Key Points
Midostaurin in combination with intensive chemotherapy was well-tolerated in fit adult patients with no upper age limit.
The combination showed high response rates irrespective of patient age, induction regimen, or the type of anthracycline used in induction.
Abstract
The pivotal RATIFY study demonstrated midostaurin (50 mg twice daily) with standard chemotherapy significantly reduced mortality in adult patients (<60 years) with newly diagnosed (ND) FLT3mut acute myeloid leukemia (AML). Considering that AML often present in older patients who show poor response to chemotherapy, this open-label, multicenter phase 3b trial was designed to further assess safety and efficacy of midostaurin plus chemotherapy in induction, consolidation, and maintenance monotherapy in young (≤60 years) and older (>60 years) patients with FLT3mut ND-AML. Compared with RATIFY, this study extended midostaurin treatment from 14 days to 21 days, substituted anthracyclines (idarubicin or daunorubicin), and introduced variation in standard combination chemotherapy dosing (“7+3” or “5+2” in more fragile patients). Total 301 patients (47.2% >60 years and 82.7% with FLT3-ITDmut) of median age 59 years entered induction phase. Overall, 295 patients (98.0%) had at least 1 adverse event (AE), including 254 patients (84.4%) with grade ≥3 AE. The grade ≥3 serious AEs occurred in 134 patients. No difference was seen in AE frequency between age groups, but grade ≥3AE frequency was higher in older patients. Overall, complete remission (CR) rate including incomplete hematologic recovery (CR + CRi) (80.7% [95% confidence interval, 75.74-84.98]) was comparable between age groups (≤60 years [83.5%]; >60 to ≤70 years [82.5%]; in patients >70 years [64.1%]) and the type of anthracycline used in induction. CR + CRi rate was lower in males (76.4%) than females (84.4%). Overall, the safety and efficacy of midostaurin remains consistent with previous findings, regardless of age, sex, or induction regimen. The trial is registered at www.clinicaltrials.gov as #NCT03379727.
Introduction
Acute myeloid leukemia (AML) represents up to 1% of all new cancer cases and is the most common type of acute leukemia in adults,1,2 with a reported estimated incidence of 4.0 and 3.7 cases per 100 000 in the United States and European Union,3 respectively. Although advances in AML treatment have been made, the risk of relapse remains a major challenge; the cumulative incidence of relapse at 3 years is ∼50% to 85% depending on patient age and genetic characteristics of the leukemia.4-9 The incidence of AML increases with age, with a median age of 68 to 70 years at diagnosis.2,10 Older patients with AML are more likely to have AML with unfavorable cytogenetics and poorer outcomes with treatment.1,11
Approximately 30% of adults with AML carry mutations in the FMS-like tyrosine kinase 3 (FLT3) gene, including internal tandem duplications (ITD, 25%) and tyrosine kinase domain (TKD, 5%) mutations.12FLT3-ITD and FLT3-TKD mutations constitutively activate FLT3 receptor and downstream signaling pathways that regulate the proliferation and differentiation of normal hematopoietic progenitor cells, leading to increased cell survival and proliferation.13 Patients with FLT3-ITDmut AML, particularly those with a high allelic ratio of FLT3-ITD relative to the wild-type FLT3, have an increased risk of relapse and poor survival compared with those without the mutation; however, the effect of FLT3-TKD mutations on patient prognosis remains uncertain.14-16 Current guidelines recommend to consider response to initial therapy and assessment of early measurable residual disease in individual risk assessment in addition to baseline genetic characterization (patients with FLT3-ITDmut AML are now categorized as intermediate-risk irrespective of allelic ratio or NPM1 mutation) for determining AML prognosis and optimal treatment.14,17
Midostaurin is an oral, small molecule inhibitor of multiple tyrosine kinases that directly inhibits FLT3 signaling and induces apoptosis in FLT3-mutated (ITD or TKD) leukemic cells.18-20 In the registrational RATIFY trial, patients with newly diagnosed FLT3-mutated AML who received midostaurin plus standard “7+3” cytarabine (200 mg/m2 per day) induction chemotherapy with daunorubicin (60 mg/m2 per day), midostaurin plus high-dose cytarabine consolidation therapy, followed by single-agent midostaurin maintenance therapy had statistically significant and clinically meaningful improvement in overall survival (OS) and event-free survival (EFS) compared with those who received placebo plus standard chemotherapy.21 The results from RATIFY led to worldwide approval of midostaurin in combination with standard induction and consolidation chemotherapy for the treatment of adult patients with newly diagnosed FLT3-mutated AML22,23 and as a single-agent maintenance therapy outside of the United States and Canada.24
