Key Points
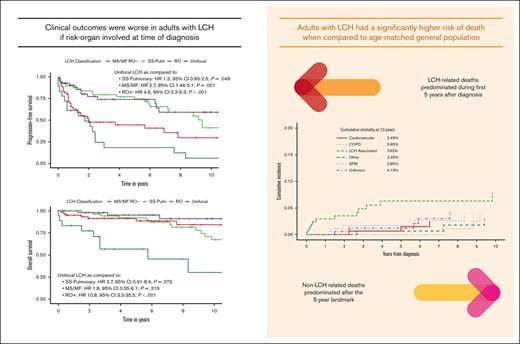
Adults with LCH have a higher risk of mortality from non-LCH causes such as chronic obstructive pulmonary disease and second primary malignancies.
Most LCH-related deaths occurred within 5 years of diagnosis, with other causes predominating beyond that period.
Abstract
Advances in the treatment of Langerhans cell histiocytosis (LCH) have resulted in a growing survivor population. There is a lack of data on long-term outcomes among adults with LCH. We conducted a retrospective record review of 219 adults (aged ≥18 years) with LCH. Most common presentation was multisystem (34.2%), followed by single-system pulmonary (32%), unifocal (28.3%), and single-system multifocal (5.5%) LCH. Risk organ involvement (the liver, spleen, or bone marrow) was seen in 8.7% of cases, and 40 of 88 (45.5%) tested cases were BRAFV600E. At a median follow-up of 74 months, 5-year progression-free survival (PFS) was 58.3% and estimated median PFS was 83 months. Median overall survival (OS) was not reached; 5- and 10-year OS rates were 88.7% and 74.5%, respectively. Risk organ involvement was associated with worse PFS (hazard ratio [HR], 4.5) and OS (HR, 10.8). BRAFV600E was not associated with risk organ involvement or survival. When compared with matched unaffected US population, individuals with LCH had a significantly higher risk of overall mortality (standardized mortality ratio [SMR], 2.66), specifically among those aged <55 years at diagnosis (SMR, 5.94) and those with multisystem disease (SMR, 4.12). Second cancers occurred in 16.4% cases, including diverse hematologic and solid organ malignancies. LCH-associated deaths constituted 36.1% of deaths and occurred within 5 years of diagnosis. After 5 years, non-LCH causes of death, including second cancers, chronic obstructive pulmonary disease, and cardiovascular diseases, predominated. Our study highlights, to our knowledge, for the first time, that adults with LCH experience early and late mortality from non-LCH causes and the need for development of targeted survivorship programs to improve outcomes.
Introduction
Langerhans cell histiocytosis (LCH) is a rare histiocytic disorder, with manifestations ranging from indolent unifocal disease to multisystem disease, causing morbidity and mortality.1 Over the last decade, our understanding of LCH has been revolutionized by the discovery of the clonal MAPK–extracellular signal-regulated kinase (ERK) pathway (RAS-RAF-MEK-ERK) and other mutations leading to near-universal ERK activation.2-4 These discoveries have reframed LCH from an inflammatory/autoimmune disease to a hematologic neoplasm. LCH is included in the “L” (Langerhans) group of histiocytosis in the 2016 classification by the International Histiocyte Society, along with Erdheim-Chester disease (ECD) and extracutaneous juvenile xanthogranuloma because of similar MAPK-ERK pathway alterations in these disorders.5
Most of the data pertaining to the clinical manifestations and outcomes of LCH are derived from the pediatric literature, with a paucity of such studies evaluating adult counterparts. The largest study to date examining adults with LCH used the Survival, Epidemiology, and End-Results (SEER) registry from the United States, encompassing 456 adults with LCH and showed a 5-year overall survival (OS) of 88.5%.6 Another registry-based study from the United Kingdom showed 5-year OS in adults with LCH to be 72%.7 A major limitation of the aforementioned tumor registry–based studies is the lack of clinical and therapeutic data that makes it difficult to distill any actionable information. Another study from the International Histiocyte Society registry data from 1972 to 2000 (N = 274) showed a 5-year OS of 92% in the entire cohort.8 A recent study from China also examined the treatments and outcomes among adults with LCH.9 However, the median follow-up duration in both the aforementioned studies was relatively short (28-38 months), which precluded the assessment of long-term outcomes and natural history of the disease. Because of a lack of randomized studies defining treatment of adults with LCH, empiric treatments ranging from surgery or radiation for localized disease and systemic chemotherapy for multisystem disease have been used, borrowing from pediatric experience. The discovery of MAPK-ERK pathway mutations has enabled the successful treatment with BRAF and MEK inhibitors in otherwise refractory cases.10-12 As therapies for LCH improve and the survivor population ages, these individuals may be at an increased risk of morbidity and mortality from causes beyond LCH. Studies examining cause-specific mortality among adults with LCH are lacking. It is also unknown whether “risk organ” disease (involving the liver, spleen, or bone marrow), as defined in pediatric literature to be associated with worse prognosis, applies to adults as well. Population- and survey-based studies have suggested an increased risk of second primary malignancies (SPMs) among patients with LCH.6,13,14 However, such registry-based studies lack granular data, precluding the assessment of the specific types of neoplasms and factors associated with their development. Therefore, we undertook this study to address key gaps in knowledge by using a large cohort of adults with LCH from a tertiary care institution.
