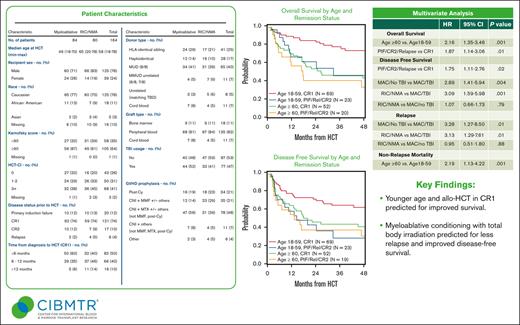
Key Points
Age of >60 years and remission status at time of allo-HCT (CR2/primary induction failure/relapse vs CR1) was predictive of inferior OS.
Use of myeloablative conditioning with total body irradiation was predictive for improved DFS and reduced risk of relapse.
Abstract
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare hematological malignancy with a poor prognosis and considered incurable with conventional chemotherapy. Small observational studies reported allogeneic hematopoietic cell transplantation (allo-HCT) offers durable remissions in patients with BPDCN. We report an analysis of patients with BPDCN who received an allo-HCT, using data reported to the Center for International Blood and Marrow Transplant Research (CIBMTR). We identified 164 patients with BPDCN from 78 centers who underwent allo-HCT between 2007 and 2018. The 5-year overall survival (OS), disease-free survival (DFS), relapse, and nonrelapse mortality (NRM) rates were 51.2% (95% confidence interval [CI], 42.5-59.8), 44.4% (95% CI, 36.2-52.8), 32.2% (95% CI, 24.7-40.3), and 23.3% (95% CI, 16.9-30.4), respectively. Disease relapse was the most common cause of death. On multivariate analyses, age of ≥60 years was predictive for inferior OS (hazard ratio [HR], 2.16; 95% CI, 1.35-3.46; P = .001), and higher NRM (HR, 2.19; 95% CI, 1.13-4.22; P = .02). Remission status at time of allo-HCT (CR2/primary induction failure/relapse vs CR1) was predictive of inferior OS (HR, 1.87; 95% CI, 1.14-3.06; P = .01) and DFS (HR, 1.75; 95% CI, 1.11-2.76; P = .02). Use of myeloablative conditioning with total body irradiation (MAC-TBI) was predictive of improved DFS and reduced relapse risk. Allo-HCT is effective in providing durable remissions and long-term survival in BPDCN. Younger age and allo-HCT in CR1 predicted for improved survival, whereas MAC-TBI predicted for less relapse and improved DFS. Novel strategies incorporating allo-HCT are needed to further improve outcomes.
Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is an aggressive hematological malignancy derived from the precursors of plasmacytoid dendritic cells. It is a relatively rare entity, accounting for ∼0.4% of all hematological malignancies alone, and carries a poor prognosis.1-3 Previously referred to as acute agranular CD4+ natural killer cell leukemia4 and blastic natural killer cell leukemia/lymphoma,5 BPDCN is currently listed as a separate entity in the revised 2022 World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms.6 BPDCN typically presents with cutaneous lesions, although it can also present with other various sites of involvement, including the bone marrow (BM), spleen, liver, lymph nodes, and central nervous system (CNS), and with leukemic dissemination with circulating lymphoid/monocytoid cells. BPDCN can affect all ages, but it is more predominant in older adults, with males more commonly affected than females.7
Treatment options for BPDCN are limited. Leukemia-based regimens have been effective, with prolonged survival seen when compared with those receiving lymphoma-based regimens.8 More recently, tagraxofusp, a CD123-directed cytotoxin consisting of human interleukin-3 fused to truncated diphtheria toxin, was US Food and Drug Administration approved for treatment of BPDCN in adults and pediatric patients based on the pivotal trial by Pemmaraju et al, which reported an overall response rate of 90%.9 Long-term remission with only chemotherapy remains uncommon, with relapse rate of nearly 80%.3
Allogeneic hematopoietic cell transplantation (allo-HCT) has been reported as an effective consolidation strategy in BPDCN.10-14 The European Society for Blood and Marrow Transplantation (EBMT) published outcomes of 34 patients with BPDCN who received an allo-HCT, showing a 3-year disease-free survival (DFS) and overall survival (OS) of 33% and 41%, respectively.14 The Japanese Society for Hematopoietic Cell Transplantation reported 14 cases of allo-HCT showing a 4-year OS of 53%.15 Kharfan-Dabaja et al reported a North American multicenter collaborative observational study of 37 cases showing a 3-year OS of 58% in recipients of allo-HCT.12 Recently, Bashir et al reported outcomes of 17 patients with BPDCN who received allo-HCT between 2000 and 2020. The 5-year OS was reported at 40%, with 80% 5-year OS when allo-HCT performed in first complete remission (CR1) compared with 0% in patients not in CR1.13
The generalizability of findings from the aforementioned retrospective studies may be limited by small sample size, highlighting the need for a larger observational study. Accordingly, we analyzed data from the Center for International Blood and Marrow Transplant (CIBMTR) registry to help guide and inform clinical decision making and determine the effectiveness of allo-HCT in BPDCN.
Methods
Data sources
The CIBMTR is a working group of >500 transplantation centers worldwide that contribute detailed data on HCT to a statistical center at the Medical College of Wisconsin. The CIBMTR collects data at 2 levels, transplant essential data (TED) in all patients, and more comprehensive report forms (CRF) in a subset of patients. Participating centers are required to report all transplantations consecutively and compliance is monitored by on-site audits. Computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. The Medical College of Wisconsin and the National Marrow Donor Program institutional review boards approved this study. The study was performed in accordance with the Declaration of Helsinki.
