Key Points
For patients with Sezary syndrome (SS), first-line combination therapies have greater time-to-next-treatment (TTNT) than monotherapies.
Extracorporeal photopheresis is associated with good TTNT benefit, particularly when delivered in the first line.
Abstract
Despite increasing availability of therapies, patients with Sezary syndrome (SS) commonly endure multi-line treatment journeys, mostly with partial responses of short duration. Measuring clinical benefit is challenging; time-to-next-treatment (TTNT) provides a robust, objective measurement of efficacy. This international observational study examines patterns of clinical care and therapeutic benefit as measured by TTNT. TTNT was calculated for monotherapies and combination therapies, with consideration to treatment line. 178 patients with SS (73% de novo, 27% secondary) were included, receiving 721 lines of systemic therapy, with median follow-up of 56.9 months. Across all lines, 58 different therapeutic regimens were prescribed (54 were systemic therapies) and classified into 17 treatment groups. The most common first-line treatments were extracorporeal photopheresis (ECP)–containing combination therapy (20%) and retinoid monotherapy (19%). Median TTNT for all first-line therapies was short (5.4 months). First-line, combination therapies had longer median TTNT than monotherapies, 10.0 vs 5.0 months (P = .004), respectively. Later delivery of combination therapies was associated with shorter clinical benefit, with median TTNT reduced to 6.2 and 2.2 months for mid-line (2nd-4th line) and late-line (≥5th line), respectively (P < .001). First-line ECP-containing treatments were associated with longer median TTNT than non-ECP–containing treatments, 9.0 vs 4.9 months (P = .007). For both ECP-monotherapy and ECP–containing combination therapy, significant reductions in TTNT were seen in later lines. These data suggest therapeutic benefit from first-line delivery of combination therapy for SS and favor early inclusion of ECP in the treatment algorithm for those who can access it.
Introduction
Sezary syndrome (SS) is a distinctive erythrodermic cutaneous T–cell lymphoma (CTCL) characterized by a clonal proliferation of leukemic malignant T-cells that matches the clone in the skin. Usually diagnosed de novo, SS may also arise as a continual progression from mycosis fungoides (MF) with blood involvement. SS is rare and associated with limited treatment options with durable effectiveness, reduced health-related quality of life1 and poor survival. Management of MF or SS is based on the stage of disease; international and national guidelines list a number of treatment options to be considered first-line, but with neither particular order of preference nor for subsequent therapies to be considered thereafter.2-8
Most treatments achieve only partial responses of short duration and lack curative potential, with only a minority achieving complete or near complete responses. Only selected patients may be suitable for allogeneic stem cell transplant (alloSCT).9 Thus, for the majority of patients, management represents a multiline treatment journey without durable remissions. Because there is no clear consensus on the optimal treatment algorithm,2-7 individual treatment pathways are highly variable among centers.10,11 To date, there is only 1 randomized clinical trial specifically for relapsed or refractory SS or advanced MF, the MAVORIC study12 compared mogamulizumab with vorinostat, and in a recently published post hoc analysis, a clear benefit in time-to-next-treatment (TTNT) was observed for patients with B2 blood involvement receiving mogamulizumab (median TTNT, 13.1 vs 3.5 months; P < .0001).13 However, beyond this, there is a paucity of prospective comparative data examining treatment efficacy for patients with SS.
TTNT provides a reliable, simple, patient-centric, surrogate end point for duration of clinical benefit, with established use in CTCL.13-26 TTNT encompasses both the clinical response and tolerability of the treatment as experienced by the patient; it is a particularly useful end point to measure the durability of clinical benefit of treatments in the real-world setting, providing an objective measure of therapeutic benefit that may be applied retrospectively.27,28 TTNT is defined as the time from commencement of 1 therapy to the commencement of the subsequent line of therapy. Uniquely, TTNT encompasses not only the duration of disease control across all disease compartments, but also the tolerability of treatment-induced toxicities and patient adherence to treatment. Treatments for SS are typically associated with disappointingly short TTNT.13-18,20,21
In this international, retrospective study from 3 quaternary centers, we investigate the current therapeutic management strategies for SS, with the aim of better informing the future management of such patients. Firstly, we describe the patterns of care for patients, spanning international clinical practice. Secondly, we investigate and compare the TTNT for monotherapy vs combination systemic therapies including extracorporeal photopheresis (ECP), monoclonal antibody–based monotherapies and alloSCT. Thirdly, we assess the impact of treatment line (while examining monotherapy and combination therapies) on the duration of clinical benefit.