Methods
Cohort selection and variable definitions
After institutional review board approval, we conducted a retrospective review of all individuals diagnosed with LCH at age ≥ 18 years at our institution from 2001 to 2021. The diagnosis of LCH was based on the prevailing consensus guidelines1,15 and included the following criteria: (1) characteristic histopathologic features of LCH via a tissue biopsy, including CD207 (langerin) and CD1a+ histiocytes in lesional tissue by immunohistochemistry or (2) typical upper lobe–predominant nodular and cystic lung lesions in a smoker via a chest computed tomography (CT). The distribution of disease subtypes and organ manifestations were abstracted from available radiographic studies, including CTs, 18F-fluorodeoxyglucose positron emission tomography (FDG PET) CTs, technetium-99m methylene diphosphonate bone scintigraphy, and magnetic resonance imaging (MRI) studies. The disease phenotypes were grouped into 4 categories: (1) unifocal (solitary lesion involving any organ system), (2) single-system pulmonary (isolated lung involvement, mostly smoking related), (3) single-system multifocal or multisystem disease (MS/MF; >1 lesion involving any organ except risk organ), and (4) risk organ disease (involvement of the bone marrow, liver, or spleen, as defined in the pediatric LCH population).16 Neurodegenerative disease was defined by characteristic clinical and MRI findings, as described previously.17 Because BRAFV600E mutations were first discovered in LCH in 2010, most patients diagnosed after this period underwent BRAF mutational status evaluation using various methodologies, including BRAFV600E-specific immunohistochemistry (VE1, Abcam), tumor-tissue, allele-specific quantitative polymerase chain reaction, next-generation sequencing, and/or plasma cell–free DNA sequencing.
Statistical analysis
Nominal and ordinal variables were described in terms of frequency and percentage, whereas quantitative data were described as mean and standard deviation or median and range, as appropriate.
Because objective response assessment was not uniformly conducted, we focused primarily on clinically relevant survival end points in this study. OS was defined as time from diagnosis of LCH until death or last follow-up. Progression-free survival (PFS) was defined as the time from diagnosis until confirmed disease progression, relapse, or death, whichever came first. Time-to-event analyses were conducted using the Kaplan-Meier method and compared using log-rank testing. We also conducted univariate and logistic regression (Cox proportional hazards) to ascertain factors predictive of PFS and OS. Given the limitations posed by small number of events, covariates considered for multivariable OS analysis were restricted to LCH subtype, insurance status, and age at diagnosis. Determination of cumulative incidence of cause-specific mortality was conducted, with death from causes other than LCH treated as competing risk.
Standardized mortality ratios (SMRs; ratio of the observed to the expected number of deaths) were calculated by comparison of expected and observed mortality rates in our cohort with those in the standard US age-, sex-, and calendar year–matched population obtained from free-access Centers for Disease Control population data.18 We calculated 95% confidence intervals (CIs) of the SMRs using the Poisson regression method described by Vandenbroucke.19 All statistical analysis was performed using R statistical software (version 4.0.5; R Core Team 2021).