Patient selection
Adult patients (aged ≥18 years) who underwent first allo-HCT for BPDCN between 2007 and 2018 were included in this analysis. Because BPDCN has not been a recognized diagnosis captured by the CIBMTR before 2013, all BPDCN cases reported between 2007 and 2013 were identified, and pathology reports were individually reviewed to ascertain diagnostic accuracy. A total of 19 cases met these criteria, and all were found to be correctly identified as BPDCN.
Graft sources included peripheral blood, BM, and umbilical cord blood. Eligible donors included HLA-identical sibling donors or unrelated donors matched at the allele-level at HLA-A, -B, -C, and -DRB1, or those receiving HLA-haploidentical, HLA-mismatched unrelated donor, or cord blood transplantation. Ex vivo T-cell–depleted grafts were excluded, as were patients receiving syngeneic twin transplants. Recipients of allo-HCT receiving in vivo T-cell depletion were included.
Definitions and study end points
The intensity of conditioning regimens was defined using consensus criteria.16 Myeloablative total body irradiation (TBI) was defined as either a single fraction of ≥500 cGy, or fractionated cumulative doses of ≥800 cGy.17 The primary end point was OS. Death from any cause was considered an event, and surviving patients were censored at the time of last follow-up. Secondary end points included nonrelapse mortality (NRM), progression/relapse, and DFS. NRM was defined as death in the absence of disease relapse/progression, which were considered competing risks. Progressive disease or recurrence of BPDCN was defined as progression after allo-HCT or recurrence after a documented CR. NRM was the competing event. For DFS, this was defined as survival after allo-HCT without relapse or progression. Relapse, progression of disease, or death were considered events. Patients alive without evidence of disease relapse or progression were censored at last follow-up. The causes of death were reported in accordance with previously described methodology.18
Statistical analysis
Probabilities of OS and DFS were calculated using the Kaplan-Meier method, and probabilities of relapse and NRM were calculated using the cumulative incidence estimator to accommodate competing risks. Multivariate analysis for NRM, relapse, DFS, and OS were performed using Cox proportional hazards models. The assumption of proportional hazards for each factor was tested using time-dependent covariates, and a backward stepwise model was used to select all significant risk factors. Factors that were significant at a 5% level were retained in the final model. The variables that were considered in the multivariable models included recipient age, Karnofsky performance status, HCT comorbidity index, disease status at transplant, conditioning regimen intensity, time from diagnosis to transplant, donor/recipient cytomegalovirus serostatus, graft-versus-host disease (GVHD) prophylaxis, donor type, graft source, and in vivo T-cell depletion.
Results
Baseline characteristics
This study included 164 patients who received allo-HCT for BPDCN at 1 of 78 CIBMTR reporting centers. The baseline patient-, disease-, and transplant-related characteristics are described in Table 1. The median follow-up was 49 months (range, 6-121 months). Recipients’ median age at the time of allo-HCT was 58 years (range, 18-78 years); 77% were male, and 64% had a Karnofsky performance status score of ≥90. The majority of allo-HCT recipients were Caucasian (76%). Disease status at the time of HCT were CR1, CR2, primary induction failure, and relapse in 74%, 10%, 12%, and 4%, respectively. No patients in this analysis received a prior autologous HCT.
Characteristics of patients receiving first HCT for BPDCN between 2007 and 2018, registered with the CIBMTR
| Characteristic . | MAC . | RIC/NMA . | Total . |
|---|---|---|---|
| Patients, n | 84 | 80 | 164 |
| Centers, n | 47 | 49 | 78 |
| Age (y) at HCT, n (%) | |||
| Median (min-max) | 49 (18-70) | 65 (20-78) | 58 (18-78) |
| 18-29 | 15 (18) | 3 (4) | 18 (11) |
| 30-39 | 16 (19) | 5 (6) | 21 (13) |
| 40-49 | 13 (16) | 2 (3) | 15 (9) |
| 50-59 | 26 (31) | 12 (15) | 38 (23) |
| 60-69 | 14 (17) | 40 (50) | 54 (33) |
| ≥70 | 0 (<1) | 18 (23) | 18 (11) |
| Reporting track, n (%) | |||
| TED | 60 (71) | 65 (81) | 125 (76) |
| CRF | 24 (29) | 15 (19) | 39 (24) |
| Recipient sex, n (%) | |||
| Male | 60 (71) | 66 (83) | 126 (77) |
| Female | 24 (27) | 14 (18) | 38 (23) |