In the RATIFY study, patients aged <60 years were eligible to participate; however, given that older patients constitute the majority of AML cases and often respond poorly to standard therapy, limited information is available about patients ≥60 years of age. Following a similar study design as RATIFY trial, a phase 2 trial by the German-Austrian AML Study Group (AMLSG) investigated the effect of midostaurin plus intensive chemotherapy in older patients (60-70 years) and the use of midostaurin as maintenance after allogenic hematopoietic cell transplant. Among 284 patients, 86 patients aged 61 to 70 years demonstrated a complete remission (CR), including incomplete hematologic recovery (CRi) (CR + CRi) rate of 77.9% and EFS and OS at 2 years of 53% (95% confidence interval [CI], 46-61) and 46% (95% CI, 35-59), respectively, in older patients.24 Recently, the final analysis of the AMLSG trial in all 440 patients (128 patients aged 61-70 years) concluded that midostaurin in combination with chemotherapy significantly improved outcomes in both younger and older patients with AML and FLT3-ITD.25 Further investigation on the safety and efficacy of midostaurin in fit patients of older age is still required.2,11,26 Owing to the absence of any objective and generally accepted definition of fitness for treatment, the decision to start older patients on intensive chemotherapy rests on the clinical judgment of the treating physician, according to local guidelines.27 When feasible, real-world practice is to offer standard induction chemotherapy even to older patients, with many institutions, mainly in Europe, using idarubicin instead of daunorubicin. Although “7+3” is the standard combination of cytarabine with any of the 2 anthracyclines, some institutions prefer less intensive chemotherapy regimen in older and/or less fit patients to decrease the risk of mortality because of therapy.28,29 Most of the remaining patients who are considered unfit for induction chemotherapy receive less intensive therapies with the backbone of hypomethylating agents associated with novel agents.14,17
Therefore, the safety of combining midostaurin with intensive chemotherapy in clinical practice, especially in older patients, needs to be investigated further. Additional questions to address are (1) whether idarubicin and daunorubicin, the anthracyclines used in the RATIFY trial, leads to similar outcomes and (2) whether reducing the intensity of induction from “7+3” to the less standard “5+2” regimen has a negative impact on response in older patients. To address these questions in FLT3mut AML, a new phase 3b study was initiated that allowed for the enrollment of both younger (aged <60 years) and older patients (aged ≥60 years) and accepted variations in the induction chemotherapy regimens administered.
Methods
Study design
This was an open-label, multicenter, single-arm, phase 3b study (EudraCT number 2016-004440-12, NCT03379727) in adult patients (with no upper age limit) with newly diagnosed FLT3-mutated AML who were eligible for “7+3” or “5+2” chemotherapy regimens (ie, consists of 7 [or 5] days of standard-dose cytarabine, and 3 [or 2] days of an anthracycline antibiotic or anthracenedione or anthraquinone, most often daunorubicin). The study protocol and all amendments were reviewed by the independent ethics committee or institutional review board for each center. The study was conducted according to ICH E6 Guideline for Good Clinical Practice that have their origin in the Declaration of Helsinki. All patients provided written informed consent before any screening procedure. The trial was conducted in 15 countries across Europe; the first patient was enrolled in February 2018, and the enrollment was closed in January 2020. The estimated study duration was ∼3.5 years, which included ∼18 months for recruitment and ∼18 to 25 months for the completion of the induction, consolidation, and maintenance therapy. Once treatment was completed or discontinued, patients were followed in the 30-day safety follow-up, but there was no further evaluation for efficacy or survival. Investigators obtained locally approved FLT3 testing results before informed consent form signature to allow participants to initiate midostaurin treatment per protocol; data on other mutational status, including NPM1 mutation status, were also collected (locally) if available.
Eligible patients received either “7+3” or “5+2” induction chemotherapy per the discretion of the investigator; once induction started, patients were not allowed to switch between induction regimens. In addition to induction chemotherapy, patients received oral midostaurin 50 mg (2 25-mg capsules) twice daily on days 8 to 28 during 1 or 2 cycles of induction chemotherapy. Patients, who achieved CR or CR /CRi after induction chemotherapy, received high-dose cytarabine plus midostaurin 50 mg (2 25-mg capsules) twice daily on days 8 to 28 for up to 4 cycles of consolidation chemotherapy. Patients, who remained in CR/CRi after completion of consolidation chemotherapy, entered a maintenance phase and received midostaurin (50 mg twice daily) as a single agent on days 1 to 28 for up to 12 cycles. There were recovery periods between 2 treatment phases. Figure 1 describes the treatment protocol in detail.
Patients
Patients, aged ≥18 years, with newly diagnosed AML (per World Health Organization 2008 classification) and documented FLT3-ITD and/or FLT3-TKD mutations, with an Eastern Cooperative Oncology Group performance status (ECOG PS) of ≤2, who had not received prior therapy for AML (except for limited emergency treatments such as leukapheresis, hydroxyurea for ≤7 days for hyperleukocytosis, cranial radiation therapy for leukostasis of the central nervous system, or growth factor/cytokine support), and who had given written informed consent, were enrolled in this study. Exclusion criteria included left ventricular ejection fraction (LVEF) <45%, any pulmonary infiltrate, corrected QT (QTc) >470 ms, history of hypersensitivity of any drug, pregnancy, etc. (details provided in supplemental Table 1).