Methods
Eligibility
Three quaternary centers participated in this ethics-approved, retrospective study: University Hospitals Birmingham (Birmingham, United Kingdom), Peter MacCallum Cancer Centre (Melbourne, Australia), and Hôpital Saint Louis (Paris, France). Eligibility required clinicopathological diagnoses of MF or SS with B2 blood involvement according to International Society for Cutaneous Lymphomas (ISCL) or European Organization of Research and Treatment of Cancer (EORTC) definition,29,30 and newly diagnosed between 1 January 2012 and 31 December 2020. Included in this cohort were patients diagnosed with SS de novo, SS preceded by MF (secondary SS), and MF with leukemic (B2) involvement.29,31 Exclusion criteria included non-Hodgkin lymphomas with secondary cutaneous involvement. Patients were managed as per each center’s SS treatment guidelines. Medical records of eligible patients were retrospectively reviewed for information regarding demographics, clinicopathological features, treatment details including commencement dates and patient outcomes. Blood involvement was retrospectively confirmed and classified according to ISCL/EORTC definition.29,30
Treatment groups
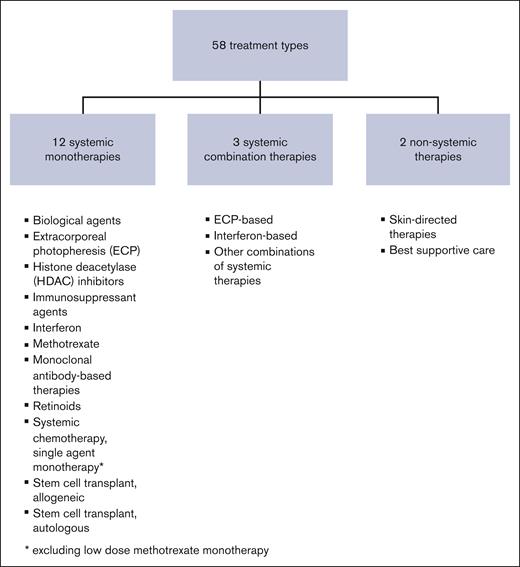
Treatments were classified into systemic monotherapies, systemic combination therapies, and non-systemic therapies (Figure 1) The systemic monotherapies were subdivided into following 12 treatment groups: biological agents, ECP, histone deacetylase inhibitors, immunosuppressant agents, interferon, methotrexate, monoclonal antibody–based therapies, multi-agent chemotherapy, retinoids, single-agent chemotherapy, and stem cell transplantations (SCT), alloSCT and autologous (auSCT) (supplemental Table 1). The systemic combination therapies were subdivided into following 3 groups: ECP-based, interferon-based, and other combinations. Non-systemic treatments included skin-directed therapies including ultraviolet B, psoralen ultraviolent A, total skin electron therapy, and supportive care only. For purposes of this study, topical corticosteroids and localized radiotherapy were not considered as separate treatment courses.
TTNT
TTNT was used as the end point to evaluate time-to-treatment failure and duration of clinical benefit. TTNT was measured from the date of commencing 1 line of treatment to the commencement date of the next treatment course, using previously published definitions.28 To mitigate against false enhancement of TTNT of the final line of therapy, particularly when the end-of-life phase is protracted, commencement of end-of-life or supportive care was included as a TTNT event.28 For all other patients not receiving a subsequent line of treatment, TTNT was censored at the date of death or last follow-up.
Monotherapies and combination therapies were analyzed separately. Where another systemic therapy was added to an existing treatment to improve disease control, a TTNT event was triggered for the existing monotherapy and represented commencement of a new line of treatment as a combination therapy.28
Statistics
The Kaplan-Meier method was used to estimate time-to-event analyses; patients were censored at death or date they were last seen if no further treatment was given. Median follow-up was calculated by reverse Kaplan-Meier. Treatments were trichotomized into first-line therapy, mid-line (defined as second to fourth lines) and late-line (defined as fifth line or greater). Fisher 2-sided exact test was used to test associations with prescribing patterns. The log-rank test was used to test associations with TTNT. Statistical analyses were performed in Stata 15.1 SE. A 2-tailed P value of .05 was considered significant.
Results
Patient characteristics and treatment lines
A total of 178 patients were eligible, of which 77 (43%) were female, with median age at diagnosis of 66 years (range, 17-94) (Table 1). On retrospective review, B2 blood involvement could be verified in all but 9 patients; of the remaining 169 patients, stage at diagnosis was stage IVA1 in 139 (82.3%), stage IVA2 in 26 (15.4%), and stage IVB in 4 (2.4%). Of the whole cohort of 178 patients, 130 (73.0%) were diagnosed with SS de novo. Forty-eight (27.0%) patients had SS preceded by MF, with median interval of 3.4 years and median number of 2 lines of MF treatment before diagnosis of B2 blood involvement. Six (3.4%) patients had documented suberythroderma at time of diagnosis with B2 blood involvement. Thirty-five (20.0%) patients had a synchronous or preexisting diagnosis of large cell transformation on skin histology. The median follow-up for all patients was 56.9 months (95% confidence interval [CI], 48.5-62.0).
