Key Points
We show that patients gradually recover CD38 antibody sensitivity while on other treatments.
We conclude that re-treatment efficacy may improve by waiting 1 year before rechallenge and alternating anti-CD38 agents.
Abstract
Monoclonal antibodies targeting CD38 are important for treatment of both newly diagnosed and relapsed multiple myeloma (MM). Daratumumab and isatuximab are anti-CD38 antibodies with the US Food and Drugs Administration approval in multiple different combinations. Despite good initial efficacy, patients inevitably develop drug resistance. Whether patients can be effectively re-treated with these antibodies in subsequent lines of therapy is unclear. Thus far, studies have mostly been limited to clinical retrospectives with short washout periods. To answer whether patients regain sensitivity after longer washouts, we used ex vivo sensitivity testing to isolate the anti-CD38 antibody-specific cytotoxicity in samples obtained from patients who had been exposed to and then off daratumumab for up to 53 months. MM cells from patients who had been off daratumumab for >1 year showed greater sensitivity than those with <1 year, although they still were less sensitive than those who were daratumumab naïve. CD38 expression on MM cells gradually recovered, although, again, not to the level of anti-CD38 antibody–naïve patients. Interestingly, low MM CD38 explained only 45% of cases identified to have daratumumab resistance. With clinical follow-up, we found ex vivo sensitivity predicted subsequent clinical response but CD38 overexpression did not. Patients clinically re-treated with anti-CD38 antibodies had <6 months of clinical benefit, but 1 patient who was daratumumab exposed but not refractory achieved complete response lasting 13 months. We conclude that transient efficacy can be achieved by waiting 1 year before CD38 antibody rechallenge, but this approach may be best used as a bridge to, or after, chimeric antigen receptor T-cell therapy.
Introduction
Multiple myeloma (MM) is an incurable malignancy of antibody-producing plasma cells, afflicting >35 000 Americans per year.1 Patients typically present with bone marrow infiltration and can have complications that include renal failure, hypercalcemia, and bone lesions. Although controllable for extended periods, almost all patients ultimately succumb to their disease. Outcomes have substantially improved from the use of immunomodulatory drugs (IMiDs), proteasome inhibitors (PIs), and anti-CD38 monoclonal antibodies administered as multidrug combination regimens. Recently, a median survival of ∼10 years has been reached with optimal treatment, including autologous stem cell transplant and lenalidomide maintenance.2 Currently, the biggest challenge in managing patients with MM is that all of them will become drug resistant over time, and those that relapse after anti-CD38 monoclonal antibodies have a limited prognosis.3
CD38 is a transmembrane glycoprotein that is highly expressed on MM cells, holding dual functions in proliferative signaling and enzymatic production of cyclic adenosine 5′-diphosphate ribose, which regulates intracellular calcium.4 CD38 has been implicated in the interactions of MM with stromal cells, osteoblasts, and endothelial cells. Under resting physiological conditions, CD38 is relatively low in most normal lymphoid and myeloid cells, creating an attractive therapeutic window in MM. Currently, 2 anti-CD38 antibodies, daratumumab and isatuximab, are available, each binding to adjacent but distinct epitopes that may confer different activities.5-7 To varying degrees, both antibodies trigger a variety of mechanisms, including of antibody-dependent cellular cytotoxicity and phagocytosis, complement-dependent cytotoxicity, and direct apoptosis induction.8,9 Interestingly, targeting CD38 may also have immunomodulatory effects because anti-CD38 antibodies have been shown to decrease suppressive regulatory T and B cells while increasing effector T cells and their clonality in patients.10,11
Daratumumab was first approved for relapsed/refractory MM, with efficacy shown from monotherapy.12 Several studies have established that outcomes with daratumumab are enhanced in combinations with PIs, IMiDs, and dexamethasone.13-16 Daratumumab has also recently been approved in regimens for newly diagnosed patients.17-20 Despite the efficacy observed in these trials, daratumumab resistance eventually develops and patients relapse, and CD38 is downregulated from the MM cell surface.21,22 Resistance has also been linked to increased complement-inhibitor proteins, Fcγ receptor polymorphisms, and CD47 upregulation.23 Isatuximab has also been approved for relapsed/refractory MM in combinations with dexamethasone and pomalidomide or carfilzomib.24,25 To this point, the 2 CD38 antibodies have not been compared in clinical trials directly, and it is not known whether switching agents is of benefit upon re-treatment.