Institutional review board approval was obtained from the Mayo Clinic institutional review board.
Results
Baseline demographics
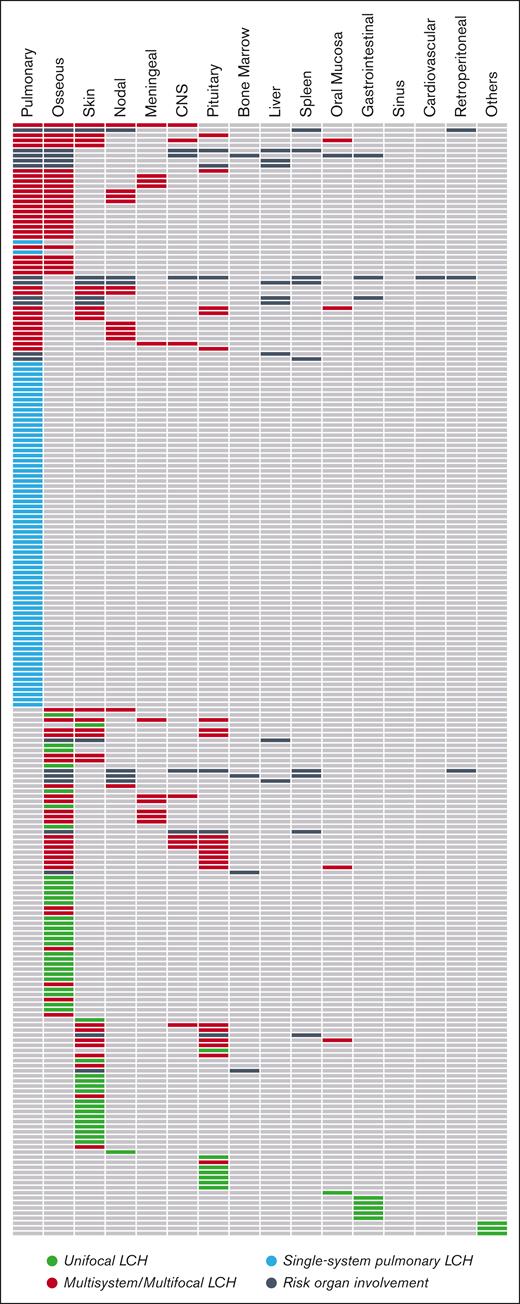
We included 219 adults with LCH in the study cohort. The mean age at diagnosis of LCH was 43.8 years (range, 19-88 years), and 51.1% (n = 112) of patients were female. At diagnosis, the most common presentation was multisystem LCH (n = 75; 34.2%), followed by single-system pulmonary (n = 70; 32%), unifocal (n = 62; 28.3%), and single-system multifocal LCH (n = 12; 5.5%). Nineteen patients (8.7%), all with MS/MF disease, had risk organ involvement at diagnosis. Dedicated chest CT, whole-body FDG PET-CT, head MRI, and technetium-99m-methylene diphosphonate bone scintigraphy were reported in 68.5% (n = 150), 49.8% (n = 109), 52.1% (n = 114), and 18.7% (n = 41) of the individuals, respectively. The utilization of extended whole-body imaging was particularly lower among patients with single-system pulmonary LCH, for whom FDG PET-CT was performed in 35.7% (n = 25). Regardless of LCH subtype, the most common organs involved by LCH included lung (n = 115; 52.5%), bone (n = 91; 41.6%), skin (n = 52; 23.7%), and the pituitary/hypothalamus (n = 35; 16%; Table 1; Figure 1A). Of the 35 patients with pituitary/hypothalamus involvement, 30 (85.7%) had both diabetes insipidus and radiographic infiltration, 4 (11.5%) had diabetes insipidus alone, and 1 (2.8%) had isolated radiographic pituitary disease. Liver involvement was reported in 9 (4.1%) cases, of whom 4 had evidence of primary sclerosing cholangitis (PSC). On conducting a matched 1:1 comparison (age at diagnosis and sex) of the peripheral blood counts between risk organ–positive and –negative cases, the former group was found to have lower hemoglobin level (11.8 g/dL vs 14.8 g/dL; P < .001) but no difference in other cell lines. Three patients had evidence of neurodegenerative LCH: 1 with memory difficulties and 2 with radiographic features on MRI of the brain. Tobacco smoking was prevalent (n = 141; 66%) and strongly associated with single-system pulmonary LCH (94.1% smokers) compared with other subtypes (P < .001). There was no association of smoking with any other organ manifestations or risk organ involvement (P > .05).
Baseline characteristics for adults with LCH (N = 219). All data are presented as n (% of total in that column)
| Characteristic . | 2001-2009 (n = 90) . | ≥ 2010 (n = 129) . | Total (N = 219) . | P . |
|---|---|---|---|---|
| Age at diagnosis, y, mean (range) | 41.5 (19-76) | 45.3 (19-88) | 43.8 (18-88) | .234 |
| Female, n (%) | 47 (52.2) | 65 (50.4) | 112 (51.1) | .789 |
| Smoking history, n = 214 (%) | 66 (76.7) | 75 (58.6) | 141 (65.9) | .006 |
| BRAFV600E, n = 88 (%) | 3 (30) | 37 (47.4) | 40 (45.5) | .297 |
| Organ involvement through follow-up, n (%) | ||||
| Lung | 62 (68.9) | 53 (41.1) | 115 (52.5) | <.001 |
| Osseous | 29 (32.2) | 62 (48.1) | 91 (41.6) | 0.019 |
| Skin | 20 (22.2) | 32 (24.8) | 52 (23.7) | .658 |
| Pituitary/hypothalamus | 14 (15.6) | 21 (16.3) | 35 (16.0) | .886 |
| Central nervous system | 6 (6.7) | 9 (7) | 15 (6.9) | .929 |
| Meningeal disease | 3(3.3) | 12 (9.3) | 15 (6.9) | .085 |
| Nodal | 10 (11.1) | 10 (7.8) | 20 (9.1) | .396 |
| Liver | 2 (2.2) | 7 (5.4) | 9 (4.1) | .240 |
| Spleen | 5 (5.6) | 4 (3.1) | 9 (4.1) | .368 |
| Gastrointestinal | 3 (3.3) | 6 (4.7) | 9 (4.1) | .629 |
