TO THE EDITOR:
Despite the curative potential of chimeric antigen receptor (CAR) T cells, a significant number of patients fail to respond, underlining the need for precise patient monitoring.1-3 Currently, assessing responses in real-world settings is mainly based on radiography at 1 and 3 months after CAR T-cell infusion.4-6 However, because disease progression is rapid in heavily pretreated patients, earlier detection of treatment failure would be favorable, so that salvage treatment can be initiated sooner and be more effective. Several studies have repeatedly shown the prognostic potential of CAR T-cell kinetics for the outcome of relapsed or refractory diffuse large B-cell lymphoma.7-10 Thus, bioanalytical platforms have been developed to detect CAR T cells in peripheral blood based on quantitative polymerase chain reaction (qPCR),11,12 droplet digital PCR,13,14 flow cytometry,15-17 or cell-free DNA.18 However, most of these assays are primarily intended for use in clinical trials and translational research and are unavailable for routine diagnostic applications at many treatment centers. We hypothesized that alongside imaging-based assessment, in the real-world setting, early quantification of CAR T cells in vivo with flow cytometry would enable earlier identification of patients who are nonresponding (NRs), and we initiated an immune monitoring program at 3 treatment sites in Germany and Spain.
Patients receiving third-line axicabtagene ciloleucel or tisagenlecleucel for relapsed or refractory diffuse large B-cell lymphoma at the University Hospitals of Erlangen (n = 16) and the LMU in Munich (n = 47) were retrospectively included in the training cohort, and 55 patients who were treated at the Vall d'Hebron University Hospital in Barcelona were included as validation cohort. Aliquots from rinsed CAR T-cell infusion bags and EDTA-anticoagulated peripheral blood (15 mL) were collected after infusion. CAR T cells were assessed using a 2-step staining approach with a biotinylated recombinant CD19 protein (see supplemental Material for gating strategy and flow cytometry panel). Significance was determined by Mann-Whitney U tests for unpaired, Wilcoxon tests for paired, and Fisher exact tests for categorical variables. Spearman correlation was used to assess associations between continuous variables and logistic regression as well as log-rank tests for associations between continuous variables and outcome data. Adjustment for multiplicity was performed using the Holm-Šídák method or a false-discovery approach (false discovery rate, 5%) as indicated. Because we did not aim at modeling or investigating the time trend of continuous values but rather searched for time points with maximal discrimination between groups, we did not use linear mixed-effects models. Receiver operating characteristic (ROC) analysis and the corresponding Youden index were used to identify and validate cutoff values. In addition, positive and negative predictive values of the training and validation cohorts have been plotted. A multivariate logistic regression was used to identify predictors of treatment response. Samples and clinical metadata were collected with approval by institutional review boards (LMU Munich no. 19-817, Erlangen no. 19-336_1-B, and Barcelona no. PR(AG)404/2020). The study was conducted in accordance with the Declaration of Helsinki.
Twenty-eight responding patients (Rs) and 35 NRs (Table 1) were enrolled in the training cohort. Most of the patients presented with advanced-stage disease and elevated lactic acid dehydrogenase at baseline (Table 1). Particularly, NRs presented with higher Eastern Cooperative Oncology Group (ECOG) performance status than Rs (Table 1).