| Race, n (%) | |||
| Caucasian | 65 (77) | 60 (75) | 125 (76) |
| African-American | 11 (13) | 7 (9) | 18 (11) |
| Asian | 2 (2) | 3 (4) | 5 (3) |
| Not reported | 6 (7) | 10 (13) | 16 (10) |
| Karnofsky performance status score, n (%) | |||
| <90 | 27 (32) | 31 (39) | 58 (35) |
| ≥90 | 56 (67) | 49 (61) | 105 (64) |
| Not reported | 1 (1) | 0 (<1) | 1 (1) |
| HCT-CI , n (%) | |||
| 0 | 27 (32) | 16 (20) | 43 (26) |
| 1-2 | 24 (29) | 26 (33) | 50 (31) |
| ≥3 | 32 (38) | 36 (45) | 68 (42) |
| Not reported | 1 (1) | 2 (3) | 3 (2) |
| Disease status before HCT, n (%) | |||
| Primary induction failure | 10 (12) | 10 (13) | 20 (12) |
| CR1 | 62 (74) | 59 (74) | 121 (74) |
| CR2 | 10 (12) | 7 (9) | 17 (10) |
| Relapse | 2 (2) | 4 (5) | 6 (4) |
| Time (mo) from diagnosis to HCT, n (%) | |||
| <6 | 50 (60) | 32 (40) | 82 (50) |
| 6-12 | 29 (35) | 37 (46) | 66 (40) |
| >12 | 5 (6) | 11 (14) | 16 (10) |
| Donor type, n (%) | |||
| HLA-identical sibling | 24 (29) | 17 (21) | 41 (25) |
| Other related | 12 (14) | 16 (20) | 28 (17) |
| Well-matched unrelated (8 of 8) | 34 (41) | 31 (39) | 65 (40) |
| Partially matched unrelated (7 of 8) | 3 (4) | 7 (9) | 10 (6) |
| Mismatched unrelated (≤6 of 8) | 1 (1) | 0 (<1) | 1 (1) |
| Unrelated (matching unknown) | 3 (4) | 5 (6) | 8 (5) |
| Cord blood | 7 (8) | 4 (5) | 11 (7) |
| Donor/recipient CMV serostatus, n (%) | |||
| +/+ | 29 (35) | 27 (34) | 56 (34) |
| +/− | 8 (10) | 11 (14) | 19 (12) |
| −/+ | 22 (26) | 24 (30) | 46 (28) |
| −/− | 23 (27) | 17 (21) | 40 (24) |
| Not reported | 2 (2) | 1 (1) | 3 (2) |
| Donor/recipient sex match, n (%) | |||
| M-M | 40 (48) | 42 (53) | 82 (50) |
| M-F | 10 (12) | 8 (10) | 18 (11) |
| F-M | 20 (24) | 24 (30) | 44 (27) |
| F-F | 14 (17) | 6 (8) | 20 (12) |
| Graft type, n (%) | |||
| BM | 9 (11) | 9 (11) | 18 (11) |
| Peripheral blood | 68 (81) | 67 (84) | 135 (82) |
| Cord blood | 7 (8) | 4 (5) | 11 (7) |
| TBI usage, n (%) | |||
| No | 40 (48) | 47 (59) | 87 (53) |
| Yes | 44 (52) | 33 (41) | 77 (47) |
| GVHD prophylaxis, n (%) | |||
| PTCy | 16 (19) | 18 (23) | 34 (21) |
| CNI + MMF w/wo others | 12 (14) | 23 (29) | 35 (21) |
| CNI + MTX w/wo others (not MMF or PTCy) | 47 (56) | 31 (39) | 78 (48) |
| CNI + others (not MMF, MTX, or PTCy) | 7 (8) | 4 (5) | 11 (7) |
| Other | 2 (2) | 4 (5) | 6 (4) |
| Year of HCT, n (%) | |||
| 2007 | 2 (2) | 0 (<1) | 2 (1) |
| 2008 | 1 (1) | 0 (<1) | 1 (1) |
| 2009 | 0 (<1) | 3 (4) | 3 (2) |
| 2010 | 2 (2) | 2 (3) | 4 (2) |
| 2011 | 8 (10) | 5 (6) | 13 (8) |
| 2012 | 7 (8) | 8 (10) | 15 (9) |
| 2013 | 9 (11) | 9 (11) | 18 (11) |
| 2014 | 11 (13) | 6 (8) | 17 (10) |
| 2015 | 9 (11) | 13 (16) | 22 (13) |
| 2016 | 12 (14) | 13 (16) | 25 (15) |
| 2017 | 12 (14) | 15 (19) | 27 (17) |
| 2018 | 11 (13) | 6 (8) | 17 (10) |
| Follow-up of survivors (mo), median (range) | 49 (12-121) | 49 (6-119) | 49 (6-121) |
| Characteristic . | MAC . | RIC/NMA . | Total . |
|---|---|---|---|
| Patients, n | 84 | 80 | 164 |
| Centers, n | 47 | 49 | 78 |
| Age (y) at HCT, n (%) | |||
| Median (min-max) | 49 (18-70) | 65 (20-78) | 58 (18-78) |
| 18-29 | 15 (18) | 3 (4) | 18 (11) |
| 30-39 | 16 (19) | 5 (6) | 21 (13) |
| 40-49 | 13 (16) | 2 (3) | 15 (9) |
| 50-59 | 26 (31) | 12 (15) | 38 (23) |
| 60-69 | 14 (17) | 40 (50) | 54 (33) |
| ≥70 | 0 (<1) | 18 (23) | 18 (11) |
| Reporting track, n (%) | |||
| TED | 60 (71) | 65 (81) | 125 (76) |
| CRF | 24 (29) | 15 (19) | 39 (24) |
| Recipient sex, n (%) | |||
| Male | 60 (71) | 66 (83) | 126 (77) |
| Female | 24 (27) | 14 (18) | 38 (23) |
| Race, n (%) | |||
| Caucasian | 65 (77) | 60 (75) | 125 (76) |
| African-American | 11 (13) | 7 (9) | 18 (11) |
| Asian | 2 (2) | 3 (4) | 5 (3) |
| Not reported | 6 (7) | 10 (13) | 16 (10) |
| Karnofsky performance status score, n (%) | |||
| <90 | 27 (32) | 31 (39) | 58 (35) |
| ≥90 | 56 (67) | 49 (61) | 105 (64) |
| Not reported | 1 (1) | 0 (<1) | 1 (1) |
| HCT-CI , n (%) | |||
| 0 | 27 (32) | 16 (20) | 43 (26) |
| 1-2 | 24 (29) | 26 (33) | 50 (31) |
| ≥3 | 32 (38) | 36 (45) | 68 (42) |
| Not reported | 1 (1) | 2 (3) | 3 (2) |
| Disease status before HCT, n (%) | |||
| Primary induction failure | 10 (12) | 10 (13) | 20 (12) |
| CR1 | 62 (74) | 59 (74) | 121 (74) |
| CR2 | 10 (12) | 7 (9) | 17 (10) |
| Relapse | 2 (2) | 4 (5) | 6 (4) |
| Time (mo) from diagnosis to HCT, n (%) | |||
| <6 | 50 (60) | 32 (40) | 82 (50) |
| 6-12 | 29 (35) | 37 (46) | 66 (40) |
| >12 | 5 (6) | 11 (14) | 16 (10) |
| Donor type, n (%) | |||