| Oral | 5 (5.6) | 2 (1.6) | 7 (3.2) | .097 |
| Bone marrow∗ | 1 (1.1) | 3 (2.3) | 4 (2.7) | .509 |
| Retroperitoneal† | 1 (1.1) | 2 (1.6) | 3 (1.4) | .783 |
| Sinus | 1 (1.1) | 1 (0.8) | 2 (0.9) | .797 |
| Heart | 0 | 1 (0.8) | 1 (0.5) | .402 |
| LCH Classification at diagnosis, n (%) | ||||
| Unifocal | 19 (21.1) | 43 (33.3) | 62 (28.3) | 0.048 |
| Single-system pulmonary | 39 (43.3) | 31 (24) | 70 (32) | 0.003 |
| MS/MF, RO− | 25 (27.8) | 43 (33.3) | 68 (31.1) | 0.382 |
| RO+ | 7 (7.8) | 12 (9.3) | 19 (8.7) | 0.693 |
| Characteristic . | 2001-2009 (n = 90) . | ≥ 2010 (n = 129) . | Total (N = 219) . | P . |
|---|---|---|---|---|
| Age at diagnosis, y, mean (range) | 41.5 (19-76) | 45.3 (19-88) | 43.8 (18-88) | .234 |
| Female, n (%) | 47 (52.2) | 65 (50.4) | 112 (51.1) | .789 |
| Smoking history, n = 214 (%) | 66 (76.7) | 75 (58.6) | 141 (65.9) | .006 |
| BRAFV600E, n = 88 (%) | 3 (30) | 37 (47.4) | 40 (45.5) | .297 |
| Organ involvement through follow-up, n (%) | ||||
| Lung | 62 (68.9) | 53 (41.1) | 115 (52.5) | <.001 |
| Osseous | 29 (32.2) | 62 (48.1) | 91 (41.6) | 0.019 |
| Skin | 20 (22.2) | 32 (24.8) | 52 (23.7) | .658 |
| Pituitary/hypothalamus | 14 (15.6) | 21 (16.3) | 35 (16.0) | .886 |
| Central nervous system | 6 (6.7) | 9 (7) | 15 (6.9) | .929 |
| Meningeal disease | 3(3.3) | 12 (9.3) | 15 (6.9) | .085 |
| Nodal | 10 (11.1) | 10 (7.8) | 20 (9.1) | .396 |
| Liver | 2 (2.2) | 7 (5.4) | 9 (4.1) | .240 |
| Spleen | 5 (5.6) | 4 (3.1) | 9 (4.1) | .368 |
| Gastrointestinal | 3 (3.3) | 6 (4.7) | 9 (4.1) | .629 |
| Oral | 5 (5.6) | 2 (1.6) | 7 (3.2) | .097 |
| Bone marrow∗ | 1 (1.1) | 3 (2.3) | 4 (2.7) | .509 |
| Retroperitoneal† | 1 (1.1) | 2 (1.6) | 3 (1.4) | .783 |
| Sinus | 1 (1.1) | 1 (0.8) | 2 (0.9) | .797 |
| Heart | 0 | 1 (0.8) | 1 (0.5) | .402 |
| LCH Classification at diagnosis, n (%) | ||||
| Unifocal | 19 (21.1) | 43 (33.3) | 62 (28.3) | 0.048 |
| Single-system pulmonary | 39 (43.3) | 31 (24) | 70 (32) | 0.003 |
| MS/MF, RO− | 25 (27.8) | 43 (33.3) | 68 (31.1) | 0.382 |
| RO+ | 7 (7.8) | 12 (9.3) | 19 (8.7) | 0.693 |
RO, risk organ (liver, spleen, and bone marrow); MS/MF RO−, multisystem/multifocal risk organ negative; RO+, risk organ positive.
One case was tissue-proven, remaining with discrete lesions on advanced imaging.
Includes cases with renal, adrenal, and retroperitoneal fat involvement.
Patterns of organ involvement among adults with LCH. Each row represents 1 unique patient. CNS, central nervous system.
Patterns of organ involvement among adults with LCH. Each row represents 1 unique patient. CNS, central nervous system.
Of the entire cohort, 88 cases (40.2%) underwent evaluation for BRAF mutational status, mostly among the cases diagnosed after 2010 (n = 78; 88.6%). Among the population evaluated for mutation status, 45.5% (n = 40) were positive for the BRAFV600E. Increasing age was associated with BRAFV600E (10-year increase odds ratio [OR], 1.42; 95% CI, 1.07-1.92; P = .017), whereas no correlation was found between BRAFV600E status and risk organ involvement (P = .936) or other LCH subtypes (P = .956).
First-line therapy was heterogeneous for the cohort and varied based on the organ manifestations at the time of diagnosis (Table 2). The most common first-line treatments used were surgical excision (30.6%), recommendation for smoking cessation (29.6%), cytotoxic chemotherapy (20.9%), and systemic corticosteroids (15.8%). BRAF or MEK inhibitors were used in a minority of patients as frontline therapy (n = 3).