Patient characteristics
| Patient characteristics . | N = 178 (%) . |
|---|---|
| Female | 77 (43.3) |
| Age (y), median (range) | 66 (17-94) |
| Stage at diagnosis∗ | |
| Stage IVA1 | 139 (82.3) |
| Stage IVA2 | 26 (15.4) |
| Stage IVB | 4 (2.4) |
| Coexistent large cell transformation at time of diagnosis with SS | 35 (20.0) |
| Prior history of MF without B2 blood involvement | 48 (27.0) |
| Number of prior lines of therapy for MF, median (range) | 2 (0-9) |
| Number of treatment lines for SS, median (range) | 4 (1-16) |
| Number of lines of systemic therapies for SS, median (range) | 3 (0-14) |
| Patient characteristics . | N = 178 (%) . |
|---|---|
| Female | 77 (43.3) |
| Age (y), median (range) | 66 (17-94) |
| Stage at diagnosis∗ | |
| Stage IVA1 | 139 (82.3) |
| Stage IVA2 | 26 (15.4) |
| Stage IVB | 4 (2.4) |
| Coexistent large cell transformation at time of diagnosis with SS | 35 (20.0) |
| Prior history of MF without B2 blood involvement | 48 (27.0) |
| Number of prior lines of therapy for MF, median (range) | 2 (0-9) |
| Number of treatment lines for SS, median (range) | 4 (1-16) |
| Number of lines of systemic therapies for SS, median (range) | 3 (0-14) |
Excludes 9 patients with documented SS but unable to retrospectively verify B stage.
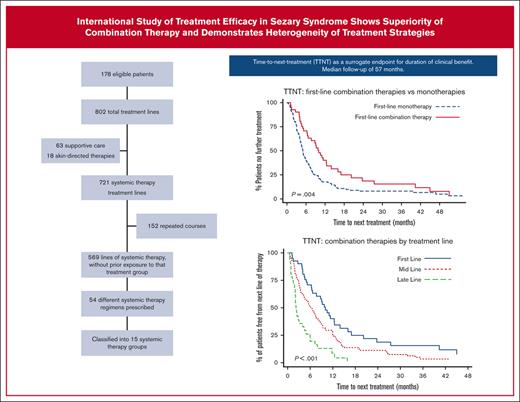
A total of 802 treatment lines were delivered (supplemental Figure 1). Of these, 63 treatment lines were supportive care only, and 18 were non-systemic, skin-directed treatments. The remaining 721 treatment lines were systemic therapies, of which 569 treatment lines of systemic therapy were administered without previous exposure to that particular treatment group, and 152 were repeated courses within the same treatment group. The median number of total treatment lines for B2 blood involvement was 4 (range, 1-16) with a median of 3 (range, 0-14) lines of systemic therapy received.
First-line setting: patterns of care and TTNT
In the first-line setting, there were 27 different therapeutic regimens prescribed, which were classified into 13 distinct treatment groups (supplemental Tables 1 and 2). Of these, the 2 most common first-line treatments were ECP–based combination therapy (36 patients, 20.2%) and retinoids (33 patients, 18.5%) (Table 2). Of these 36 ECP–based combination therapies, the most common were ECP-interferon in 13 (36.1%) and ECP-retinoid (bexarotene) in 10 (27.8%). Nine patients did not receive systemic therapy first-line (skin-directed treatments only, 4; supportive care only, 5) and were excluded from subsequent TTNT analyses of first-line systemic therapies.
First-line treatment groups (n = 178), with the TTNT after first-line systemic therapies (n = 169)
| First-line treatment groups . | N = 178 first-line treatments (%) . | Median TTNT (months) for first-line systemic therapies (95% CI) . | % free from next line of treatment at 1 year (95% CI) . | % free from next line of treatment at 2 years (95% CI) . |
|---|---|---|---|---|
| Monotherapies | ||||
| Extracorporeal photopheresis (ECP) monotherapy | 16 (9.0) | 8.0 (2.0-10.7) | 20.8 (5.2-43.6) | 20.8 (5.2-43.6) |
| Histone deacetylase (HDAC) inhibitor monotherapy | 4 (2.3) | 3.6 | 25 (0.9-66.5) | 25 (0.9-66.5) |
| Interferon monotherapy | 21 (11.8) | 4.8 (3.0-7.4) | 14.3 (3.6-32.1) | 4.8 (0.3-19.7) |
| Methotrexate monotherapy | 28 (15.7) | 5.0 (3.0-7.4) | 26.8 (12.0-44.1) | 15.3 (4.9-31.2) |
| Monoclonal antibody–based monotherapy∗ | 4 (2.3) | 2.0 | 0 | 0 |
| Retinoid monotherapy | 33 (18.5) | 4.4 (3.2-7.7) | 24.2 (11.4-39.6) | 6.1 (1.1-17.6) |