Study objectives and assessments
The primary objective of this study was to further assess the safety of midostaurin in induction, consolidation, and maintenance therapy, including the “7+3” regimen, daunorubicin (60-90 mg/m2 per day), the substitution of daunorubicin by idarubicin (12 mg/m2 per day), cytarabine (100-200 mg/m2 per day), and also allowing the “5+2” reduced dose regimen. Safety was monitored by assessing the proportion of patients with adverse events (AEs), serious AEs (SAEs), AEs leading to discontinuation, and deaths. The secondary objectives was to assess the clinical efficacy of midostaurin in combination with chemotherapy regimens in induction and consolidation and the clinical efficacy of midostaurin maintenance phase (measured by proportion of patients with CR/CRi rate as per local assessment).
Statistical analysis
No statistical hypotheses were tested in this study, and no imputation was applied for any missing data. The planned enrollment for this study was ∼300 patients, based on a sample size of 300 patients providing a 95% probability of observing at least 1 patient with an AE, assuming a true probability of occurrence of 1%. Safety, patient demographics, and baseline characteristics data were summarized descriptively for all patients who received at least 1 dose of midostaurin. All AEs were assessed according to the Common Terminology Criteria for AEs version 4.03. Patient safety data were further analyzed according to age (≥60 years and <60 years) and/or induction drug (daunorubicin or idarubicin). Given that midostaurin is a substrate of cytochrome P450 3A4, a post hoc analysis of safety data in patients who received midostaurin and concomitant antifungal agents (strong cytochrome P450 3A4 inhibitors) was also conducted. For all safety analyses, the safety set was used. The safety set (N = 301) was defined as all patients who received at least 1 dose of midostaurin. Clinical efficacy rate was defined as the proportion of participants with CR or CRi as per local assessment and was calculated based on the full analysis set (N = 300), which is defined as all patients who received at least 1 dose of midostaurin and had a documented FLT3 mutation (ITD or TKD). The rate of CR/CRi with 95% CI were assessed in all patients who had a documented FLT3 mutation and received at least 1 dose of midostaurin.
Results
Patient disposition and baseline characteristics
Of the 301 patients, 210 (69.8%) completed the induction phase (183 patients received 1 cycle and 27 patients received 2 cycles) and entered consolidation. Ninety-three (44.3%) patients of those 210 patients, who entered the consolidation phase, entered maintenance (Figure 2). For patients who did not enter maintenance phase, the most common reasons for study discontinuation during the consolidation phase included physician decision (primarily to undergo hematopoietic stem cell transplant [HSCT]), progressive disease (PD)/lack of efficacy, and AEs. A total of 89 patients (29.6%) underwent HSCT, including 6 patients moving to HSCT after induction failure, 75 patients from the consolidation phase after CR + CRi, and 8 patients moving to HSCT during the maintenance phase; however, please note that there was 1 more patient (protocol deviation; the patient is excluded from analysis), who underwent HSCT before the induction phase.
Patient demographics and baseline characteristics are summarized in Table 1. The median age was 59 years (range, 19-85), with 47.2% of patients aged >60 years. Majority of patients were female (53.2%) and had ECOG PS of 1 (51.5%). Most patients (82.7%) had an FLT3-ITD mutation, with 17.6% having an FLT3-TKD mutation. During induction, 80.4% of patients received midostaurin plus “7+3” and 19.6% received midostaurin plus “5+2”. In the induction phase, 135 patients (44.9%) received daunorubicin (110 and 25 patients as part of “7+3” and “5+2,” respectively) and 166 patients (55.1%) received idarubicin (132 and 34 patients as part of “7+3” and “5+2,” respectively). The majority of the patients (84/135 [62.2%]), who received daunorubicin, were younger (≤60 years); whereas most patients (91/166 [54.8%]), receiving idarubicin, were older (>60 years). Patients receiving daunorubicin demonstrated better performance status (88.9% vs 79.5% had ECOG PS ≤ 1 and 10.4% vs 18.1% had ECOG PS = 2) and were more likely to be female (57.8% vs 49.4%) than patients receiving idarubicin (supplemental Table 2).