First-line treatments for adults with LCH
| N = 196 . | U . | SS-Pulm . | MS/MF RO− . | RO+ . | Total, n (%) . |
|---|---|---|---|---|---|
| Surgical resection or excision | 43 | 1 | 14 | 2 | 60 (30.6) |
| Smoking cessation or reduction | 2 | 42 | 12 | 2 | 58 (29.6) |
| Systemic cytotoxic therapy | 4 | 5 | 20 | 12 | 41 (20.9) |
| Cladribine based | 2 | 2 | 12 | 10 | 26 (13.3) |
| Vinblastine-/vincristine-based with steroid | 0 | 0 | 5 | 2 | 7 (3.6) |
| Cytarabine based | 0 | 1 | 3 | 0 | 4 (2) |
| Others | 2 | 2 | 1 | 0 | 5 (2.6) |
| Systemic corticosteroids | 1 | 16 | 8 | 6 | 31 (15.8) |
| Radiation therapy | 8 | 0 | 10 | 1 | 19 (9.7) |
| Topical immunosuppression | 2 | 0 | 4 | 0 | 6 (3.1) |
| Targeted therapy∗ | 1 | 1 | 2 | 0 | 4 (2) |
| Immune suppression with antiresorptive therapy | 0 | 0 | 1 | 0 | 1 (0.5) |
| N = 196 . | U . | SS-Pulm . | MS/MF RO− . | RO+ . | Total, n (%) . |
|---|---|---|---|---|---|
| Surgical resection or excision | 43 | 1 | 14 | 2 | 60 (30.6) |
| Smoking cessation or reduction | 2 | 42 | 12 | 2 | 58 (29.6) |
| Systemic cytotoxic therapy | 4 | 5 | 20 | 12 | 41 (20.9) |
| Cladribine based | 2 | 2 | 12 | 10 | 26 (13.3) |
| Vinblastine-/vincristine-based with steroid | 0 | 0 | 5 | 2 | 7 (3.6) |
| Cytarabine based | 0 | 1 | 3 | 0 | 4 (2) |
| Others | 2 | 2 | 1 | 0 | 5 (2.6) |
| Systemic corticosteroids | 1 | 16 | 8 | 6 | 31 (15.8) |
| Radiation therapy | 8 | 0 | 10 | 1 | 19 (9.7) |
| Topical immunosuppression | 2 | 0 | 4 | 0 | 6 (3.1) |
| Targeted therapy∗ | 1 | 1 | 2 | 0 | 4 (2) |
| Immune suppression with antiresorptive therapy | 0 | 0 | 1 | 0 | 1 (0.5) |
MS/MF RO−, multisystem/multifocal risk organ negative; RO+, risk organ positive; SS-pulm, single-system pulmonary; U, unifocal.
Vemurafenib, dabrafenib, cobimetinib, and an on-trial AKT inhibitor. Additional cases as second-line therapy.
Survival analysis and patterns of disease progression
The median follow-up duration for the entire cohort was 74 months (95% CI, 63-86). At the last follow-up, 17.7% of individuals with unifocal LCH (n = 11) progressed to single-system multifocal or multisystem disease (supplemental Figure 1). Of those with single-system multifocal disease, 25% (n = 3) progressed to multisystem disease. The proportion of individuals that progressed from single-system pulmonary LCH to multisystem disease was low (2.8%; n = 2).
The estimated median PFS for the entire cohort was 83 months (95% CI, 68-113), with a 5-year PFS of 58.3% (95% CI, 51-66.8). Factors associated with worse PFS on univariate analysis were MS/MF disease (P = .001) and the presence of risk organ involvement at diagnosis (P < .001). Single-system multifocal LCH had worse PFS compared with unifocal disease (P = .0003; supplemental Figure 2). We further explored differences in the outcomes between bone and non-bone LCH within unifocal and single-system multifocal groups and found no difference in the PFS between the 2 groups (supplemental Figure 3). No significant association was found between PFS rates and the following variables: BRAFV600E status (P = .173), age at diagnosis (P = .239), and diagnosis period (before vs after 2010; P = .605; Figure 2). When examining insurance status, individuals without insurance were associated with worse PFS compared with those who were privately insured (P < .01; supplemental Figure 4). In multivariable analysis using age, insurance status, and LCH classification as covariates, MS/MF disease (hazard ratio [HR], 2.8; 95% CI, 1.5-5.2; P < .001) and risk organ involvement (HR, 4.5; 95% CI, 2.2-9.2; P < .001) were associated with worse PFS. Additional PFS analyses using the initiation of treatment as the initial time point or comparison among patients treated at the primary study site vs elsewhere did not change any of the results materially.
PFS estimates for adults with LCH. (A) Entire cohort, (B) based on year of diagnosis, (C) based on BRAF V600E mutational status, and (D) based on classification of disease subtype. MS/MF RO-, multisystem or multifocal risk organ negative; neg, negative; pos, positive; SS-Pulm, single-system pulmonary; RO+, risk organ positive.
PFS estimates for adults with LCH. (A) Entire cohort, (B) based on year of diagnosis, (C) based on BRAF V600E mutational status, and (D) based on classification of disease subtype. MS/MF RO-, multisystem or multifocal risk organ negative; neg, negative; pos, positive; SS-Pulm, single-system pulmonary; RO+, risk organ positive.
The median OS for the entire cohort was not reached, with a 5-year OS rate of 88.7% (95% CI, 83.9-93.7) and a projected 10-year OS rate of 74.5% (95% CI, 66.4-83.6). Factors associated with worse OS on univariate analysis included older age at diagnosis (10-year increments; P < .001) and risk organ involvement (P < .001). A trend toward worse OS among patients harboring BRAFV600E (P = .055) and those without health insurance (P = .058) was observed, and there was no correlation between OS and diagnosis period (before vs after 2010, P = .628; Figure 3). Multivariable analysis considering the LCH classification, insurance status, and age at diagnosis demonstrated significantly worse overall mortality among patients with risk organ involvement (HR, 10.8; 95% CI, 3.3-35.5; P < .001) and older age at diagnosis (10-year increment HR, 1.09; 95% CI, 1.06-1.12; P < .001).
OS estimates for adults with LCH. (A) Entire cohort, (B) based on year of diagnosis, (C) based on BRAF V600E mutational status, and (D) based on classification of disease subtype.