| Systemic chemotherapy, multiagent monotherapy | 8 (4.5) | 4.7 (0.5-6.0) | 0 | 0 |
| Systemic chemotherapy, single-agent monotherapy† | 14 (7.9) | 4.9 (2.4-7.0) | 0 | 0 |
| Combination therapies | ||||
| ECP–based combination therapy | 36 (20.2) | 9.8 (6.3-12.5) | 40.1 (24.0-55.8) | 14.9 (40.9-30.0) |
| Interferon–based combination therapy | 4 (2.3) | 9.2 | 50.0 (5.8-84.5) | 50.0 (5.8-84.5) |
| Other combinations of systemic therapies | 1 (0.6) | — | 0 | 0 |
| Non-systemic therapies | ||||
| Skin–directed monotherapy only | 4 (2.3) | — | — | — |
| Supportive care only | 5 (2.8) | — | — | — |
| First-line treatment groups . | N = 178 first-line treatments (%) . | Median TTNT (months) for first-line systemic therapies (95% CI) . | % free from next line of treatment at 1 year (95% CI) . | % free from next line of treatment at 2 years (95% CI) . |
|---|---|---|---|---|
| Monotherapies | ||||
| Extracorporeal photopheresis (ECP) monotherapy | 16 (9.0) | 8.0 (2.0-10.7) | 20.8 (5.2-43.6) | 20.8 (5.2-43.6) |
| Histone deacetylase (HDAC) inhibitor monotherapy | 4 (2.3) | 3.6 | 25 (0.9-66.5) | 25 (0.9-66.5) |
| Interferon monotherapy | 21 (11.8) | 4.8 (3.0-7.4) | 14.3 (3.6-32.1) | 4.8 (0.3-19.7) |
| Methotrexate monotherapy | 28 (15.7) | 5.0 (3.0-7.4) | 26.8 (12.0-44.1) | 15.3 (4.9-31.2) |
| Monoclonal antibody–based monotherapy∗ | 4 (2.3) | 2.0 | 0 | 0 |
| Retinoid monotherapy | 33 (18.5) | 4.4 (3.2-7.7) | 24.2 (11.4-39.6) | 6.1 (1.1-17.6) |
| Systemic chemotherapy, multiagent monotherapy | 8 (4.5) | 4.7 (0.5-6.0) | 0 | 0 |
| Systemic chemotherapy, single-agent monotherapy† | 14 (7.9) | 4.9 (2.4-7.0) | 0 | 0 |
| Combination therapies | ||||
| ECP–based combination therapy | 36 (20.2) | 9.8 (6.3-12.5) | 40.1 (24.0-55.8) | 14.9 (40.9-30.0) |
| Interferon–based combination therapy | 4 (2.3) | 9.2 | 50.0 (5.8-84.5) | 50.0 (5.8-84.5) |
| Other combinations of systemic therapies | 1 (0.6) | — | 0 | 0 |
| Non-systemic therapies | ||||
| Skin–directed monotherapy only | 4 (2.3) | — | — | — |
| Supportive care only | 5 (2.8) | — | — | — |
Alemtuzumab, 4
Excluding low dose methotrexate monotherapy
Of the 169 patients who received systemic therapy first-line, the median TTNT was 5.4 months (95% CI, 4.7-67). For the 2 most commonly prescribed first-line therapies, the median TTNT of retinoid monotherapy was 4.4 months, whereas the median TTNT was more than doubled for ECP–based combination therapy, at 9.8 months (Table 3).
TTNT for systemic therapies delivered across all treatment lines
| Systemic therapy groups . | N = 721 lines of systemic therapy . | Median line of therapy (IQR) . | Median TTNT (months) (95% CI) . | % free from next line of treatment at 1 year (95% CI) . | % free from next line of treatment at 2 years (95% CI) . |
|---|---|---|---|---|---|
| Monotherapies | |||||
| Biological monotherapy | 10 | 8 (4-11) | — | — | — |
| Extracorporeal photopheresis (ECP) monotherapy | 47 | 2 (1-4) | 5.2 (4.1-7.6) | 14.2 (5.8-26.3) | 11.4 (4.0-23.1) |
| Histone deacetylase (HDAC) inhibitor monotherapy | 43 | 5 (3-8) | 60 (3.3-7.9) | 12.8 (4.7-25.0) | 6.4 (1.3-17.7) |
| Immunosuppressant monotherapy | 1 | — | — | — | — |
| Interferon monotherapy | 46 | 2 (1-3) | 5.0 (4.0-7.4) | 18.5 (8.7-31.1) | 11.5 (4.2-22.9) |
| Methotrexate monotherapy | 47 | 1 (1-3) | 5.0 (3.3-6.5) | 29.3 (16.8-43.0) | 19.5 (9.3-32.5) |
| Monoclonal antibody–based monotherapy | 99 | 4 (3-6) | 8.7 (6.2-11.6) | 36.3 (26.2-46.5) | 20.4 (11.5-31.2) |
| Retinoid monotherapy | 73 | 2 (1-3) | 6.0 (4.2-8.5) | 31.2 (20.8-42.2) | 13.2 (6.3-22.6) |