Recent clinical studies have asked whether patients benefit from re-treatment with anti-CD38 antibodies. A single prospective study described the efficacy of single-agent isatuximab in 32 patients who were refractory to daratumumab and had been off treatment for at least 6 weeks.26 The median time from the last daratumumab until isatuximab start was 13 weeks, and 59.4% had daratumumab in the last treatment line. The clinical benefit was minimal response in 1 patient and stable disease (SD) is 53.1%. Disease control >8 weeks was 58.3% in patients with at least 6 months off daratumumab, compared with 28.6% for those off <3 months, although the statical analysis of this difference was not reported. CD38 expression increased with time off and was associated with clinical benefit. In retrospective analyses, outcomes from re-treatment with CD38 antibodies have been mixed in combinations with various IMiDs or PIs, but the duration of response has mostly been short.27,28 Similar results have been found for patients previously treated with isatuximab.29,30 Taken together, there can be benefit from re-treatment with CD38 antibodies, but it remains unknown how much time off is optimal to improve outcomes.
We hypothesized that by increasing lengths of time, resistance to anti-CD38 antibodies in MM is reversible off therapy, and patients could benefit from optimizing re-treatment with daratumumab or isatuximab. To test this hypothesis, we used a previously reported laboratory assay termed Myeloma Drug Sensitivity Testing (My-DST) for profiling drug responses, including anti-CD38 antibodies, ex vivo. My-DST is performed on short term cultures of mononuclear cells (MNCs) from patients’ bone marrow aspirates, which include the immune cells from the endogenous microenvironment, enabling the measurement of antibody-dependent cellular cytotoxicity.31 Previously, we showed that drug sensitivity with My-DST correlated with clinical depth of response after 4 cycles of subsequent treatment.31 Thus, our objectives were to (1) determine what amount of time is optimal for re-treatment sensitivity, (2) measure CD38 levels with increasing periods off anti-CD38 antibody treatment, and (3) determine whether ex vivo measurement results predicted clinical outcomes from re-treatment.
Methods
My-DST
Bone marrow and blood samples were collected from patients at the University of Colorado, and Weill-Cornell Medicine Institutional Review Board approval and informed consent from the patients were obtained. Patients were eligible if they had previously been treated with daratumumab. Patient identification information was removed. MNCs were isolated from the samples by density gradient centrifugation using SepMate Ficoll-Plaque tubes. Samples were used fresh or cryopreserved in freezing medium composed of Iscove modified Dulbecco medium, 45% fetal bovine serum, and 10% dimethyl sulfoxide. Daratumumab and isatuximab were obtained from the University of Colorado Pharmacy. Unselected MNCs were incubated in triplicate wells with daratumumab, isatuximab, or untreated controls for 48 hours followed by flow cytometry to measure MM cell–specific viability. Sensitivity to drugs was determined by the loss of MM cell viability with at least 3 replicates per condition, normalized to the untreated controls, as described.31
Flow cytometry
Protein marker expression and MM cell viability were determined via flow cytometry. Flow cytometry was performed on a FACSCelesta with a high-throughput sampler. Results were analyzed using FlowJo software. Before staining, samples were incubated with Fc receptor–blocking reagent (Miltenyi). To identify viable MM cells, staining was performed with multiepitope anti-CD38-FITC (Cytognos, chosen to avoid masking from clinically administered antibodies), anti-CD138-BV421 MI15, anti-CD45-BV510 HI30, anti-CD19-BV786 SJ25C1, anti-CD46-APC E4.3, anti-CD55-PE IA10, anti-CD59-BV605 H19, anti-BCMA-PerCPCy5.5 19F2, and Live/Dead Fixable Near-IR Stain (Thermo Fisher Scientific). In parallel, natural killer cells were stained with anti-CD56-APCR700 and anti-CD16-BV786. All antibodies were from BD Biosciences, except where noted.
Statistics
All data are presented as mean and standard deviation. Two-tailed Student t test was used for comparing 2 means, and for >2 means, analysis of variance with Tukey correction was used. Significance levels are shown by ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. The stratification of patient characteristics was done with Fisher exact test or Student t test. Survival comparisons used Kaplan-Meier curves and univariate Cox proportional hazard methods.