OS estimates for adults with LCH. (A) Entire cohort, (B) based on year of diagnosis, (C) based on BRAF V600E mutational status, and (D) based on classification of disease subtype.
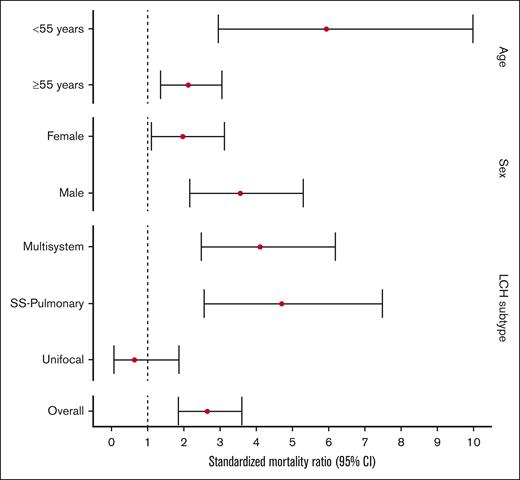
When compared with the matched unaffected US population, individuals in our LCH cohort had a significantly higher risk of overall mortality (SMR, 2.66; 95% CI, 1.78-3.54). Particularly, subgroups at highest risk of death included those aged <55 years at time of diagnosis (SMR, 5.94; 95% CI, 2.43-9.45) and those who had multisystem disease (SMR, 4.12; 95% CI, 2.27-5.97). The mortality for people with unifocal LCH was not significantly different from that of the general population (SMR, 0.65; 95% CI, 0.06-1.87; Figure 4; supplemental Table 1).
SMRs of adults with LCH compared with those of age-, sex-, and calendar year–matched control US population.
SMRs of adults with LCH compared with those of age-, sex-, and calendar year–matched control US population.
SPM and cause-specific mortality
A total of 36 patients (16.4%) had at least 1 additional malignant neoplasm reported in our cohort. Of these, 18 patients developed subsequent SPMs with a median latency of 5 years (range, 1-18 years), including 10 patients with solid organ neoplasms, 7 patients with hematologic neoplasms, and a single case with diffuse large B-cell lymphoma and malignant melanoma (supplemental Table 2). Molecular data on SPMs were available for a subset, and 1 of the cases with LCH and subsequent acute myeloid leukemia (AML) shared SRSF2 mutations, highlighting common clonal origin (supplemental Table 3). SPMs were mainly identified during evaluation of new clinical findings (n = 13); however, 3 cases were incidentally detected during routine follow-up studies and 2 during performance of cancer screening strategies. The risk of SPMs was higher among individuals who received systemic chemotherapy compared with those who did not (P = .01). There was no significant association of SPM with age at LCH diagnosis, BRAF-mutation status, risk organ involvement, or LCH subtype. There was no significant difference in the OS between those who developed SPM after diagnosis of LCH compared with those who did not (P = .594). Most common solid tumors included breast cancer (n = 3) and non–small cell lung cancer (n = 2). Six of 7 hematologic neoplasms were myeloid malignancies, encompassing chronic myelomonocytic leukemia (CMML; n = 2), myelodysplastic syndrome (MDS; n = 2), and AML (n = 2). One case each with CMML and MDS progressed to AML. All 4 cases that developed AML had received cladribine for LCH, with or without other treatments sequentially. Of the 37 patients who received cladribine at any line of therapy, 10.8% developed a second myeloid neoplasm.
Four patients were concomitantly diagnosed with LCH and another malignancy: 3 had solid organ tumors, and 1 patient had follicular lymphoma. For 14 patients, LCH was diagnosed after another malignancy, of which 12 were solid organ neoplasms and 2 were lymphomas (supplemental Table 2).
At the time of the last follow-up, 36 (16.4%) deaths were reported (19 multisystem, 15 single-system pulmonary, and 2 unifocal). Most common causes of death were non–LCH related (n = 16; 44.4%), including second cancers (n = 5), chronic obstructive pulmonary disease (COPD; n = 4), cardiovascular disease (n = 3), trauma (n = 2), and septic shock (n = 2). Seven of the deaths (19.4%) were accounted for via external reports to the primary treatment teams and the causes of death were unknown. LCH-associated deaths represented 36.1% (n = 13) of deaths, all of which occurred within the first 5 years from diagnosis (supplemental Table 4). After 5 years from diagnosis, non–LCH-related causes of mortality predominated (supplemental Figure 5). Progressive pulmonary LCH was the leading cause of death for 4 patients, 3 of whom had developed severe pulmonary arterial hypertension. Among 19 deaths occurring among individuals with multisystem LCH, 9 (47%) were due to progressive disease. Of the 4 patients diagnosed with PSC, 3 died because of PSC-associated complications, whereas a single patient underwent liver transplantation with excellent outcome. Among the 3 cases with neurodegenerative LCH features, 2 had multisystem disease that resulted in transition to hospice, whereas 1 had disease remission after systemic therapy with cladribine. Most common associated cancers leading to death were hematologic, consisting of AML (n = 3) occurring in multisystem LCH cases and Hodgkin lymphoma (n = 1) occurring in a case with unifocal LCH. All 4 COPD-related deaths occurred among individuals with otherwise minimal radiographic evidence of single-system pulmonary LCH. The cumulative cause-specific mortality at 10 years was 7.8% for LCH, 3.9% for COPD, and 3.8% for SPMs (Figure 5).