| HLA-identical sibling | 24 (29) | 17 (21) | 41 (25) |
| Other related | 12 (14) | 16 (20) | 28 (17) |
| Well-matched unrelated (8 of 8) | 34 (41) | 31 (39) | 65 (40) |
| Partially matched unrelated (7 of 8) | 3 (4) | 7 (9) | 10 (6) |
| Mismatched unrelated (≤6 of 8) | 1 (1) | 0 (<1) | 1 (1) |
| Unrelated (matching unknown) | 3 (4) | 5 (6) | 8 (5) |
| Cord blood | 7 (8) | 4 (5) | 11 (7) |
| Donor/recipient CMV serostatus, n (%) | |||
| +/+ | 29 (35) | 27 (34) | 56 (34) |
| +/− | 8 (10) | 11 (14) | 19 (12) |
| −/+ | 22 (26) | 24 (30) | 46 (28) |
| −/− | 23 (27) | 17 (21) | 40 (24) |
| Not reported | 2 (2) | 1 (1) | 3 (2) |
| Donor/recipient sex match, n (%) | |||
| M-M | 40 (48) | 42 (53) | 82 (50) |
| M-F | 10 (12) | 8 (10) | 18 (11) |
| F-M | 20 (24) | 24 (30) | 44 (27) |
| F-F | 14 (17) | 6 (8) | 20 (12) |
| Graft type, n (%) | |||
| BM | 9 (11) | 9 (11) | 18 (11) |
| Peripheral blood | 68 (81) | 67 (84) | 135 (82) |
| Cord blood | 7 (8) | 4 (5) | 11 (7) |
| TBI usage, n (%) | |||
| No | 40 (48) | 47 (59) | 87 (53) |
| Yes | 44 (52) | 33 (41) | 77 (47) |
| GVHD prophylaxis, n (%) | |||
| PTCy | 16 (19) | 18 (23) | 34 (21) |
| CNI + MMF w/wo others | 12 (14) | 23 (29) | 35 (21) |
| CNI + MTX w/wo others (not MMF or PTCy) | 47 (56) | 31 (39) | 78 (48) |
| CNI + others (not MMF, MTX, or PTCy) | 7 (8) | 4 (5) | 11 (7) |
| Other | 2 (2) | 4 (5) | 6 (4) |
| Year of HCT, n (%) | |||
| 2007 | 2 (2) | 0 (<1) | 2 (1) |
| 2008 | 1 (1) | 0 (<1) | 1 (1) |
| 2009 | 0 (<1) | 3 (4) | 3 (2) |
| 2010 | 2 (2) | 2 (3) | 4 (2) |
| 2011 | 8 (10) | 5 (6) | 13 (8) |
| 2012 | 7 (8) | 8 (10) | 15 (9) |
| 2013 | 9 (11) | 9 (11) | 18 (11) |
| 2014 | 11 (13) | 6 (8) | 17 (10) |
| 2015 | 9 (11) | 13 (16) | 22 (13) |
| 2016 | 12 (14) | 13 (16) | 25 (15) |
| 2017 | 12 (14) | 15 (19) | 27 (17) |
| 2018 | 11 (13) | 6 (8) | 17 (10) |
| Follow-up of survivors (mo), median (range) | 49 (12-121) | 49 (6-119) | 49 (6-121) |
CMV, cytomegalovirus; CNI, calcineurin inhibitors; CRF, comprehensive report form; Cy, cyclophosphamide; F, female; HCT-CI; HCT comorbidity index; M, male; max, maximum; MMF, mycophenolate mofetil; min, minimum; MTX, methotrexate; PTCy; posttransplant cyclophosphamide; TED, transplant essential data; w/wo, with/without.
Most patients received granulocyte colony-stimulating factor mobilized peripheral blood stem cells (82%), with 11% receiving BM grafts, and 7% receiving umbilical cord blood. Matched related donors (25%) and 8-of-8 HLA-matched unrelated donors (40%) were the most common types of donors. Myeloablative conditioning (MAC) and reduced intensity and nonmyeloablative conditioning (RIC/NMA) were used in 53% and 47% of cases, respectively. For patients aged <60 years, 80% (72 of 90) received MAC whereas for patients aged ≥60 years, 79% (57 of 72) received RIC/NMA. In total, 77 patients (47%) received TBI as part of their conditioning, with 52% of all recipients of MAC receiving myeloablative TBI and 38% of recipients of RIC/NMA receiving low-dose TBI. TBI doses for MAC ranged from 550 cGy administered as single fraction, to 1600 cGy total. One patient received MAC with low-dose TBI (400 cGy). RIC/NMA TBI doses ranged from 200 to 400 cGy. Commonly used MAC regimens included cyclophosphamide TBI (n = 28), busulfan fludarabine (n = 24), and busulfan cyclophosphamide (n = 16) whereas commonly used RIC/NMA regimens included fludarabine melphalan (n = 26), fludarabine-cyclophosphamide TBI (n = 20), fludarabine TBI (n = 23), and fludarabine busulfan (n = 16). A total of 31 patients (17%) received in vivo T-cell depletion as part of their conditioning (supplemental Table 3). Most received calcineurin-based GVHD prophylaxis (76%) whereas 21% received posttransplant cyclophosphamide.
Data regarding pre-HCT induction therapy and disease sites of involvement were limited to comprehensive report form (CRF) data only, which was available for 39 of 164 patients. Of these 39 patients, 54% had extramedullary disease at diagnosis, 54% had disease involvement of the BM, 36% with skin involvement, 3% with CNS, and 31% had other sites of extramedullary disease. Most patients (82%) received only 1 line of treatment before allo-HCT. Leukemia style induction was used in 71% of patients, with 28% receiving hyperfractionated cyclophosphamide, vincristine, doxorubicin [Adriamycin], and dexamethasone (hyper-CVAD), and 60% receiving cytarabine-based therapy. Only 3 patients received tagraxofusp before allo-HCT.