Results
Recovered ex vivo sensitivity and CD38 expression with time off daratumumab
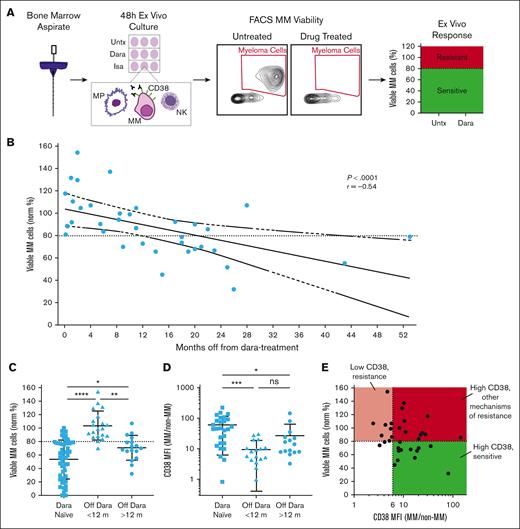
Re-treatment with anti-CD38 antibodies can be efficacious in patients refractory to daratumumab, but short washout periods have been used with limited benefits.26-28 To improve this, we used My-DST to test antibody sensitivity and target expression with longer periods of time off daratumumab (Figure 1A). Thirty-seven samples were accrued from 29 patients who previously had daratumumab in the relapsed setting and been off for periods up to 53 months (supplemental Table 1). Ex vivo depth of response to 48 hours daratumumab treatment correlated with the time elapsed since last anti-CD38 dose (r = −0.61; P < .0001; Figure 1B). The median number of treatment lines for this cohort was 5 (range, 2-11); 90% were daratumumab-refractory, and 42% were pentarefractory to IMiDs, PIs, and daratumumab (Table 1). From our prior study of anti-CD38 antibody–naïve patients, 20 nM daratumumab produced maximum efficacy at 48 hours and established a working sensitivity cutoff of 80% MM cell survival (ie, 20% decrease compared with untreated controls).31 Similar results were observed from titrating isatuximab for 4 anti-CD38 antibody treatment–naïve patients (supplemental Figure 1). Contrasting with our previously reported results from 52 anti-CD38 antibody–naïve patients, only 1 of 20 samples from patients off their last daratumumab dose for <12 months met our response cutoff after 48 hours, but 13 of 17 samples met the sensitivity cutoff from patients with >12 months off daratumumab (Figure 1C). Thus, ex vivo sensitivity of MM cells to daratumumab partially recovered after a 1-year washout.
Ex vivo sensitivity to daratumumab is gradually recovered after the drug is discontinued. (A) Schematic depiction of the My-DST workflow. Unselected MNCs were isolated from donated patient biopsy samples, and then cells were incubated with or without drug treatment for 48 hours before flow cytometry. Viability was normalized to that of Untx controls, and our established cutoff of 80% MM cell viability compared with untreated was used to classify patient samples as sensitive (green) or resistant (red). (B) Drug-sensitivity testing results for daratumumab (Dara) sensitivity in bone marrow samples from patients who were refractory. Each data point represents the mean number of viable MM cells from 3 replicates normalized to the mean number of viable MM cells in untreated control wells for a patient sample. Pearson r value is shown with its associated P value; the solid line represents linear regression, with dashed lines for 95% confidence intervals. (C) Comparison of the Dara sensitivity in patients who had not previously been treated with an anti-CD38 antibody (Dara-naïve) with that of patients who were Dara refractory but off that treatment for less than or greater than 1 year. (D) CD38 MFI on MM cells divided by CD38 MFI on non-MM cells across settings showed that patients who were Dara-naïve had higher CD38 expression than patients who were Dara refractory, but patients with >12 months off anti-CD38 treatment did partially regain CD38 expression. (E) CD38 expression on cells of patients who were Dara refractory compared with ex vivo Dara sensitivity, showing that a fold overexpression of at least sixfold was generally necessary for sensitivity. Statistical comparisons of the mean were conducted using analysis of variance with Tukey correction for multiple comparisons. FACS, fluorescence-activated cell sorting; NK, natural killer cell; Norm %, normalized percent of controls; Untx, untreated.
Ex vivo sensitivity to daratumumab is gradually recovered after the drug is discontinued. (A) Schematic depiction of the My-DST workflow. Unselected MNCs were isolated from donated patient biopsy samples, and then cells were incubated with or without drug treatment for 48 hours before flow cytometry. Viability was normalized to that of Untx controls, and our established cutoff of 80% MM cell viability compared with untreated was used to classify patient samples as sensitive (green) or resistant (red). (B) Drug-sensitivity testing results for daratumumab (Dara) sensitivity in bone marrow samples from patients who were refractory. Each data point represents the mean number of viable MM cells from 3 replicates normalized to the mean number of viable MM cells in untreated control wells for a patient sample. Pearson r value is shown with its associated P value; the solid line represents linear regression, with dashed lines for 95% confidence intervals. (C) Comparison of the Dara sensitivity in patients who had not previously been treated with an anti-CD38 antibody (Dara-naïve) with that of patients who were Dara refractory but off that treatment for less than or greater than 1 year. (D) CD38 MFI on MM cells divided by CD38 MFI on non-MM cells across settings showed that patients who were Dara-naïve had higher CD38 expression than patients who were Dara refractory, but patients with >12 months off anti-CD38 treatment did partially regain CD38 expression. (E) CD38 expression on cells of patients who were Dara refractory compared with ex vivo Dara sensitivity, showing that a fold overexpression of at least sixfold was generally necessary for sensitivity. Statistical comparisons of the mean were conducted using analysis of variance with Tukey correction for multiple comparisons. FACS, fluorescence-activated cell sorting; NK, natural killer cell; Norm %, normalized percent of controls; Untx, untreated.