Discussion
We present a large retrospective cohort study to examine long-term outcomes and cause-specific mortality among adults with LCH. Despite advances in treatments and understanding of the disease, we found that adults with LCH had an increased overall risk of death compared with the general population. The SMR for individuals with unifocal LCH was not statistically different from that of the general population, highlighting the excellent prognosis of this LCH subtype. Younger individuals (aged < 55 years) had a particularly increased risk of death compared with the general population (SMR, 5.94). Prior studies, including ours, have shown increasing age to be associated with worse outcomes within the LCH cohort; however, the comparison with the general population provides a concrete estimate of the burden of mortality in this young subgroup, in which the risk of death from competing causes is lower than older counterparts. Additionally, the 5-year OS in our cohort was similar to that reported by the Histiocyte Society registry–based study in 2003 (88% vs 92%)8 and identical to the recent SEER-based report.6 We found the rates of progression from other subtypes of LCH to multisystem disease to be low 20,21; however, the 10-year projected OS in our cohort dropped further to ∼75%. Although the deaths in the earlier period were primarily related to LCH, the deaths beyond 5 years of diagnosis were not directly related to LCH. These data suggest that there is an increased burden of late mortality among adults with LCH, which may not be driven directly by the histiocytic neoplasm.
Late mortality among people with cancer can often be due to new-onset morbidities, which can partly be ascribed to therapeutic exposures. An understanding of the causes of death can lead to the development of mitigation strategies. In our cohort, the leading causes of late mortality were COPD, SPMs, and cardiovascular disease. Interestingly, all 4 of the COPD-related deaths occurred in the single-system pulmonary LCH cohort with minimal features of LCH at the last thoracic imaging. This finding highlights that patients with LCH are at risk of mortality from other smoking-related complications that are unrelated to LCH, which may not reverse by smoking cessation. This information is critical for counseling patients with single-system pulmonary LCH in the clinic regarding ongoing screening and management of these late health problems. We found a high prevalence of second myeloid malignancies, especially CMML, MDS, and AML. Three deaths occurred from AML, all among the multisystem cohort. Studies from our group and others have highlighted a similar association between AML and LCH, thought to be related to the use of etoposide in the past.22-24 We also found 2 cases of CMML occurring subsequent to LCH diagnosis; such an association was previously described among people with ECD.25-27 The most common therapeutic exposure among patients who developed myeloid malignancies in our cohort was cladribine; however, long-term studies of cladribine in hairy cell leukemia did not show an increased risk of myeloid malignancies.28,29 It is notable that the cumulative dose of cladribine is much higher for LCH compared with that for hairy cell leukemia, in which typically only 1 cycle is administered. In a recent SEER-based analysis, we found increased risk of second miscellaneous cancers, including MDS and adenocarcinoma, unspecified, but could not evaluate specific type of other cancers in detail.6 A recent collborative study from our group with a larger cohort size found BRAF V600E to be associated with SPMs.30 These data, in combination with our own case with similar SRSF2 mutation in LCH and AML, suggest a biological link between LCH and other myeloid malignancies, thus necessitating further studies to assess whether these myeloid neoplasms are clonally related to LCH.
There were a variety of solid organ neoplasms occurring before, concomitantly with, and subsequent to the diagnosis of LCH. These findings have been reported in other studies, with an especially increased risk of lung cancer among individuals with pulmonary LCH, highlighting a relationship with tobacco smoking.31-33 Most of the second cancers were identified based on workup ensuing from abnormal clinical/laboratory findings, underscoring the need for clinicians to have high index of suspicion for such diagnoses. Cardiovascular disease as a cause of death could be related to the aging process itself, concomitant smoking, LCH treatments, or disease related inflammation. Second myeloid malignancies and cardiovascular disease could also be related to underlying clonal hematopoiesis, which was reported to have a high prevalence in ECD and warrants evaluation in LCH.34,35
Our study also confirmed the association of risk organ involvement with poor prognosis, a notion that is established in pediatric LCH but not among adults. The mortality was especially high among patients with PSC. There is a lack of data on optimal treatment of this high-risk subgroup in adults. In a recent and the only clinical trial for adults with LCH in the last 2 decades, an intensive treatment regimen consisting of high-dose systemic methotrexate and cytarabine was used.36 Although this regimen was associated with high overall response rates (88%), liver and spleen involvement remained associated with poor survival. Therefore, the risk organ phenotype of LCH represents an area of unmet need, and future studies evaluating novel therapeutic approaches, especially BRAF and MEK inhibitors, are warranted.10-12 Future studies should also aim to determine genomic predictors of risk organ involvement so that such cases can be captured earlier.
In pediatric LCH, the terms unifocal and multifocal are used only in the context of bone disease. The consensus classification terminology for adult LCH, in contrast, does not limit the definition to osseous disease only.1 In our cohort, we explored the impact of these 2 definitions on outcomes and found that the PFS within unifocal and single-system multifocal LCH did not vary based on bone vs nonbone disease, thereby reinforcing the definitions as proposed by the adult LCH classification. Future studies should aim to harmonize definitions between the two age groups based on prognostic implications.