OS and DFS
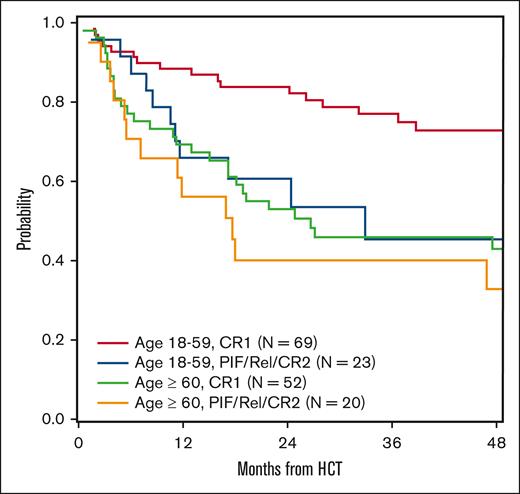
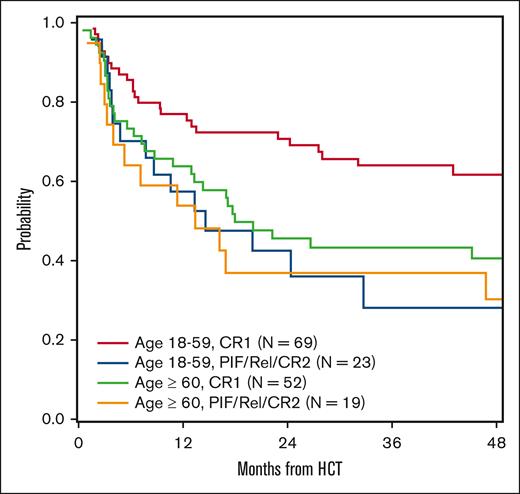
The 5-year OS and DFS were 51.2% (95% confidence interval [CI], 42.5-59.8) and 44.4% (95% CI, 36.2-52.8), respectively (supplemental Table 1). On multivariate analyses, age of ≥60 years was predictive for inferior OS (hazard ratio [HR], 2.16; 95% CI, 1.35-3.46; P = .001). Additionally, allo-HCT performed for non-CR1 (primary induction failure/CR2/relapse) was predictive for inferior OS (HR, 1.87; 95% CI, 1.14-3.06; P = .01) and DFS (HR, 1.75; 95% CI, 1.11-2.76; P = .02; Table 2; Figures 1 and 2).
Multivariate analysis results
| . | N . | HR (95% CI) . | P value . |
|---|---|---|---|
| OS | |||
| Age (y) at HCT | |||
| <60 | 92 | Reference | |
| ≥60 | 72 | 2.16 (1.35-3.46) | .001 |
| Disease status | |||
| CR1 | 121 | Reference | |
| PIF/CR2/relapse | 43 | 1.87 (1.14-3.06) | .01 |
| DFS | |||
| Disease status | |||
| CR1 | 121 | Reference | |
| PIF/CR2/relapse | 42 | 1.75 (1.11-2.76) | .02 |
| Conditioning intensity/TBI usage | .003 | ||
| MAC/TBI | 43 | Reference | |
| MAC/no TBI | 41 | 2.89 (1.41-5.94) | .004 |
| RIC/NMA | 79 | 3.09 (1.59-5.98) | .001 |
| RIC/NMA vs MAC/no TBI | 1.07 (0.66-1.73) | .79 | |
| Relapse | |||
| Conditioning intensity/TBI usage | .03 | ||
| MAC/TBI | 43 | Reference | |
| MAC/no TBI | 41 | 3.28 (1.27-8.5) | .01 |
| RIC/NMA | 79 | 3.13 (1.29-7.61) | .01 |
| RIC/NMA vs MAC/no TBI | 0.95 (0.51-1.80) | .88 | |
| NRM | |||
| Age (y) at HCT | |||
| <60 | 92 | Reference | |
| ≥60 | 71 | 2.19 (1.13-4.22) | .02 |
| . | N . | HR (95% CI) . | P value . |
|---|---|---|---|
| OS | |||
| Age (y) at HCT | |||
| <60 | 92 | Reference | |
| ≥60 | 72 | 2.16 (1.35-3.46) | .001 |
| Disease status | |||
| CR1 | 121 | Reference | |
| PIF/CR2/relapse | 43 | 1.87 (1.14-3.06) | .01 |
| DFS | |||
| Disease status | |||
| CR1 | 121 | Reference | |
| PIF/CR2/relapse | 42 | 1.75 (1.11-2.76) | .02 |
| Conditioning intensity/TBI usage | .003 | ||
| MAC/TBI | 43 | Reference | |
| MAC/no TBI | 41 | 2.89 (1.41-5.94) | .004 |
| RIC/NMA | 79 | 3.09 (1.59-5.98) | .001 |
| RIC/NMA vs MAC/no TBI | 1.07 (0.66-1.73) | .79 | |
| Relapse | |||
| Conditioning intensity/TBI usage | .03 | ||
| MAC/TBI | 43 | Reference | |
| MAC/no TBI | 41 | 3.28 (1.27-8.5) | .01 |
| RIC/NMA | 79 | 3.13 (1.29-7.61) | .01 |
| RIC/NMA vs MAC/no TBI | 0.95 (0.51-1.80) | .88 | |
| NRM | |||
| Age (y) at HCT | |||
| <60 | 92 | Reference | |
| ≥60 | 71 | 2.19 (1.13-4.22) | .02 |
PIF, primary induction failure.
OS by age and remission status. Rel, relapse; PIF, primary induction failure.
DFS by age and remission status. Rel, relapse; PIF, primary induction failure.