| Systemic chemotherapy, multiagent monotherapy∗ | 57 | 5 (3-7) | 3.1 (2.8-3.8) | 1.9 (0.2-8.9) | 0 |
| Systemic chemotherapy, single-agent monotherapy | 100 | 4 (2-5) | 4.3 (3.2-5.0) | 6.9 (2.8-13.3) | 1.1 (0.1-5.5) |
| Stem cell transplant, allogeneic | 24 | 4 (3-5.5) | — | 80.1 (55.2-92.1) | 72.1 (44.0-87.8) |
| Stem cell transplant, autologous | 1 | — | — | — | — |
| Combination therapies | |||||
| ECP–based combination therapy | 142 | 2 (1-3) | 6.7 (5.4-8.4) | 28.2 (20.9-35.9) | 10.9 (6.2-17.1) |
| Interferon–based combination therapy | 14 | 3 (1-5) | 2.5 (1.5-9.2) | 21.4 (5.2-44.8) | 21.4 (5.2-44.8) |
| Other combinations of systemic therapies | 17 | 6 (4-8) | 2.1 (1.2-4.0) | 9.5% (0.8-32.2) | 0 |
| Systemic therapy groups . | N = 721 lines of systemic therapy . | Median line of therapy (IQR) . | Median TTNT (months) (95% CI) . | % free from next line of treatment at 1 year (95% CI) . | % free from next line of treatment at 2 years (95% CI) . |
|---|---|---|---|---|---|
| Monotherapies | |||||
| Biological monotherapy | 10 | 8 (4-11) | — | — | — |
| Extracorporeal photopheresis (ECP) monotherapy | 47 | 2 (1-4) | 5.2 (4.1-7.6) | 14.2 (5.8-26.3) | 11.4 (4.0-23.1) |
| Histone deacetylase (HDAC) inhibitor monotherapy | 43 | 5 (3-8) | 60 (3.3-7.9) | 12.8 (4.7-25.0) | 6.4 (1.3-17.7) |
| Immunosuppressant monotherapy | 1 | — | — | — | — |
| Interferon monotherapy | 46 | 2 (1-3) | 5.0 (4.0-7.4) | 18.5 (8.7-31.1) | 11.5 (4.2-22.9) |
| Methotrexate monotherapy | 47 | 1 (1-3) | 5.0 (3.3-6.5) | 29.3 (16.8-43.0) | 19.5 (9.3-32.5) |
| Monoclonal antibody–based monotherapy | 99 | 4 (3-6) | 8.7 (6.2-11.6) | 36.3 (26.2-46.5) | 20.4 (11.5-31.2) |
| Retinoid monotherapy | 73 | 2 (1-3) | 6.0 (4.2-8.5) | 31.2 (20.8-42.2) | 13.2 (6.3-22.6) |
| Systemic chemotherapy, multiagent monotherapy∗ | 57 | 5 (3-7) | 3.1 (2.8-3.8) | 1.9 (0.2-8.9) | 0 |
| Systemic chemotherapy, single-agent monotherapy | 100 | 4 (2-5) | 4.3 (3.2-5.0) | 6.9 (2.8-13.3) | 1.1 (0.1-5.5) |
| Stem cell transplant, allogeneic | 24 | 4 (3-5.5) | — | 80.1 (55.2-92.1) | 72.1 (44.0-87.8) |
| Stem cell transplant, autologous | 1 | — | — | — | — |
| Combination therapies | |||||
| ECP–based combination therapy | 142 | 2 (1-3) | 6.7 (5.4-8.4) | 28.2 (20.9-35.9) | 10.9 (6.2-17.1) |
| Interferon–based combination therapy | 14 | 3 (1-5) | 2.5 (1.5-9.2) | 21.4 (5.2-44.8) | 21.4 (5.2-44.8) |
| Other combinations of systemic therapies | 17 | 6 (4-8) | 2.1 (1.2-4.0) | 9.5% (0.8-32.2) | 0 |
Excluding low dose methotrexate monotherapy
Notably, there were no observable differences in the first-line prescribing patterns or the median TTNT, among subgroups or stages. Of the 169 patients receiving first-line systemic therapy, 124 were diagnosed with de novo SS vs 45 with secondary SS, with first-line combination therapies received in 28 (22.6%) vs 13 (28.9%), respectively. Similarly, no clear differences were observed in the prescribing patterns for the 132 patients diagnosed with stage IVA1 vs the 25 patients with stage IVA2 who received systemic therapies in the first-line setting; combination therapies were received by 30 (22.7%) vs 7 (28.0%), respectively. Including all patients, median TTNT in the first-line setting for de novo SS and secondary SS were 5.2 (95% CI, 4.5-6.5) vs 6.0 (95% CI, 4.4-8.0) months, respectively. Numerically, the first-line median TTNT appeared to favor patients with stage IVA1 over stage IVA2 (6.0 [95% CI, 5.0-7.6] vs 4.4 [95% CI, 3.6-6.9] months, respectively) but did not reach statistical significance (P = .2).