Patient demographics and baseline characteristics
| Characteristics . | All patients (N = 301) . |
|---|---|
| Age, median (range), y | 59.0 (19-85) |
| ≤60 y, n (%) | 159 (52.8) |
| >60 y, n (%) | 142 (47.2) |
| Time (d) since initial diagnosis to start of CT, median (range) | 5.0 (0.0-76.0) |
| Male, n (%) | 141 (46.8) |
| Female, n (%) | 160 (53.2) |
| White, n (%) | 225 (74.8) |
| BMI, median (range), kg/m2 | 25.7 (17.0-53.4) |
| Creatinine, median (range), μmol/L∗ | 61.9 (0.6-187)† |
| Lactate dehydrogenase, median (range), U/L∗ | 330.0 (0.8-7745)‡ |
| Bilirubin, median (range), μmol/L∗ | 13.7 (0.7-66.2)§ |
| ECOG PS, n (%) | |
| 0 | 97 (32.2) |
| 1 | 155 (51.5) |
| 2 | 44 (14.6) |
| Missing | 5 (1.7) |
| AML mutational status, n (%) | |
| FLT3 mutation (ITD and/or TKD)‖ | 300 (99.7) |
| FLT3-ITD | 249 (82.7) |
| FLT3-TKD | 53 (17.6) |
| NPM1 | 126 (41.9) |
| Other | 43 (14.3) |
| Peripheral blood and bone marrow at baseline¶, median (range) | |
| Hemoglobin, g/L | 83.0 (58.0-126.0) |
| Platelet count, 109/L | 23.0 (3.0-225.0) |
| Bone marrow blasts, % | 76.0 (1.0-99.0) |
| Absolute neutrophil count, 109/L | 0.2 (0.0-33.0) |
| WBC count (109/L) | 0.6 (0.01-198.4) |
| Induction chemotherapy group, n (%) | |
| 7+3 | 242 (80.4) |
| 5+2 | 59 (19.6) |
| Induction chemotherapy group by age, n (%) | |
| 7+3 | |
| ≤60 y, n (%) | 145 (48.2) |
| >60 y, n (%) | 97 (32.2) |
| 5+2 | |
| ≤60 y, n (%) | 14 (4.7) |
| >60 y, n (%) | 45 (15.0) |
| Induction chemotherapy group by drug, n (%) | |
| 7+3 | |
| Daunorubicin | 110 (36.5) |
| Idarubicin | 132 (43.9) |
| 5+2 | |
| Daunorubicin | 25 (8.3) |
| Idarubicin | 34 (11.3) |
| Characteristics . | All patients (N = 301) . |
|---|---|
| Age, median (range), y | 59.0 (19-85) |
| ≤60 y, n (%) | 159 (52.8) |
| >60 y, n (%) | 142 (47.2) |
| Time (d) since initial diagnosis to start of CT, median (range) | 5.0 (0.0-76.0) |
| Male, n (%) | 141 (46.8) |
| Female, n (%) | 160 (53.2) |
| White, n (%) | 225 (74.8) |
| BMI, median (range), kg/m2 | 25.7 (17.0-53.4) |
| Creatinine, median (range), μmol/L∗ | 61.9 (0.6-187)† |
| Lactate dehydrogenase, median (range), U/L∗ | 330.0 (0.8-7745)‡ |
| Bilirubin, median (range), μmol/L∗ | 13.7 (0.7-66.2)§ |
| ECOG PS, n (%) | |
| 0 | 97 (32.2) |
| 1 | 155 (51.5) |
| 2 | 44 (14.6) |
| Missing | 5 (1.7) |
| AML mutational status, n (%) | |
| FLT3 mutation (ITD and/or TKD)‖ | 300 (99.7) |
| FLT3-ITD | 249 (82.7) |
| FLT3-TKD | 53 (17.6) |
| NPM1 | 126 (41.9) |
| Other | 43 (14.3) |
| Peripheral blood and bone marrow at baseline¶, median (range) | |
| Hemoglobin, g/L | 83.0 (58.0-126.0) |
| Platelet count, 109/L | 23.0 (3.0-225.0) |
| Bone marrow blasts, % | 76.0 (1.0-99.0) |
| Absolute neutrophil count, 109/L | 0.2 (0.0-33.0) |
| WBC count (109/L) | 0.6 (0.01-198.4) |
| Induction chemotherapy group, n (%) | |
| 7+3 | 242 (80.4) |
| 5+2 | 59 (19.6) |
| Induction chemotherapy group by age, n (%) | |
| 7+3 | |
| ≤60 y, n (%) | 145 (48.2) |
| >60 y, n (%) | 97 (32.2) |
| 5+2 | |
| ≤60 y, n (%) | 14 (4.7) |
| >60 y, n (%) | 45 (15.0) |
| Induction chemotherapy group by drug, n (%) | |
| 7+3 | |
| Daunorubicin | 110 (36.5) |
| Idarubicin | 132 (43.9) |
| 5+2 | |
| Daunorubicin | 25 (8.3) |
| Idarubicin | 34 (11.3) |
AML, acute myeloid leukemia; BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group performance status; HSCT, hematopoietic stem cell transplant; ITD, internal tandem duplication.