The impact of conditioning intensity and use of TBI were also examined in multivariate analysis. The use of MAC with TBI predicted for superior DFS compared with MAC without TBI (HR, 2.89; 95% CI, 1.41-5.94; P = .004), and RIC/NMA (HR, 3.09; 95% CI, 1.59-5.98; P = .001). RIC/NMA did not offer any significant impact on DFS when compared with MAC without TBI (HR, 1.07; 95% CI, 0.66-1.73; P = .79). Conditioning intensity and use of TBI were not significant variables in multivariable model for OS.
NRM and chronic GVHD
The 1-year and 5-year cumulative incidence of NRM were 16.6% (95% CI, 11.3-22.7), and 23.3% (95% CI, 16.9-30.4), respectively, whereas 1-year and 3-year rates of chronic GVHD were 45.5% (95% CI, 37.8-53.3) and 54.4% (95% CI, 46.4-62.3) respectively (supplemental Table 1). On multivariate analyses, only age ≥60 years was predictive for worse NRM (HR, 2.19; 95% CI, 1.13-4.42; P = .02). Conditioning intensity and use of TBI were tested and not found to be significant in multivariable model for NRM.
Relapse
The cumulative incidence of relapse/progression at 1 year and 5 years were 16.6% (95% CI, 11.3-22.7) and 32.2% (95% CI, 24.7-40.3), respectively. MAC with TBI appeared to have a protective effect against relapse in multivariate analysis when compared with MAC without TBI (HR, 3.28; 95% CI, 1.27-8.50; P = .01) and RIC/NMA (HR, 3.13; 95% CI, 1.29-7.61; P = .01). RIC/NMA did not affect risk of relapse when compared with MAC without TBI (HR, 0.95; 95% CI, 0.51-1.80; P = .88).
Causes of death
The most common cause of death was relapse of the primary disease (40%), followed by GVHD (22%), organ failure (10%), and infection (8%; supplemental Table 2).
Discussion
This CIBMTR registry study confirms the efficacy of allo-HCT in BPDCN. Better OS and DFS as well as reduced relapse risk were noted when allo-HCT was performed in CR1, confirming previously published findings.10-14 Additionally, we identified older age, defined by age of ≥60 years at time of allo-HCT, as a significant risk for inferior OS and increased risk of NRM. The use of MAC with TBI predicted for improved DFS and reduced risk of relapse.
The median age at time of allo-HCT was 58 years (range, 18-78 years), which represent a relatively older group compared with previous studies by EBMT (age, 41 years [range, 10-70 years]) and Kharfan-Dabaja et al (age, 50 years [range, 14-74 years]),12,14 and closer to the median age of BPDCN at diagnosis. In contrast to prior reports from EBMT and Kharfan-Dabaja et al, which did not show older age as an adverse prognostic variable on survival or NRM, we observed inferior OS and increased NRM in patients aged ≥60 years. It should be noted that each study assessed different age cutoffs for their respective analysis, with EBMT using as age of 55 years, and Kharfan-Dabaja et al using an age of 41 years.12,14 In our analysis, despite 79% of all patients aged ≥60 years receiving a RIC/NMA regimen, we did not identify age as a significant influence on relapse risk. Strategies to enhance outcomes in allo-HCT recipients who are older represent an unmet need, given the reported median age ranging between 53 and 67 years.3,19
The survival benefit for allo-HCT performed in CR1 is consistent with prior published studies by the EBMT, Kharfan-Dabaja et al, and Bashir et al.12-14 The favorable impact of CR1 highlights the importance of prompt referral of patients diagnosed with BPDCN for HCT evaluation. Although time to transplantation was not found to significantly affect survival in our analysis, early referral for consideration of allo-HCT is recommended to enhance the likelihood of proceeding to allo-HCT in CR1, which results in significantly decreased risk for relapse compared with transplantation beyond CR1.
The benefit of allo-HCT in CR1 also highlights the importance of effective induction strategies for newly diagnosed BPDCN. Yun et al reported better CR rates with frontline hyper-CVAD regimens compared with cyclophosphamide, doxorubicin, vincristine (Oncovin), and prednisolone (CHOP)-based regimens or tagraxofusp (91% vs 50% vs 50%),20 similar to data reported by Pemmaraju et al, who also showed better CR rates with frontline hyper-CVAD regimens compared with CHOP-based regimens or tagraxofusp (80% vs 59% vs 43%).21 New therapies are currently being investigated in salvage and frontline settings for BPDCN including CD123 antibody–drug conjugate,22 chimeric antigen receptor T-cell therapies,23 and venetoclax-based therapies.24 Finally, there exists the need for measurable residual disease (MRD) testing in BPDCN. Currently MRD testing in BPDCN is not standard and has been identified as 1 of the highest unmet needs by the North American Blastic Plasmacytoid Dendritic Cell Neoplasm Consortium.25 The standardization of MRD testing in BPDCN could influence choice of conditioning intensity, risk stratify the risk of relapse depending on degree of remission, further help with patient selection, and determine subsets of patients who could potentially benefit from posttransplant therapies and heightened surveillance.
The beneficial impact of conditioning regimen in our analysis was limited to MAC with TBI. Overall, 53% of patients in this study received MAC regimens, compared with 54% in the study by Kharfan-Dabaja et al whereas studies by Bashir et al and the EBMT (Table 3) reported a higher proportion of MAC use at 76% and 74%, respectively.12-14 Of those receiving MAC, the CIBMTR reported a higher proportion of MAC with TBI with 55%, compared with the EBMT at 50%, and Kharfan-Dabaja et al at 24%. The impact on DFS and relapse was limited only to MAC with TBI, and the same benefit was not observed when comparing MAC without TBI to RIC. BPDCN lesions were previously reported to be radiosensitive,26 thus, that could be 1 possible theory of the additive benefit of TBI. Another possible explanation is the known incidence of CNS involvement of BPDCN, reported to be 13% to 22% depending on disease course of BPDCN.27 This is similar to the role of myeloablative TBI in acute lymphoblastic leukemia, which has demonstrated benefit in reducing relapse risk and improving progression-free survival compared with chemotherapy-only conditioning regimens.28 It is also important to note that neither conditioning intensity nor use of TBI was found to have any significant effect on NRM or OS.