Key baseline characteristics, toxicity, and survival according to treatment response based on positron emission tomographies/computed tomographies on day 90 of the training cohort
| Variable . | All (n = 63) . | Rs (n = 28) . | NRs (n = 35) . | Univariate . |
|---|---|---|---|---|
| Age, median (range in y) | 64 (19-83) | 64.5 (36-83) | 60 (19-80) | .08 |
| Sex, male, n (%) | 38 (60) | 17 (61) | 21 (60) | >.99 |
| ECOG, 0 to 1, n (%) | 45 (71) | 25 (89) | 20 (57) | .01 |
| Treatment history, prior therapies, median number (range) | 3 (2-9) | 3.5 (2-5) | 2 (2-9) | .07 |
| Tumor burden | ||||
| Tumor volume, median, cm3 (range) | 56 (0-1380.5) | 33.5 (0-528.7) | 92 (0-1380.5) | .06 |
| Ann Arbor stage III-IV, n (%) | 49 (78) | 20 (71) | 29 (83) | .36 |
| LDH > ULN before lymphodepletion, n (%) | 39 (62) | 14 (50) | 25 (71) | .12 |
| CAR product | ||||
| Tisa-cel, n (%) | 40 (64) | 15 (54) | 25 (71) | .19 |
| Axi-cel, n (%) | 23 (36) | 13 (46) | 10 (29) | |
| CRS, grade ≥2, n (%) | 25 (40) | 11 (39) | 14 (40) | >.99 |
| ICANS, grade ≥2, n (%) | 12 (19) | 6 (21) | 6 (17) | .75 |
| Toxicity management, steroid administration, d 0 to 7, n (%) | 25 (40) | 12 (43) | 13 (37) | .59 |
| Survival | ||||
| PFS, median (range in mo) | 3.0 (0.6-35.3) | Not reached (1.7-35.3) | 1.3 (0.6-4.1) | <.0001 |
| OS, median (range in mo) | 12.1 (0.2-35.3) | Not reached (1.7-35.3) | 4.1 (0.2-22.2) | <.0001 |
| Variable . | All (n = 63) . | Rs (n = 28) . | NRs (n = 35) . | Univariate . |
|---|---|---|---|---|
| Age, median (range in y) | 64 (19-83) | 64.5 (36-83) | 60 (19-80) | .08 |
| Sex, male, n (%) | 38 (60) | 17 (61) | 21 (60) | >.99 |
| ECOG, 0 to 1, n (%) | 45 (71) | 25 (89) | 20 (57) | .01 |
| Treatment history, prior therapies, median number (range) | 3 (2-9) | 3.5 (2-5) | 2 (2-9) | .07 |
| Tumor burden | ||||
| Tumor volume, median, cm3 (range) | 56 (0-1380.5) | 33.5 (0-528.7) | 92 (0-1380.5) | .06 |
| Ann Arbor stage III-IV, n (%) | 49 (78) | 20 (71) | 29 (83) | .36 |
| LDH > ULN before lymphodepletion, n (%) | 39 (62) | 14 (50) | 25 (71) | .12 |
| CAR product | ||||
| Tisa-cel, n (%) | 40 (64) | 15 (54) | 25 (71) | .19 |
| Axi-cel, n (%) | 23 (36) | 13 (46) | 10 (29) | |
| CRS, grade ≥2, n (%) | 25 (40) | 11 (39) | 14 (40) | >.99 |
| ICANS, grade ≥2, n (%) | 12 (19) | 6 (21) | 6 (17) | .75 |
| Toxicity management, steroid administration, d 0 to 7, n (%) | 25 (40) | 12 (43) | 13 (37) | .59 |
| Survival | ||||
| PFS, median (range in mo) | 3.0 (0.6-35.3) | Not reached (1.7-35.3) | 1.3 (0.6-4.1) | <.0001 |
| OS, median (range in mo) | 12.1 (0.2-35.3) | Not reached (1.7-35.3) | 4.1 (0.2-22.2) | <.0001 |
ASTCT, American Society for Transplantation and Cellular Therapy, Axi-cel, axicabtagene ciloleucel; CRS, cytokine release syndrome; ICANS, immune-effector cell neurotoxicity syndrome; LDH, lactic acid dehydrogenase; mo, months; OS, overall survival; PFS, progression-free survival; tisa-cel, tisagenlecleucel; ULN, upper limit of normal.
Response was assessed by positron emission tomography/computed tomographies 3 months after infusion. Tumor volume was manually segmented at baseline for up to 6 target lesions according to Lugano criteria and is reported in cubic centimeters. ULN for LDH was 240 U/L (LMU) or 220 U/L (Erlangen). Toxicity was graded according to the ASTCT consensus grading of Lee et al, 2019.23P values were calculated using the Mann-Whitney U test for continuous or Fisher exact test for categorical variables and log-rank testing for survival data. Values in bold indicate significant P = 0.05.