Baseline was defined as the latest assessment before the start of midostaurin (including assessment during chemotherapy treatment period).
n = 300.
n = 274.
n = 297.
ITD and TKD categories are not mutually exclusive. Two patients had both FLT3-ITD and TKD mutations. One patient did not have a FLT3 mutation and was excluded from the efficacy analysis.
Baseline was defined as the latest assessment prior to start of midostaurin
Exposure to treatment
The overall median duration of exposure to midostaurin was 101 days (range, 1-597) in the safety population. The median duration was 21 days (range, 1-44) in the induction phase, 42 days (range, 2-88) in the consolidation phase, and 321.5 days (range, 6-372) in the maintenance phase.
Safety
Overall, 295 patients (98.0%) had at least 1 AE, regardless of relationship with study treatment, including 254 patients (84.4%) who had a grade ≥3 AE. The number of patients experiencing AEs was higher in the induction (94.7%) and consolidation (92.9%) phases than in the maintenance phase (62.4%); however, no unexpected AEs were observed in this study other than those typically associated with intensive chemotherapy for AML. Overall, the most frequent AEs by preferred term (PT) in the induction phase (≥20% of patients) were diarrhea (32.6%), pyrexia (30.9%), nausea (25.2%), and febrile neutropenia (24.3%). In the consolidation phase, the most frequent AEs were pyrexia (34.8%), febrile neutropenia (32.9%), nausea (31.9%), neutropenia (24.3%), and headache (22.4%). The AE frequencies were lower in the maintenance phase with the most frequent being nausea (19.4%) and neutropenia (12.9%). Proportion of patients experiencing at least 1 SAE was higher in the induction (25.2%) and consolidation (31.9%) phases than during the maintenance (7.5%) phase. SAEs were reported in 137 patients (45.5%), with 134 patients (44.5%) experiencing grade ≥3 events. The grade 3 SAEs included febrile neutropenia (10.6%), sepsis (4.7%), pneumonia (4.0%), septic shock (3.7%), and respiratory failure (2.3%) (supplemental Table 3).
Overall, the safety profile was comparable in patients who received daunorubicin (n = 135) or idarubicin (n = 166) in the induction phase. However, the proportion of patients experiencing grade ≥3 AEs (80.7% vs 87.3%), ≥1 SAE (41.5% vs 48.8%), and on-treatment deaths (4.4% vs 7.8%) were cumulatively lower in daunorubicin vs idarubicin groups for the entire treatment period (supplemental Table 4).
Overall, the safety profile was comparable between younger (≤60 years) and older patients (>60 years) for the entire treatment duration (supplemental Table 5). However, older patients had a higher frequency of grade ≥3 AEs (90.1% vs 79.2%), SAEs (54.2% vs 37.7%), and AEs leading to treatment discontinuation (16.9% vs 10.1%) than younger patients. A total of 35 patients died, including 19 patients who died on-treatment and 16 died >30 days after last treatment. Among those 19 patients (6.3%) who died on-treatment (primary reasons: cardiac arrest in 3, respiratory failure in 2, and the remaining 14 deaths were due to a different cause each time), 15 were older patients (9 patients aged >60 to ≤70 years and 6 patients aged >70 years), whereas 4 were younger (<60 years). Sixteen of these 19 patients died during induction and 3 during consolidation phases, with no deaths occurring during the maintenance phase. Of those 19 deaths, 4 (patients aged >60 years) were considered treatment-related. Those 4 patients received idarubicin as the induction drug (treatment group: midostaurin [50 mg twice daily] + cytarabine + idarubicin), and the reasons for death were myocardial infarction (during induction phase), cardiac arrest (consolidation), interstitial lung disease (induction), and septic shock (induction), respectively.
An overview of AEs (>15%) by PT and age group is presented in Table 2. Although nausea (43.4% vs 36.6%) and headache (27.0% vs 11.3%) were more frequent in younger patients, stomatitis (21.4% vs 26.8%), asthenia (9.4% vs 16.2%), and edema peripheral (4.4% vs 15.5%) were more frequent in older patients. However, no meaningful differences were seen in the frequencies of AEs (grade ≥3) by PT between the 2 age groups.