Selected studies of allo-HCT in BPDCN
| Publication . | Study . | N . | Remission status at allo-HCT (n) . | Donor type (n) . | Regimen intensity (n) . | Outcomes . |
|---|---|---|---|---|---|---|
| Roos-Weil et al14 | EBMT | 34 | CR1 = 19 >CR1 = 15 | MSD = 11 MUD = 23 | MAC = 25 RIC/NMA = 9 | 3-y OS: 41% 3-y NRM: 30% 3-y relapse: 32% |
| Kharfan-Dabaja et al12 | North America | 37 | CR1 = 28 >CR1 = 9 | MSD=16 MUD = 12 Other: 9∗ | MAC = 20 RIC/NMA = 17 | 3-y OS: 58% 1-y NRM: 25% 3-y relapse: 10% |
| Bashir et al13 | MDACC | 17 | CR1 = 10 >CR1 = 7 | MSD = 5 MUD = 5 Other: 7† | MAC = 13 RIC/NMA = 4 | 5-y OS: 40% 1-y NRM: 29% 5-y relapse: 22% |
| Murthy et al (this study) | CIBMTR | 164 | CR = 121 >CR1 = 43 | MSD = 41 MUD = 65 Other = 58‡ | MAC = 84 RIC = 80 | 5-y OS: 51.2% 5-y NRM: 23.3% 5-y relapse: 32.2% |
| Publication . | Study . | N . | Remission status at allo-HCT (n) . | Donor type (n) . | Regimen intensity (n) . | Outcomes . |
|---|---|---|---|---|---|---|
| Roos-Weil et al14 | EBMT | 34 | CR1 = 19 >CR1 = 15 | MSD = 11 MUD = 23 | MAC = 25 RIC/NMA = 9 | 3-y OS: 41% 3-y NRM: 30% 3-y relapse: 32% |
| Kharfan-Dabaja et al12 | North America | 37 | CR1 = 28 >CR1 = 9 | MSD=16 MUD = 12 Other: 9∗ | MAC = 20 RIC/NMA = 17 | 3-y OS: 58% 1-y NRM: 25% 3-y relapse: 10% |
| Bashir et al13 | MDACC | 17 | CR1 = 10 >CR1 = 7 | MSD = 5 MUD = 5 Other: 7† | MAC = 13 RIC/NMA = 4 | 5-y OS: 40% 1-y NRM: 29% 5-y relapse: 22% |
| Murthy et al (this study) | CIBMTR | 164 | CR = 121 >CR1 = 43 | MSD = 41 MUD = 65 Other = 58‡ | MAC = 84 RIC = 80 | 5-y OS: 51.2% 5-y NRM: 23.3% 5-y relapse: 32.2% |
MDACC, MD Anderson Cancer Center; MSD, matched sibling donor; MUD, matched unrelated donor.
Umbilical cord blood = 4, haploidentical = 3, mismatch unrelated = 2.
Umbilical cord blood = 3, haploidentical = 4.
Umbilical cord blood = 11, haploidentical = 28, mismatch unrelated = 10, multidonor = 1, unknown = 8.
There are limitations that are inherent to registry studies including the retrospective nature of the study, among others. Because data were obtained from a registry, we could not compare outcomes with those of patients who did not undergo allo-HCT. Another limitation is the lack of pertinent baseline pretransplantation information, such as cytogenetics, presence (or lack thereof) of somatic mutations, and details of induction or salvage therapies prescribed before allo-HCT. Additionally, there was a lack of comprehensive information captured in the CIBMTR registry regarding post-HCT therapies including donor lymphocyte infusions and any postremssion or maintenance therapies. This information was not available for most of our study participants, thus, we did not include this information in our analyses. The lack of consensus disease staging and response criteria is also a notable limitation. The impact of new therapies on allo-HCT outcomes is limited in this analysis because of the timeframe of data collected, notably tagraxofusp. Only 3 patients in the CIBMTR analysis received tagraxofusp. With more patients receiving frontline tagraxofusp29 and post-HCT tagraxofusp being investigated,30 more information on the effect of tagraxofusp and other novel therapies on HCT outcomes will become more evident in time.
Conclusion
Allo-HCT is an effective treatment for BPDCN, particularly in patients in CR1. This highlights the importance of prompt transplant referral for patients with BPDCN. The use of MAC with TBI was found to improve DFS and reduce relapse without adversely effecting NRM or OS. Strategies are needed to improve survival, especially in allo-HCT recipients who are older. With newer therapies showing promise in BPDCN, integrating these approaches with allo-HCT will be crucial to further improve outcomes of allo-HCT in BPDCN.
Acknowledgments
The Center for International Blood and Marrow Transplant Research is supported primarily by the Public Health Service U24CA076518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases; 75R60222C00011 from the Health Resources and Services Administration; and N00014-21-1-2954 and N00014-23-1-2057 from the Office of Naval Research. Support is also provided by Be The Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, Gateway for Cancer Research, Pediatric Transplantation and Cellular Therapy Consortium, and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc; Adaptimmune; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Allogene; AlloVir, Inc; Amgen, Inc; Angiocrine; Astellas Pharma US; Atara Biotherapeutics; BeiGene; bluebird bio, Inc; Bristol Myers Squibb Co; CareDx Inc; CSL Behring; CytoSen Therapeutics, Inc; Elevance Health; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida Cell, Ltd; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc; Karius; Kiadis Pharma; Kite, a Gilead company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; Merck & Co; Mesoblast; Millennium, the Takeda Oncology Co; Miltenyi Biotec, Inc; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc; Ossium Health, Inc; Pfizer, Inc; Pharmacyclics, LLC, an AbbVie company; PPD Development, LP; Regimmune; Sanofi; Sarah Cannon; Sobi, Inc; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc; and Xenikos BV.