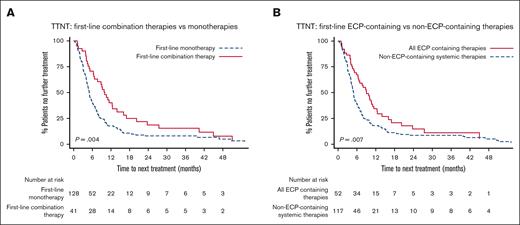
In univariable analysis, first-line combination therapies had significantly longer median TTNT than monotherapies of 10.0 months vs 5.0 months, respectively (P = .004) (Figure 2A). This remained statistically significant after adjusting for stage (data not shown).
TTNT after first-line systemic therapies. (A) TTNT after first-line combination therapies vs first-line monotherapies. Patients receiving first-line combination therapy had significantly longer TTNT (median TTNT, 10.0 vs 5.0 months; P = .004). (B) TTNT after first-line ECP-containing treatment vs first-line non-ECP–containing treatments. Patients receiving ECP-containing therapy had significantly longer TTNT (median TTNT, 9.0 vs 4.9 months; P = .007).
TTNT after first-line systemic therapies. (A) TTNT after first-line combination therapies vs first-line monotherapies. Patients receiving first-line combination therapy had significantly longer TTNT (median TTNT, 10.0 vs 5.0 months; P = .004). (B) TTNT after first-line ECP-containing treatment vs first-line non-ECP–containing treatments. Patients receiving ECP-containing therapy had significantly longer TTNT (median TTNT, 9.0 vs 4.9 months; P = .007).
Comparing first-line ECP-containing treatments (ECP monotherapy plus ECP-combination therapies) vs non-ECP–containing treatments, revealed a statistically significant increase in TTNT favoring ECP, with median TTNT of 9.0 months vs 4.9 months, respectively (P = .007) (Figure 2B). Median TTNT of first-line ECP–based combination therapies was 9.8 months, 8.0 months for ECP monotherapy, and 4.9 months for non-ECP–containing systemic therapies (P = .024).
Across all lines of therapy: patterns of care and TTNT
Across all lines of therapy, there were 58 different treatment regimens prescribed, which were classified into 17 treatment groups (Figure 1; supplemental Table 1). Of the 15 systemic therapy groups, ECP–based combination therapy remained the most commonly prescribed treatment across all treatment lines, received by 96 patients (53.9%) (supplemental Table 2). Thirty-one of these 96 patients received multiple lines of ECP-combination therapy during their treatment journey. A total of 142 lines of ECP–based combination therapy were delivered, with all but 2 delivered within the first 7 lines of treatment; the most common combinations were ECP-interferon in 53 (37.3%), and ECP-retinoid (bexarotene) in 40 (28.2%).
Twenty-four patients underwent alloSCT, with median number of 4 (range, 2-13) prior lines of therapy (Table 3). Of all the systemic therapies, alloSCT was associated with the greatest duration of clinical efficacy, with predicted freedom from next line of treatment of 80.1% (95% CI, 55.2-92.1) at 1 year and 72.1% (95% CI, 44.0-87.8) at 2 years (Table 3; supplemental Figure 2). Two patients (9%) died within the first 100 days of allograft. Notably, of the 68 patients aged >70 years at the time of diagnosis, only 1 underwent alloSCT.
Seventy-six patients received monoclonal antibody–based therapies, with a total of 99 courses delivered across all treatment lines. The median TTNT for all lines (n = 99) was 8.7 (95% CI, 6.2-11.6) months with 20.4% (95% CI, 11.5-31.2) free from next line of therapy at 2 years (Table 3). The analysis was repeated when limiting the denominator to the first exposure to monoclonal antibody–based monotherapy (n = 76) to remove the potential effect of retreatment bias, 18.6% (95% CI, 9.5-30.1) of patients were predicted to remain free from the next line of therapy at 2 years (supplemental Figure 2). Mogamulizumab (n = 38) was the most commonly prescribed monoclonal antibody–based therapy, with median TTNT of 13.1 months (95% CI, 5.0-25.0). The TTNT for the antibody drug conjugate brentuximab vedotin (n = 11) and alemtuzumab (n = 11) were shorter, but the small number of treatments delivered in those groups across various treatment lines did not allow for a meaningful comparative analysis.