Overview of adverse event (by preferred term) by grades and by age — safety set
| AEs (>15%) by PT n (%) . | All grades . | Grade ≥3 . | ||
|---|---|---|---|---|
| ≤60 y (n = 159) . | >60 y (n = 142) . | ≤60 y (n = 159) . | >60 y (n = 142) . | |
| Febrile neutropenia | 57 (35.8) | 56 (39.4) | 51 (32.1) | 46 (32.4) |
| Neutropenia | 35 (22.0) | 35 (24.6) | 32 (20.1) | 33 (23.2) |
| Anemia | 28 (17.6) | 24 (16.9) | 21 (13.2) | 17 (12.0) |
| Thrombocytopenia | 30 (18.9) | 22 (15.5) | 29 (18.2) | 21 (14.8) |
| Diarrhea | 63 (39.6) | 60 (42.3) | 2 (1.3) | 4 (2.8) |
| Nausea | 69 (43.4) | 52 (36.6) | 7 (4.4) | 6 (4.2) |
| Stomatitis | 34 (21.4) | 38 (26.8) | 10 (6.3) | 9 (6.3) |
| Constipation | 31 (19.5) | 34 (23.9) | 0 | 0 |
| Vomiting | 36 (22.6) | 33 (23.2) | 0 | 2 (1.4) |
| Pyrexia | 75 (47.2) | 65 (45.8) | 3 (1.9) | 8 (5.6) |
| Asthenia | 7 (4.4) | 23 (16.2) | 0 | 2 (1.4) |
| Edema peripheral | 15 (9.4) | 22 (15.5) | 0 | 1 (0.7) |
| Headache | 43 (27.0) | 16 (11.3) | 4 (2.5) | 0 |
| Rash | 37 (23.3) | 28 (19.7) | 1 (0.6) | 2 (1.4) |
| AEs (>15%) by PT n (%) . | All grades . | Grade ≥3 . | ||
|---|---|---|---|---|
| ≤60 y (n = 159) . | >60 y (n = 142) . | ≤60 y (n = 159) . | >60 y (n = 142) . | |
| Febrile neutropenia | 57 (35.8) | 56 (39.4) | 51 (32.1) | 46 (32.4) |
| Neutropenia | 35 (22.0) | 35 (24.6) | 32 (20.1) | 33 (23.2) |
| Anemia | 28 (17.6) | 24 (16.9) | 21 (13.2) | 17 (12.0) |
| Thrombocytopenia | 30 (18.9) | 22 (15.5) | 29 (18.2) | 21 (14.8) |
| Diarrhea | 63 (39.6) | 60 (42.3) | 2 (1.3) | 4 (2.8) |
| Nausea | 69 (43.4) | 52 (36.6) | 7 (4.4) | 6 (4.2) |
| Stomatitis | 34 (21.4) | 38 (26.8) | 10 (6.3) | 9 (6.3) |
| Constipation | 31 (19.5) | 34 (23.9) | 0 | 0 |
| Vomiting | 36 (22.6) | 33 (23.2) | 0 | 2 (1.4) |
| Pyrexia | 75 (47.2) | 65 (45.8) | 3 (1.9) | 8 (5.6) |
| Asthenia | 7 (4.4) | 23 (16.2) | 0 | 2 (1.4) |
| Edema peripheral | 15 (9.4) | 22 (15.5) | 0 | 1 (0.7) |
| Headache | 43 (27.0) | 16 (11.3) | 4 (2.5) | 0 |
| Rash | 37 (23.3) | 28 (19.7) | 1 (0.6) | 2 (1.4) |
AE, adverse event; PT, preferred term.
Further analysis in patients treated with daunorubicin/idarubicin by age revealed that older patients who received idarubicin experienced a higher frequency of grade ≥3 AEs (93.4%) and treatment-related SAEs (29.7%) than patients treated with daunorubicin (irrespective of age group) or young patients treated with idarubicin (supplemental Table 6).
Overall, the treatment-related AE profile was similar to AE, with the most frequently occurring treatment-related AEs of grade ≥3 being febrile neutropenia (18.3%), neutropenia (14.3%), and thrombocytopenia (11.0%) (supplemental Figure 1; supplemental Table 7).
Of 301 patients, 227 received concomitant antifungal agents. Overall, the frequency of AEs was comparable between patients who received concomitant antifungal agents and those who did not (99.1% vs 94.6%, respectively) during the entire treatment period; however, patients treated with antifungal agents had a higher frequency of grade ≥3 AEs (87.2% vs 75.7%), SAEs (48.5% vs 36.5%), and AEs leading to treatment discontinuation (10.6% vs 6.8%) than those who did not. There were 3 treatment-related deaths in patients who received concomitant antifungal agents (supplemental Table 8).
Throughout the entire treatment period, 60 patients (23.9%; n = 251) had an increase in corrected QT interval by Fredericia (QTcF) >30 ms and ≤60 ms, whereas 27 patients (10.8%; n = 251) had an increase of >60 ms. Forty-seven patients had a new QTcF prolongation: 28 patients (11.9%; n = 236) >450 to ≤480 ms, 9 patients (3.5%; n = 255) new >480 to ≤500 ms, and 10 patients (3.9%; n = 257) (supplemental Table 9). However, QT prolongation was qualified as an AE (any grade) in 11.3% only, whereas only 5.3% patients had grade ≥3 QT prolongation. None of the instances of QTcF prolongation led to clinical sequelae.