A large study from pediatric LCH showed that patients with BRAFV600E were younger, had more high-risk features, and had a higher risk of disease reactivation than the non-BRAFV600E counterparts, but such studies for adults were lacking.37 In our study, however, we did not find any correlation between the BRAF status and clinical phenotypes or outcomes. Interestingly, we found that BRAFV600E was significantly associated with increasing age among adults. Although the lack of such an association could be because of insufficient sample size, these findings raise the hypothesis that the pathogenesis and biology of BRAFV600E LCH may be different between pediatric and adult population. Additionally, we found that the lack of health insurance was associated with poorer outcomes on univariate analysis, a finding similar to that seen in the recent UK study7 showing worse survival among those residing in areas of socioeconomic deprivation. There is a need to examine the impact of various socioeconomic determinants of health and their effect on access to care among individuals with LCH.
Our study has a few notable limitations. Given the retrospective nature of the study, we were not able to conduct uniform response assessment and staging studies; therefore, the focus of the study was on clinically relevant end points such as PFS and OS. The study cohort spanned over 2 decades, and we could not account for changes in practice over the study period. Furthermore, given the low number of events, the multivariable analysis resulted in wide CIs. The study cohort was derived from a single, tertiary care institution and may not be reflective of the general LCH population. We did not have data on MAPK-ERK pathway mutations beyond BRAFV600E, so we could not evaluate the impact of other mutations on outcomes. The strengths of our study are the large cohort of confirmed cases of adults with LCH and the long follow-up duration that enables evaluation of long-term outcomes. To our knowledge, for the first time in LCH, our study used a methodology comparing the outcomes of people with LCH with that of a cohort of matched cancer-free individuals to ascertain the excess risk of mortality within the population.
In summary, our study shows that although the majority of patients with LCH remain alive at 5 years, they are at an increased risk of overall mortality compared with the general population. The prognosis of unifocal LCH is excellent and comparable with that of cancer-free individuals. Risk organ involvement highly correlated with disease progression and mortality, whereas BRAFV600E was not associated with outcomes. Although the most common cause of death was LCH in the first 5 years, other causes such as COPD and second cancers predominated during the subsequent period. Although we tried to dichotomize the cause-specific mortality into LCH-related and non–LCH-related health conditions, it is entirely possible that several of the deaths in the latter group are indeed a late complication of LCH such as second myeloid malignancies. Given that some of the causes of death are preventable or can be captured through screening studies, these findings have critical implications for the care of adults with LCH beyond their cancer-specific treatment. The posttreatment counseling for this population should include a plan for reducing health risk behaviors, such as smoking, and testing for second cancers at the onset of concerning symptoms. With the increasing use of targeted therapies that are administered for extended periods, the late morbidity and mortality patterns may evolve further especially as the survivor population ages; therefore, it is critical to continue investigations into new-onset health conditions and health-related quality of life to improve overall patient outcomes.
Acknowledgments
This study was supported, in part, by the University of Iowa/Mayo Clinic Lymphoma SPORE P50 CA97274 and by the Walter B. Frommeyer Jr Award in Investigative Medicine (G.G.).
Authorship
Contribution: G.G and A.A.A.-M. collected the data; G.G. wrote the first manuscript draft with assistance from A.A.A.-M.; statistical analysis was conducted by A.A.A.-M.; J.P.A., C.D., J.R.Y., S.D., R.V., K.L.R., J.H.R., C.R.B., C.J.D.-P., L.M.G., G.J.R., S.Z., W.O.T., M.J.K., A.R., N.N.B., M.V.S., and R.S.G. critically revised the manuscript for important intellectual content; and all authors were involved in drafting the manuscript and approved the final version.
Conflict-of-interest disclosure: G.G. served on an advisory board for Opna Bio LLC. R.V. has received research funding from Pfizer, Bristol Myers Squibb, and Sun Pharma. W.O.T. reported receiving grants from Mallinckrodt Pharmaceuticals and National Institutes of Health R01 NS113803 01A1. N.N.B. served on advisory boards for Vividion Therapeutics, Kymera, Secura Bio, Affimed GmbH, Astellas Pharma US, and Acrotech Biopharma LLC; reports no personal compensation and no relevance to this study; and serves on the scientific advisory committee for Acrotech Biopharma LLC, which has no relevance to this study and no personal compensation given. The remaining authors declare no competing financial interests.
A complete list of the members of the Mayo Clinic-University of Alabama at Birmingham Histiocytosis Working Group appears in the supplemental Appendix.
Correspondence: Ronald S. Go, Mayo Clinic, 200 1st St SW, Rochester, MN 55905; e-mail: go.ronald@mayo.edu; and Gaurav Goyal, Hematology-Medical Oncology, University of Alabama at Birmingham, 1802 6th Ave, South Suite 2555 NP, Birmingham, AL 35294; e-mail: ggoyal@uabmc.edu.
References
Author notes
Deidentified, raw data are available upon request from the corresponding authors, Gaurav Goyal (ggoyal@uabmc.edu) and Ronald S. Go (go.ronald@mayo.edu).
The full-text version of this article contains a data supplement.