Impact of treatment line on TTNT
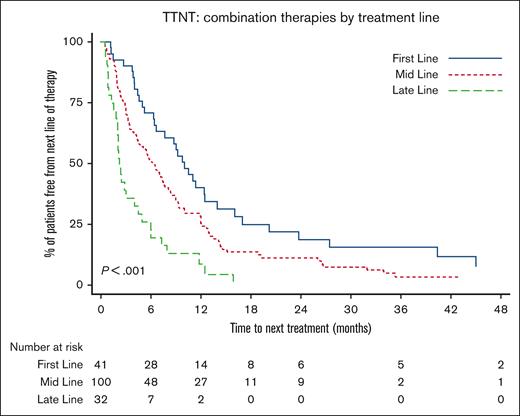
For combination therapies, earlier delivery was associated with longer clinical benefit. The median TTNT of combination therapies delivered first-line was 10.0 months (95% CI, 6.4-12.5), compared to the median TTNT of 6.2 months (95% CI, 4.4-7.7) when delivered mid-line (defined as second to fourth lines), or 2.2 months (1.8-4.0) in late-line (defined as fifth line and greater) (P < .001) (Figure 3).
Impact of treatment line on TTNT for combination therapies. Patients receiving combination therapy as first-line, mid-line (second to fourth lines), or late-line (fifth line and greater) therapy had significantly different TTNT (median TTNT, 10.0 vs 6.2 vs 2.2 months; P < .001). The longest TTNT was with first-line use.
Impact of treatment line on TTNT for combination therapies. Patients receiving combination therapy as first-line, mid-line (second to fourth lines), or late-line (fifth line and greater) therapy had significantly different TTNT (median TTNT, 10.0 vs 6.2 vs 2.2 months; P < .001). The longest TTNT was with first-line use.
Earlier delivery of ECP in the treatment journey also resulted in longer TTNT, holding true for both ECP–based combination therapy and ECP-monotherapy. For ECP–based combination therapy, the median TTNT when delivered first-line was 9.8 months (95% CI, 6.3-12.5) months, mid-line 6.5 months (95% CI, 4.7-8.4), late-line 2.8 months (95% CI, 0.9-7.3) (P = .004) (supplemental Figure 3a). For ECP-monotherapy, the median TNTT when delivered first-line was 8.0 months (95% CI, 2.0-10.7), mid-line 6.9 months (95% CI, 3.9-7.9), late-line 5.0 months (95% CI, 1.8-5.1) (P = .031) (supplemental Figure 3b).
Notably, treatment line did not significantly affect TTNT for monoclonal antibody–based monotherapies, interferon monotherapy, methotrexate monotherapy, retinoid monotherapy, multi-agent systemic chemotherapy, or single-agent systemic chemotherapy (data not shown).
Discussion
To our knowledge, this is the largest reported study comparing TTNT as an objective retrospective measure of the duration of clinical benefit in patients with SS. In the existing published literature, population of patients with SS and MF are frequently combined, typically with substantially fewer patients with SS, clouding the interpretation of treatment efficacy for these individual subgroups. This collaborative study focuses exclusively on patients with B2 blood involvement to allow a deeper insight into the clinical benefit of current state-of-the-art therapies.
To date, there is limited published data comparing the efficacy of different treatments in patients with SS. International guidelines list the treatment options to be considered first-line, however, these give no preference to the sequencing of treatments because the comparative benefit of individual treatments and combinations remain unknown. We found that the management approaches differ, even among experts at large institutions. This variation in management choices likely reflects uncertainty regarding the comparative efficacy of available treatments, lack of consensus on optimal treatment sequencing, regional regulatory prescribing restrictions, and individual physician practice.4,10,32,33
We confirmed the breadth of systemic therapies currently used in clinical practice, with 13 different therapeutic strategies (comprising 27 treatment regimens) prescribed first-line (Table 2) and 17 different strategies (comprising 58 distinct treatment regimens) across all treatment lines (supplemental Table 1). We report that in the first-line setting, retinoid monotherapy and ECP–based combination therapies were the 2 most commonly prescribed therapies, however, together only constituted approximately one-third of the total of first-line therapies. Multi-line treatment journeys were confirmed for these patients, the median number of treatment lines received was 4 (range, 1-16). Excluding non-systemic therapies, the median number of systemic treatments received was 3, with some patients receiving up to 14 lines of systemic therapy. Re-treatment with the same therapy was also common.
When examining all treatment groups across all treatment lines, we observed an overall modest duration of clinical benefit from available therapies of only 5.4 months. This outcome was not surprising and had previously been reported.14 However, early combination therapy was able to achieve a median TTNT of 10.0 months. Similarly, ECP when delivered alone or in combination at first-line, achieved median TTNT of 8.0 and 9.8 months, respectively. These findings underscore the importance of ECP for patients with SS, and we advocate for this being a key part of the early treatment algorithm for this patient group.16,34 The benefit of delivering front-line ECP in combination with modern era drug partners requires urgent prospective evaluation.
Beyond ECP, we postulate that the observed prolongation of disease control with combination therapy most likely reflects the combined symptomatic and disease control benefits, which in turn outweigh any additional toxicity arising from a need to intensify treatment regimens. Not surprisingly, we found that TTNT of combination therapies is influenced by the timing of treatment delivery, with later delivery in the treatment sequence being associated with shorter duration of clinical benefit. Given the retrospective nature of this study, we acknowledge that patient eligibility for combination therapy and the selection of combination therapy are likely to have been influenced by patient- and clinician-specific factors which were not captured in this analysis. Nonetheless, this finding argues for the prioritization of combination therapies in the first line for this patient cohort.