Efficacy
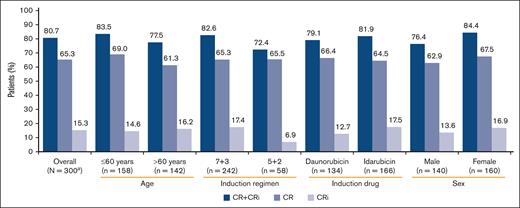
Of the 301 patients who received treatment, 1 patient did not have an FLT3 mutation and was not included in the efficacy analyses. Of 300 patients, 196 (65.3%) had a CR, whereas 46 patients (15.3%) had a CR with CRi, resulting in an overall CR + CRi rate of 80.7% (95% CI, 75.74-84.98) (Figure 3). CR + CRi rates were notably similar between age and the type of anthracycline used subgroups and slightly higher in patients receiving the “7+3” regime and males. Overall, there were 54 patients (17.9%) who experienced relapse, including 10 patients who had relapse >30 days after the last study treatment.
Response to midostaurin plus CT by patient subgroup (full analysis set). One patient who received treatment did not meet all study criteria and was not included in efficacy analyses. BL, baseline; CR, complete remission; CRi, complete remission with incomplete hematologic recovery; CT, chemotherapy.
Response to midostaurin plus CT by patient subgroup (full analysis set). One patient who received treatment did not meet all study criteria and was not included in efficacy analyses. BL, baseline; CR, complete remission; CRi, complete remission with incomplete hematologic recovery; CT, chemotherapy.
Assessment of hematologic recovery over time showed a reduction in median bone marrow blasts and improvement in median peripheral neutrophils and platelets to within normal range by the end of induction cycle 1 (Figure 4). A similar trend was observed irrespective of the anthracyclines used or the induction regimen (“7+3” or “5+2”) (supplemental Figures 2 and 3).
Hematologic recovery over time. Representing median values (A) bone marrow blast cells, (B) peripheral neutrophils, and (C) peripheral platelets over time. BL, baseline; CC, consolidation cycle; EOT, end of treatment; IC, induction cycle; MC, maintenance cycle.
Hematologic recovery over time. Representing median values (A) bone marrow blast cells, (B) peripheral neutrophils, and (C) peripheral platelets over time. BL, baseline; CC, consolidation cycle; EOT, end of treatment; IC, induction cycle; MC, maintenance cycle.
Discussion
Although the RATIFY study evaluated midostaurin in combination with standard chemotherapy in patients with FLT3-mutated AML aged ≤60 years, this study extends the findings by allowing for enrollment of both younger and older adult patients, as well as variations in chemotherapy regimens. In fact, 47% of patients were aged ≥60 years, and the treatment was even administered to the patients aged up to 85 years if they were deemed fit. Compared with RATIFY, this study allowed for a higher dose of daunorubicin, the substitution of daunorubicin by idarubicin, a lower dose of cytarabine, and the “5+2” reduced dose regimen for induction. Although the “5+2” dose regimen is not considered a standard, some institutions prefer this attenuated schedule for patients who may be less likely to tolerate full dosing with “7+3” regimen. Moreover, this reduced induction could allow extending the use of chemotherapy and midostaurin to more fragile patients. However, we continue considering that “7+3” plus midostaurin remains the standard of care. Approximately 80% of patients received the more intense “7+3” induction regimen, regardless of the type of anthracycline used. Notably, ∼55% of patients received idarubicin as part of their induction regimen.
Despite the variations in chemotherapy regimens and a longer treatment period for midostaurin (days 8-28 of each cycle) compared with that of the RATIFY study (days 8-21 of each cycle), the safety profile of midostaurin plus chemotherapy in older and younger patients was consistent with what has been previously reported,21 with no new safety signals identified. As expected, the percentages of patients with AEs and grade ≥3 AEs in the maintenance phase (62.4% and 20.4%, respectively) were notably lower than those in the earlier phases of treatment, supporting that midostaurin maintenance is well tolerated. No significant differences were observed in the overall AE frequencies across age groups. A comparison of the safety profile in younger (≤60 years) and older patients (>60 years) revealed no relevant differences, other than those that can be expected in older patients, such as a higher mortality rate (10.6% vs 2.5%), higher incidence of SAEs (54.2% vs 37.7%), and grade ≥3 AEs (90.1% vs 79.2%) compared with younger patients. This could plausibly be explained by the frailty of older patients treated with “7+3” or “5+2” induction chemotherapy compared with younger patients. It is also important to note that on-treatment deaths (16/19) mostly occurred during the induction phase and can be attributed to chemotherapy. There was no death reported in the maintenance phase. The higher rate of mortality in older patients is consistent with the reported literature in older patients with AML in this setting.30,31 A separate safety analysis for patients treated with daunorubicin or idarubicin did not show any significant differences between these 2 groups or with the overall analysis. Finally, there were no clinically relevant events of severe QTcF prolongation.
