. Patients with preexisting lung disease may have much longer recovery times from the same level of COVID-19–related lung tissue damage compared with otherwise normal patients with COVID-19 pneumonia. CD4 count and IgG level were not found to be associated with the ability to mount an immune response to COVID-19 or recover quickly. However, it is possible that treatments other than rituximab that damage T cells and cause immunosuppression beyond reductions or impairment of B cells can limit the ability to find risk factors in patients with HCL/HCLv infected with COVID-19. We did not determine T-cell immunity in our patients but recently reported significant T-cell response to vaccine in a patient with lymphoma after rituximab with impaired humeral immunity.20 Finally, the subvariant type of COVID-19 was not identified in our patients, but we expected that cases during the Delta and earlier surges would be more serious than later Omicron surges. In CLL, rates of hospitalizations and mortality were higher at the beginning of the pandemic.21 Even within the Omicron period, it was reported that CLL hospitalizations and mortality were lower during surges of the Omicron BA.2 and BA.5 subvariants compared with the earlier Omicron BA.1 subvariant.22
Study of COVID-19 at the close of the pandemic
The COVID-19 pandemic has been devastating to millions of otherwise healthy people and thousands of immunosuppressed patients with hematologic malignancies. Even as the pandemic dissipates, studies of COVID-19 in patients with HCL/HCLv should be helpful for managing these and other chronic leukemia/lymphomas through infections, including later surges of COVID-19 and other viruses.
Acknowledgments
The authors thank Barbara Debrah for managing clinical and research data, Alexia Torres, Sonya Duke, and Jennifer Hauprich for coordinating patient testing and sample submission, and John Riley for supervising flow cytometry to quantify lymphocyte subsets. Ann McCoy and Monica Epstein served as research nurses early in the study. Andy Gillespie and Helen Owens served as research nursing supervisors. This study was supported by the intramural research programs of the National Cancer Institute, National Institute of Allergy and Infectious Diseases, and the National Institute of Dental and Craniofacial Research.
Authorship
Contribution: R.J.K. designed and performed research, analyzed data, and wrote the manuscript; T.Y. performed research and analyzed data; L.J., J.F., H.E., O.S.O., M.G., J.M., and H.Z. performed research; and P.D.B., J.I.C., H.-W.W., C.M.Y., and E.A. performed research and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert J. Kreitman, Laboratory of Molecular Biology, National Cancer Institute, National Institutes of Health, Building 10, Room 13N248A, 9000 Rockville Pike, Bethesda, MD 20892; e-mail: kreitmar@mail.nih.gov.
References
Author notes
Data are available upon request from the corresponding author, Robert J. Kreitman (kreitmar@mail.nih.gov).