Monoclonal antibody–based therapies are a recent investigational focus in CTCL and reflect an important evolution of the therapeutic landscape. In our cohort, monoclonal antibody–based therapies represented only 2.3% of first-line therapies, likely because of access and prescribing restrictions. Despite this, we observed a promising “tail” on the TTNT curve after monoclonal antibody–based therapies, with 18.6% of patients with SS remaining free from next line of therapy at 2 years. Mogamulizumab therapy achieved an impressive TTNT of 13.1 months and is consistent with the post hoc analyses of the prospective MAVORIC trial which reported a median TTNT of 12.6 months for patients with B1 blood involvement and 13.1 months for those with B2 blood involvement.13 The small numbers of patients receiving other monoclonal antibody–based treatments in this study hampered any comparative evaluations.
Although alloSCT is the only curative treatment option for patients with SS,23,35-38 only 13.5% of this cohort received this treatment. This likely reflects the risks of significant treatment-related morbidity and mortality that precludes the majority of patients with SS with advanced age or comorbidities from accessing this treatment. Uniquely, this study explored the TTNT of alloSCT specific to the SS population. Our data have demonstrated an impressive TTNT, with predicted freedom from next line of treatment measuring 80.1% at 1 year and 72.1% at 2 years. However, given the increasing availability of monoclonal antibody–based treatments and the ongoing development of novel therapies, the dilemma of optimal treatment sequencing of alloSCT remains unresolved.35
The limitations of this retrospective study are acknowledged and the impact of comorbidities, drug availability, and physician preference for the selection of specific therapies are unclear and beyond the scope of this study. Toxicities and quality of life were not able to be assessed in this retrospective study design, although it is generally accepted that patient tolerance and adherence are reflected in the TTNT measure. Ideally, the TTNT findings in this study should be validated prospectively and we, therefore, eagerly await the results from the prospective, multinational observational “PROCLIPI” study39,40 (clinicaltrials.gov Identifier: NCT02848274).
Conclusion
Drawing from real-world data from 3 international quaternary centers, the patterns of care and treatment selection for patients with SS are highly variable, with 13 different treatment options being used in the first-line setting. Overall, the median TTNT of first-line systemic treatments is short, measuring <6 months. However, a clear TTNT benefit was observed from the first-line delivery of combination therapies over monotherapies. Moreover, ECP-containing therapies were associated with longer TTNT, especially when used first-line. As expected, alloSCT provided the most durable clinical benefit, with >70% remaining free from further treatment at 2 years.
Acknowledgments
This work is dedicated to the memory of Robert Twigger, Cutaneous Lymphoma Support Nurse and Database Manager in the Cutaneous Lymphoma Service at Peter MacCallum Cancer Centre, whose work on consistent and detailed data collection assisted many research projects, including this one.
Authorship
Contributions: B.A.C., H.M.P., M.B., and J.J.S. designed research; B.A.C., G.D., Z.H., M.B., and J.J.S. performed research; B.A.C., G.D., Z.H., C.v.d.W., C.M., C.R.-W., M.M., and J.J.S. collected data; B.A.C., H.M.P., F.E., and J.J.S. analyzed and interpreted data; F.E. and B.A.C. performed statistical analysis; and B.A.C., H.M.P., C.v.d.W., and J.J.S. wrote the manuscript.
Conflict-of-interest disclosure: G.D. reports being on the advisory boards of Kyowa Kirin and Recordati Rare Diseases. H.M.P. is a member of advisory boards and receives honoraria from Kyowa Kirin, Mallinkrodt, Therakos, Innate Pharma, and Takeda. M.B. reports being on the advisory boards for Innate Pharma, Kyowa Kirin, Takeda, and Helsinn/Recordati. M.M. is a medical director and clinical trial investigator of Eurofins, BIO-EC (France). J.S. reports receiving consulting and/or honoraria from Takeda, Recordati, Helsinn, Kyowa Kirin, Mallinkrodt, Therakos, Affirmed. The remaining authors declare no competing financial interests.
Correspondence: Belinda A. Campbell, Department of Radiation Oncology, Peter MacCallum Cancer Centre, Locked Bag 1, A'Beckett St, Melbourne, VIC 8006, Australia; e-mail: belinda.campbell@petermac.org.
References
Author notes
Presented in part at the 2021 meeting of the European Organization of Research and Treatment of Cancer (EORTC) Cutaneous Lymphoma Task Force (CLTF), Marseille, France.
Data are available on reasonable request from the corresponding author, Belinda A Campbell (belinda.campbell@petermac.org).
The full-text version of this article contains a data supplement.