In this study, midostaurin in combination with intensive chemotherapy provided high response rates, irrespective of patient age, induction regimen (“7+3” or “5+2”), or the type of anthracycline used (daunorubicin or idarubicin) during the induction therapy. Overall, 80.7% of patients achieved CR + CRi and 65.3% of patients achieved CR, with similar rates observed in patient subgroup analyses based on age, induction regimen, induction drug, and gender. These response rates support the results observed in the RATIFY study, in which 59% of patients who received midostaurin achieved CR.21 The results from this study also align with those from a phase 2 study (the German-Austrian AMLSG trial) of midostaurin in combination with standard induction and consolidation chemotherapy, followed by SCT, which allowed for enrollment of adult patients aged up to 70 years; similar CR + CRi rates were observed after induction in younger and older patients, with a manageable safety profile.24,25 Furthermore, the multivariate analysis from the AMLSG trial showed significant beneficial effect of midostaurin on EFS and OS in both younger and older patients (61-70 years).25
There are a few limitations to this study. Because midostaurin plus chemotherapy is considered the standard of care for FLT3-mutated AML, there was no placebo arm for this trial, and measures of efficacy (CR + CRi rate) may not reflect the true magnitude of response to midostaurin, which has been shown to improve EFS and OS in the pivotal study. Given the primary end point of this study was safety, patients were not followed after treatment discontinuation, and survival analyses are not available. Despite these limitations, the results from this study support the safety and efficacy profile of midostaurin plus chemotherapy observed in the RATIFY study and more recent phase 2 studies, independent of patient age, gender, or induction regimen. As a result, the data suggest that midostaurin plus chemotherapy, including variations to standard chemotherapy regimens that reflect real-world clinical practice, is a safe treatment option with high response rate for adult patients of all ages with FLT3-mutated AML. We consider the findings from this safety study are meaningful for clinical practice.
Acknowledgments
The authors thank the patients and their families and investigators and study teams at each participating site. They also thank Twarita Chakraborty, Novartis Healthcare Private Limited, Hyderabad for providing medical writing/editorial support in accordance with Good Publication Practice (GPP2022) guidelines (https://www.ismpp.org/gpp-2022). The study was funded by Novartis.
Authorship
Contribution: J.S. provided clinical care, participated in research design, performed the research, analyzed the results, and contributed to writing the manuscript; P.M., X.T., L.G., T.C., D.C., O.L., C.M., M.L., and F.F. provided clinical care, performed the research, analyzed the results, and edited the manuscript; B.-R.B. participated in research design, involved in statistical analysis planning, performed data analysis, and wrote the manuscript; G.G. participated in research design and edited the manuscript; S.H. participated in research design, analyzed the results, and edited the manuscript; and A.R. and A.V. provided clinical care, participated in research design, analyzed the results, and edited the manuscript.
Conflict-of-interest disclosures: J.S. receives consultancy fee for advisory board from Novartis; honoraria for lectures and speaker bureau fee from Astellas and AbbVie; honoraria for lectures and speaker bureau fee and support for attending meetings and/or travel from Jazz Pharmaceuticals; and honoraria for advisory board from Genenta Science. T.C. receives consultancy fee for advisory board, honoraria for lectures and speaker bureau; and support for attending meetings and/or travel from Novartis; honoraria for lectures and speaker bureau from Astellas; consultancy fee and advisory board fee from AbbVie; consultancy fee, honoraria for lectures and speaker bureau, support for attending meetings and/or travel, and advisory board fee from Celgene/Bristol Myers Squibb; consultancy fee, advisory board fee, honoraria for lectures and speaker bureau, and support for attending meetings and/or travel from Jazz Pharmaceuticals; support for attending meetings and/or travel from Pfizer; and advisory board fee from Agios Servier. L.G. has a clinical study contract from Novartis; M.L. participates on a data safety monitoring board or advisory board for Novartis, Merck Sharp and Dohme, Jazz Pharma, AbbVie, Sanofi, Daiichi-Sankyo, Gilead Sciences, and Grifols. A.R. receives honoraria for lectures and speaker bureau, support for attending meetings and/or travel, and participation on a data safety monitoring board or advisory board fee from Novartis, Amgen, Pfizer, Astellas, Jazz, Janssen, Incyte, Kite-Gilead, Roche, and Omeros. B.-R.B., G.G., and S.H. are the employees of Novartis and along with the remaining authors declare no competing financial interests.
Correspondence: Jorge Sierra, Department of Hematology, Hospital de la Santa Creu i Sant Pau, Mas Casanovas 90, Barcelona 08041, Spain; e-mail: jsierra@santpau.cat.
References
Author notes
Novartis is committed to sharing with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. The availability of this trial data is according to the criteria and process described on www.clinicalstudydatarequest.com.
The full-text version of this article contains a data supplement.