Authorship
Contribution: H.S.M., M.A.K.-D., S.A., U.D., S.G., A.K., F.V.M., T.N., M.P., and W.S. conceptualized and designed the study; the Center for International Blood and Marrow Transplant Research was responsible for financial support, collection and assembly of data, and data analysis; H.S.M. and M.A.K.-D. wrote the first draft of the manuscript; and all authors were responsible for data interpretation, helped revise the manuscript, and gave final approval of the manuscript.
Conflict-of-interest disclosure: H.S.M. reports advisory board participation from CRISPR Therapeutics, Senti Biosciences, Jazz Pharmaceuticals, and Incyte Corporation. S.A. reports compensation (research funding) from Seattle Genetics, Merck, Xencor, and Tessa Therapeutics, and has a membership on Tessa Therapeutic’s advisory committee. S.G. reports advisory board participation for Bristol Myers Squibb, Sanofi, Astellas, Daiichi Sankyo, Kite Pharma, Janssen, and AstraZeneca, and speaker’s bureau participation for Seattle Genetics. T.N. reports clinical trial support (institution) from Novartis and clinical trial support (drug only supply to the institution) from Karyopharm. M.B. reports research support to institution from Novartis. M.R.G. reports receiving consulting fees from AbbVie, Amgen, Astellas, Blueprint Medicines, Bristol Myers Squibb, Cardinal Health, CTI BioPharma, Daiichi Sankyo, Gamida Cell, Genentech, Gilead, GlaxoSmithKline/Sierra Oncology, Incyte, Invitae, Jazz, Karius, Novartis, Ono Pharmaceutical, Pfizer, Pharmacosmos, Premier, Servier/Agios, and Stemline Therapeutics; research support from Incyte and Janssen; and stock ownership for Medtronic. N.K. reports honorarium from Optum for educational event. H.L. reports advisory board meeting participation for Agios, Pfizer, Nkarta, CTI Biopharm, and BeiGene; research support from Bristol Myers Squibb, Karyopharm, and Miltenyi Biotec; being a consultant for NGM BioPharma; and being a speaker/lecturer for Society of Immunotherapy of Cancer and Chinese American Hematologist and Oncologist Network. M.U. reports advisory board participation for Stemline and Gilead. N.B. reports advisory board participation or consultancy services for Magenta Therapeutics, Medexus Pharma, CTI BioPharma, CareDx and Sanofi. V.R.B. reports safety monitoring committee participation for Protagonist; consulting fees from Genentech, Rigel, Agios, Incyte, Servier Pharmaceuticals LLC, Omeros, Takeda, Partnership for Health Analytic Research, LLC (which in turn, receives funds from Jazz Pharmaceuticals), and AbbVie; research funding (institutional) from AbbVie, Pfizer, Incyte, Jazz, Tolero Pharmaceuticals, Inc, and National Marrow Donor Program; and drug support (institutional) from Oncoceutics for a trial. S.C. reports research funding (institutional) from Bristol Myers Squibb, Amgen, Janssen, Novartis, Syndax, Ionis, Sanofi, and GlaxoSmithKline, and honorarium from GlaxoSmithKline, Sanofi (advisory board), and Omeros (speaker’s bureau). E.C. reports consultative council participation for Amgen. B.D. reports research funding (institutional) from Janssen, Angiocrine, Pfizer, Poseida, MEI, and Orcabio, and consultancy/adviser for Jazz, Gamida Cell, MJH BioScience, Arivan Research, and BEAM Therapeutics. J.J. reports advisory board meeting participation for Elzonris in January 2022. M.M.K. reports being a consultant for Secura Bio (unrelated to current study). N.P. reports consultancy for AbbVie, Genentech, Takeda, and Foundation One, and research support from AbbVie, Genentech, and Incyte. D.M. reports advisory board participation for MorphoSys and Seagen; research funding from Genentech, MorphoSys, and ADC Therapeutics; and honorarium from AstraZeneca. P.N.M. reports being a consultant for Kite and speaker’s bureau participation for Incyte. A.M. reports an investigator grant from Gilead. D.A.R. reports advisory board and speaker’s bureau participation for Stemline Therapeutics. A.S. reports being a consultant for Spotlight Therapeutics in 2020, Medexus Inc in 2021, and Vertex Pharmaceuticals in 2021 and 2022; research funding from CRISPR Therapeutics in 2021 and 2022; and research collaboration with Magenta Therapeutics since 2021. C.U. reports honoraria for advisory board or speaker’s bureau participation from Blueprint and Takeda (unrelated to current study). P.K. reports compensation (advisory board participation) for Jazz, Kite, and Pfizer, and clinical trial support from Amgen and Ziopharm. C.S.H. reports a government conflict of interest. The remaining authors declare no competing financial interests.
Correspondence: Wael Saber, Division of Hematology/Oncology, Department of Medicine, Medical College of Wisconsin, 9200 W. Wisconsin Ave, Suite C5500, Milwaukee, WI 53226; e-mail: wsaber@mcw.edu.
References
Author notes
∗W.S. and M.A.K.-D. contributed equally to this work and are joint senior authors.
The Center for International Blood and Marrow Transplant Research (CIBMTR) supports accessibility of research in accord with the National Institutes of Health Data Sharing Policy and the National Cancer Institute Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases deidentified data sets that comply with all relevant global regulations regarding privacy and confidentiality.
The full-text version of this article contains a data supplement.