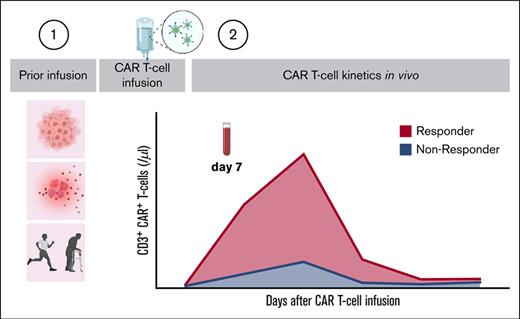
By using ROC analysis, we primarily investigated whether CAR T-cell dose levels at specific time points or kinetic variables such as area under the curve, peak expansion, or effector-to-target ratio qualify as a biomarker for response to treatment (Figure 1A-F; supplemental Figure 2). Notably, CAR T cells on day 7 displayed the highest level of significance (Figure 2B) as well as the highest area under the curve of the ROC curve (Figure 1G). We identified 19 CAR T cells per μL blood on day 7 with the highest Youden index as a cutoff value for discriminating between Rs and NRs (Figure 1G-H). Furthermore, we found an association between CAR T-cell levels on day 7 and early response to treatment on day 30 (supplemental Figure 3A-C) as well as survival (supplemental Figure 3F-G).
Early CAR T-cell expansion predicted early and late treatment response and was associated with survival. (A) Median absolute dose as documented in the certificate of analysis (tisagenlecleucel [tisa-cel]) or in the prescribing information (axicabtagene ciloleucel [axi-cel]), comparing Rs (n = 28) and NRs (n = 35). (B) Median absolute CAR T-cell expansion in the peripheral blood, comparing Rs and NRs. Only the significant P values from Mann-Whitney U tests are shown. If corrected for multiple comparisons using the Holm–Šídák method (α = 0.05), only the P values for days 7 and 14 remain. Number of Rs at specific time points: day 4, n = 22; day 7, n = 24; day 14, n = 26; day 28, n = 24; day 60, n = 18; and day 90, n = 22. Number of NRs at specific time points: day 4, n = 24; day 7, n = 32; day 14, n = 32; day 28, n = 24; day 60, n = 17; and day 90, n = 16. (C-D) Absolute CAR T-cell frequencies estimated over time as median area under the curve (AUC) (C) and median peak expansion (D) between days 0 and 90 according to treatment response on day 90 in Rs compared with those in NRs. P values from Mann-Whitney U tests are shown. AUC: R, n = 28; NR, n = 33. Peak: R, n = 28; NR, n = 35. Effector-to-target ratios were estimated as the ratio of CAR T-cell peak expansion (per μl) per tumor volume (cm3). (E) Effector-to-target ratios were estimated by dividing peak expansion by tumor volume. Median effector-to-target ratios according to the treatment response on day 90 in Rs compared with that in NRs. The P value from an unpaired t test is shown. R, n = 28; NR, n = 35. (F) Association of absolute number of CAR T cells on day 7 with treatment response on day 90. The P value from a logistic regression analysis is shown. (G) Association of absolute number of CAR T cells on day 7 and response to treatment on day 90. The P value from ROC is shown. (H) Proportion of high or low CAR T-cell expansion according to the cutoff value on day 7 among Rs and NRs. Relative frequency of patients , the P value from Fisher exact test, positive predictive values (PPV) and negative predictive values (NPV) are shown. (I-K) Association of absolute number of CAR T cells on day 7 and treatment response on day 90 in the validation cohort. P values from Mann-Whitney U tests (I), logistic regression analysis (J), and ROC analysis (K) are shown. R, n = 36; NR, n = 19. (L) Proportion of high or low CAR T-cell expansion according to the cutoff value on day 7 among Rs and NRs in the validation cohort. Relative frequency of patients and P values from Fisher exact tests are shown. (M) Corrplot of baseline parameters with CAR T-cell kinetics; n = 104. The size of the Spearman correlation coefficient r is represented by circle color and size. Only the significant correlations after adjusting P values for multiple comparisons using a false-discovery approach with the 2-stage step-up method of Benjamini, Krieger, and Yekutieli (false discovery rate, 5%) are shown. (N) Multivariate analysis of clinical characteristics of advanced-stage disease and inflammation at baseline obtained on the day of lymphodepletion, as well as administered CAR T-cell product and CAR T-cell levels on day 7 with treatment response on day 90 after infusion; n = 104. A forward stepwise logistic regression was used to identify possible predictors out of the following candidate variables: number of prior therapies, ECOG, Ann Arbor stage, lactic acid dehydrogenase (LDH) level, C-reactive protein (CRP), ferritin level, CAR T-cell product, and CAR T-cell levels on day 7. The odds ratio, 95% confidence intervals, and P values from a logistic regression analysis are shown. Range of time points is provided in supplemental Figure 1.
Early CAR T-cell expansion predicted early and late treatment response and was associated with survival. (A) Median absolute dose as documented in the certificate of analysis (tisagenlecleucel [tisa-cel]) or in the prescribing information (axicabtagene ciloleucel [axi-cel]), comparing Rs (n = 28) and NRs (n = 35). (B) Median absolute CAR T-cell expansion in the peripheral blood, comparing Rs and NRs. Only the significant P values from Mann-Whitney U tests are shown. If corrected for multiple comparisons using the Holm–Šídák method (α = 0.05), only the P values for days 7 and 14 remain. Number of Rs at specific time points: day 4, n = 22; day 7, n = 24; day 14, n = 26; day 28, n = 24; day 60, n = 18; and day 90, n = 22. Number of NRs at specific time points: day 4, n = 24; day 7, n = 32; day 14, n = 32; day 28, n = 24; day 60, n = 17; and day 90, n = 16. (C-D) Absolute CAR T-cell frequencies estimated over time as median area under the curve (AUC) (C) and median peak expansion (D) between days 0 and 90 according to treatment response on day 90 in Rs compared with those in NRs. P values from Mann-Whitney U tests are shown. AUC: R, n = 28; NR, n = 33. Peak: R, n = 28; NR, n = 35. Effector-to-target ratios were estimated as the ratio of CAR T-cell peak expansion (per μl) per tumor volume (cm3). (E) Effector-to-target ratios were estimated by dividing peak expansion by tumor volume. Median effector-to-target ratios according to the treatment response on day 90 in Rs compared with that in NRs. The P value from an unpaired t test is shown. R, n = 28; NR, n = 35. (F) Association of absolute number of CAR T cells on day 7 with treatment response on day 90. The P value from a logistic regression analysis is shown. (G) Association of absolute number of CAR T cells on day 7 and response to treatment on day 90. The P value from ROC is shown. (H) Proportion of high or low CAR T-cell expansion according to the cutoff value on day 7 among Rs and NRs. Relative frequency of patients , the P value from Fisher exact test, positive predictive values (PPV) and negative predictive values (NPV) are shown. (I-K) Association of absolute number of CAR T cells on day 7 and treatment response on day 90 in the validation cohort. P values from Mann-Whitney U tests (I), logistic regression analysis (J), and ROC analysis (K) are shown. R, n = 36; NR, n = 19. (L) Proportion of high or low CAR T-cell expansion according to the cutoff value on day 7 among Rs and NRs in the validation cohort. Relative frequency of patients and P values from Fisher exact tests are shown. (M) Corrplot of baseline parameters with CAR T-cell kinetics; n = 104. The size of the Spearman correlation coefficient r is represented by circle color and size. Only the significant correlations after adjusting P values for multiple comparisons using a false-discovery approach with the 2-stage step-up method of Benjamini, Krieger, and Yekutieli (false discovery rate, 5%) are shown. (N) Multivariate analysis of clinical characteristics of advanced-stage disease and inflammation at baseline obtained on the day of lymphodepletion, as well as administered CAR T-cell product and CAR T-cell levels on day 7 with treatment response on day 90 after infusion; n = 104. A forward stepwise logistic regression was used to identify possible predictors out of the following candidate variables: number of prior therapies, ECOG, Ann Arbor stage, lactic acid dehydrogenase (LDH) level, C-reactive protein (CRP), ferritin level, CAR T-cell product, and CAR T-cell levels on day 7. The odds ratio, 95% confidence intervals, and P values from a logistic regression analysis are shown. Range of time points is provided in supplemental Figure 1.
Confirmatory, we asked whether CAR T cells in patients below the cutoff on day 7 might expand at a later time point and still affect efficacy. Indeed, we found higher fold changes of CAR T-cell levels from day 7 to day 14 in the low-expansion group, suggesting ongoing CAR T-cell expansion (supplemental Figure 3D). However, the fold change did not differ between NRs and Rs, supporting our previous finding that very early CAR T-cell expansion has an impact on response to treatment (supplemental Figure 3E).
We were able to validate the cutoff value on day 7 in an independent cohort (Figure 1I-J; supplemental Table 2). ROC analysis revealed the same sensitivity for the cutoff value in the validation cohort compared with that in the training cohort with a decreased specificity, and Fisher exact test revealed similar predictive values (Figure 1K-L; supplemental Figure 4A). Again, log-rank tests demonstrated longer survival in the high- than in the low-expansion groups (supplemental Figure 4B-C).
Consistent with other studies, we found that parameters describing high disease burden (ECOG performance status1,2 and lactic acid dehydrogenase1,2) or baseline inflammation (CRP19,20 and ferritin21) were significantly increased in NRs (supplemental Figure 5A), in both the training and the validation cohorts. Exploratory, we found that ECOG performance status and ferritin levels at baseline were negatively associated with CAR T-cell levels on day 7 (Figure 1M). Notably, CAR T-cell levels on day 7 greater than the cutoff value remained a significant variable for response to treatment in a multivariate analysis of the entire cohort (Figure 4N).
A deeper understanding of resistance to CAR T-cell treatment is a prerequisite for the early identification of patients not benefiting from anti-CD19 CAR T-cell products and for guiding the selection of the most powerful salvage therapy for the individual. Our results demonstrate that early quantification of CAR T cells, especially a cutoff value of 19 CAR T cells per μL blood on day 7, is a strong predictor of treatment response and patient survival in training and validation cohorts. However, some patients still respond despite having day-7 CAR T-cell levels below the cutoff value. This advocates for an early and non–T-cell–toxic salvage treatment, such as lenalidomide or immune checkpoint inhibitors, in the low-expansion group that preserves CAR T-cell expansion.
In contrast to various other predictive markers of early treatment failure, especially qPCR techniques, this assay has several strengths: (1) assessment shortly after CAR T-cell infusion allows for rescue treatments to begin earlier; (2) fast turnaround and real-time analysis with a readily reproducible protocol make these assays readily accessible in all treatment centers; and (3) expression of the CAR is continuously monitored, particularly in terms of the combined characterization of CD4 and CD8 subsets, coexpression profiles for cell differentiation, and immune checkpoints.
Although the sensitivity of flow cytometry is lower than that of qPCR, this does not affect the reported cutoff value of 19 CAR T cells per μL blood because this is clearly above the detection limit of the flow cytometry assay (1:2000; minimum number of CAR+ events, 0.05% or at least 20 events). Furthermore, concurrent assessment of CAR T-cell levels with flow cytometry and qPCR revealed comparative results.15,22
Despite the commercialization of CAR T cells, with numerous others expected to be approved in the future, patient-centered monitoring of CAR T-cell kinetics outside of clinical trials has largely been overlooked. Our proposed flow-based assay would enable treatment failure to be detected earlier, in contrast to assessment by radiography. These findings can accelerate the widespread clinical application of thus far limited real-time immune monitoring approaches and support the development of personalized dynamic risk-profiling strategies for guiding decisions on subsequent therapies.
Acknowledgments: The authors thank Sabine Sandner-Thiede, Simone Pentz, Elke Habben, Ewelina Zientara, Bianca Kirschbaum (University Hospital, LMU Munich), Luisa Albert, Lena Tarantik, and Dorothea Gebhardt (University Hospital Erlangen) for their outstanding technical support. The authors also acknowledge the iFlow Core Facility of the University Hospital, LMU Munich (INST 409/225-1 FUGG), and the Core Unit Cell Sorting and Immunomonitoring Erlangen (Florentine Schonath) for assistance with generating flow cytometry data.
The work was supported by a German Research Council research grant provided within the Sonderforschungsbereich Transregio SFB-TRR 388/1 2021 452881907, and German Research Council research grant 451580403 (M.S.). The work was further supported by the Bavarian Elite Graduate Training Network (M.S. and G.B.), the Wilhelm Sander-Stiftung (M.S.) (project no. 2018.087.1), the Else Kröner-Fresenius-Stiftung (V.B., K.R., V.L.B., and M.S.), the German Cancer Consortium (V.B.), the German Cancer Aid (F.M.) (grant 70113695), the Interdisciplinary Center for Clinical Research at the University Hospital of the University of Erlangen-Nuremberg (S.V.) (project no. D43), and the Bavarian Center for Cancer Research.
Contribution: V.B., V.L.B., A.M., S.V., and M.S. designed the research; V.B., G.B., S.B., R.J., B.T., V.L.B., and S.V. performed the research; V.B., G.B., S.B., R.J., M.W., K.H., C. Schmidt, L.F., F.H., K.R., F.D., C. Schmidkonz, T.B., W.G.K., F.M., V.L.B., and S.V. collected samples and data; V.B., G.B., S.B., D.M., M.v.B.-B., V.L.B., A.M., S.V., and M.S. analyzed and interpreted the data; V.B., S.V., and M.S. wrote the manuscript; and all the authors revised the manuscript.
Conflict-of-interest disclosure: V.B. receives research funding from Bristol Myers Squibb (BMS)/Celgene, Kite/Gilead, Janssen, Novartis, Roche, and Takeda and research funding and honoraria/consultancy fee from Kite/Gilead, Janssen, and Novartis. G.I. receives honoraria/consultancy fee from AbbVie, AstraZeneca, BMS, Kite/Gilead, Janssen, Miltenyi, Novartis, Roche, and Sandoz. K.R. receives research funding and travel support from Kite/Gilead and honoraria from Novartis. F.M. receives honoraria and travel support from BMS, Kite/Gilead, and Novartis. P.B. receives honoraria from Allogene, Amgen, BMS, Kite/Gilead, Janssen, Jazz Pharmaceuticals, Miltenyi, Novartis, and Nektar. M.v.B.-B. receives research funding, honoraria, and consultancy fee from Astellas, BMS, Kite/Gilead, Miltenyi, Mologen, Merck Sharp & Dohme, Novartis, and Roche. V.L.B. reports research funding and/or honoraria from Amgen, Celgene/BMS, Kite/Gilead, Novartis, and Pfizer. M.S. reports research funding, speaker’s bureau fee, and/or consultancy fee from Amgen, AstraZeneca, Aven Cell, BMS/Celgene, CDR-Life, Kite/Gilead, GlaxoSmithKline, Ichnos Sciences, Incyte Biosciences, Janssen, Miltenyi Biotec, Molecular Partners, Novartis, Pfizer, Roche, Seattle Genetics, and Takeda. None of the declared conflicts of interest are related to the financing of the content of this manuscript. The remaining authors declare no conflicting financial interest.
Correspondence: Viktoria Blumenberg, Department of Medicine III, University Hospital LMU Munich, Marchioninistrasse 15, 81377 Munich, Germany; e-mail: viktoria.blumenberg@med.uni-muenchen.de; Simon Völkl, Department of Internal Medicine 5, University Hospital Erlangen, Schwabachanlage 12, 91054 Erlangen, Germany; e-mail: Simon.Voelkl@uk-erlangen.de; and Marion Subklewe, Department of Medicine III, University Hospital LMU Munich, Marchioninistrasse 15, 81377 Munich, Germany; e-mail: marion.subklewe@med.uni-muenchen.de.
References
Author notes
∗S.V. and M.S. contributed equally to this work.
Original data are available on request from the corresponding authors, Viktoria Blumenberg (viktoria.blumenberg@med.uni-muenchen.de), Simon Völkl (simon.voelkl@uk-erlangen.de), and Marion Subklewe (marion.subklewe@med.uni-muenchen.de).
The full-text version of this article contains a data supplement.

![Early CAR T-cell expansion predicted early and late treatment response and was associated with survival. (A) Median absolute dose as documented in the certificate of analysis (tisagenlecleucel [tisa-cel]) or in the prescribing information (axicabtagene ciloleucel [axi-cel]), comparing Rs (n = 28) and NRs (n = 35). (B) Median absolute CAR T-cell expansion in the peripheral blood, comparing Rs and NRs. Only the significant P values from Mann-Whitney U tests are shown. If corrected for multiple comparisons using the Holm–Šídák method (α = 0.05), only the P values for days 7 and 14 remain. Number of Rs at specific time points: day 4, n = 22; day 7, n = 24; day 14, n = 26; day 28, n = 24; day 60, n = 18; and day 90, n = 22. Number of NRs at specific time points: day 4, n = 24; day 7, n = 32; day 14, n = 32; day 28, n = 24; day 60, n = 17; and day 90, n = 16. (C-D) Absolute CAR T-cell frequencies estimated over time as median area under the curve (AUC) (C) and median peak expansion (D) between days 0 and 90 according to treatment response on day 90 in Rs compared with those in NRs. P values from Mann-Whitney U tests are shown. AUC: R, n = 28; NR, n = 33. Peak: R, n = 28; NR, n = 35. Effector-to-target ratios were estimated as the ratio of CAR T-cell peak expansion (per μl) per tumor volume (cm3). (E) Effector-to-target ratios were estimated by dividing peak expansion by tumor volume. Median effector-to-target ratios according to the treatment response on day 90 in Rs compared with that in NRs. The P value from an unpaired t test is shown. R, n = 28; NR, n = 35. (F) Association of absolute number of CAR T cells on day 7 with treatment response on day 90. The P value from a logistic regression analysis is shown. (G) Association of absolute number of CAR T cells on day 7 and response to treatment on day 90. The P value from ROC is shown. (H) Proportion of high or low CAR T-cell expansion according to the cutoff value on day 7 among Rs and NRs. Relative frequency of patients , the P value from Fisher exact test, positive predictive values (PPV) and negative predictive values (NPV) are shown. (I-K) Association of absolute number of CAR T cells on day 7 and treatment response on day 90 in the validation cohort. P values from Mann-Whitney U tests (I), logistic regression analysis (J), and ROC analysis (K) are shown. R, n = 36; NR, n = 19. (L) Proportion of high or low CAR T-cell expansion according to the cutoff value on day 7 among Rs and NRs in the validation cohort. Relative frequency of patients and P values from Fisher exact tests are shown. (M) Corrplot of baseline parameters with CAR T-cell kinetics; n = 104. The size of the Spearman correlation coefficient r is represented by circle color and size. Only the significant correlations after adjusting P values for multiple comparisons using a false-discovery approach with the 2-stage step-up method of Benjamini, Krieger, and Yekutieli (false discovery rate, 5%) are shown. (N) Multivariate analysis of clinical characteristics of advanced-stage disease and inflammation at baseline obtained on the day of lymphodepletion, as well as administered CAR T-cell product and CAR T-cell levels on day 7 with treatment response on day 90 after infusion; n = 104. A forward stepwise logistic regression was used to identify possible predictors out of the following candidate variables: number of prior therapies, ECOG, Ann Arbor stage, lactic acid dehydrogenase (LDH) level, C-reactive protein (CRP), ferritin level, CAR T-cell product, and CAR T-cell levels on day 7. The odds ratio, 95% confidence intervals, and P values from a logistic regression analysis are shown. Range of time points is provided in supplemental Figure 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/22/10.1182_bloodadvances.2023010364/3/m_blooda_adv-2023-010364-gr1.jpeg?Expires=1767709829&Signature=pEgLP80YOC14oaW1799nmj8P~UQBr6WIa2PVs4EZvj5Rms9CHHbIlM5RgPaSRoC4E9usqeRCBHSJBTQ4TiYvAmFirsb2c0gjFu6-G6GLZm~oFQN31MuJ07LVjITBmnzNleXO-D-22~oJNOEdNbtnBe9MdCixvsC5TEsBmQr-lDfx-Hc5l1Rpeqv1X3q2mf1fq3AvXaqYX-llMZsGqViNKunWvgxuUd1Yv~1~sKjjE68k4RBaI33nzFFig3ZRNtl5~7RNbgFYW8wheBxjzwndIhZLvSsLbEKmbRCpxaFrlW1fU1DEBnCSOB2VQrIAwL8zcAGeI1ycgR~DhpJe9m68RA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)