Clinical characteristics of the cohort of patients refractory to daratumumab
| . | All patients who were Dara refractory, n = 29 (median, range) . |
|---|---|
| Median age (y) | 64 (46-87) |
| R-ISS III (at diagnosis) (%) | 32 |
| High-risk cytogenetics (%) | 48 |
| Disease duration (y) | 4.9 (0.8-15.8) |
| Prior treatment lines | 5 (2-11) |
| Lenalidomide refractory (%) | 96 |
| Bortezomib refractory (%) | 83 |
| Pomalidomide refractory (%) | 72 |
| Carfilzomib refractory (%) | 69 |
| Daratumumab refractory (%) | 90 |
| Pentarefractory (%) | 42 |
| Time off Dara (y) | 0.7 (0.02-4.1) |
| . | All patients who were Dara refractory, n = 29 (median, range) . |
|---|---|
| Median age (y) | 64 (46-87) |
| R-ISS III (at diagnosis) (%) | 32 |
| High-risk cytogenetics (%) | 48 |
| Disease duration (y) | 4.9 (0.8-15.8) |
| Prior treatment lines | 5 (2-11) |
| Lenalidomide refractory (%) | 96 |
| Bortezomib refractory (%) | 83 |
| Pomalidomide refractory (%) | 72 |
| Carfilzomib refractory (%) | 69 |
| Daratumumab refractory (%) | 90 |
| Pentarefractory (%) | 42 |
| Time off Dara (y) | 0.7 (0.02-4.1) |
High-risk cytogenetics include deletion of chromosome 17p, translocations t(4;14) and t(14;16).
Dara, daratumumab; R-ISS, Revised MM International Staging System.
The gradual daratumumab resensitization observed by My-DST in the daratumumab-refractory setting led us to evaluate CD38 expression as a biomarker for ex vivo sensitivity. CD38 is known to decrease after starting daratumumab and partially recover 6 months after discontinuation.21 To extend those findings, we compared CD38 between daratumumab-naïve patients and those who were refractory to daratumumab with longer time periods off anti-CD38 therapy. To depict the therapeutic window, the fold difference for CD38 on MM cells compared with that for normal CD38–/low cells (non-MM cells) was calculated for each patient sample experiment. We observed downregulation of CD38 on MM cells in the patients who were <12 months off daratumumab compared with that in drug-naïve patients, with partial recovery in those off therapy for >12 months (Figure 1D). The mean CD38 increase was 60.9-fold on MM cells compared with that on non-MM cells in daratumumab-naïve patients, 9.5-fold in daratumumab-exposed patients who were off treatment for <12 months, and 27.1-fold in patients off treatment for >12 months, mirroring the timing of recovered drug sensitivity.
Interestingly, ex vivo daratumumab sensitivity and CD38 expression did not correlate directly but showed a threshold of sixfold CD38 elevation on MM cells as necessary but not sufficient for drug sensitivity. Notably, low MM CD38 explained antibody resistance in only 9 of 20 samples (45%) identified to have daratumumab resistance, and other mechanisms of resistance appeared to be important in 11 of 20 (55%) (Figure 1E). To evaluate 1 such mechanism, we also measured the expression of complement-inhibitor proteins on the surface of MM cells compared with that on background cells. Similar to the previous report that CD55 and CD59 are upregulated on MM cells at progression and at 6 months afterwards,21 we found that 9 of 11 patients with >12 months since daratumumab have more than twofold overexpression of CD55 or CD59 compared with that of non-MM cells (supplemental Figure 2). Another immune-mediated mechanism could be less or dysfunctional natural killer cell populations (supplemental Table 2). Overall, it appears that overexpression of CD38 acts as an initial gatekeeper for sensitivity, but other mechanisms are important in the majority of patients, as supported by other studies.23
Circulating tumor cells show resensitization to anti-CD38 antibodies over time
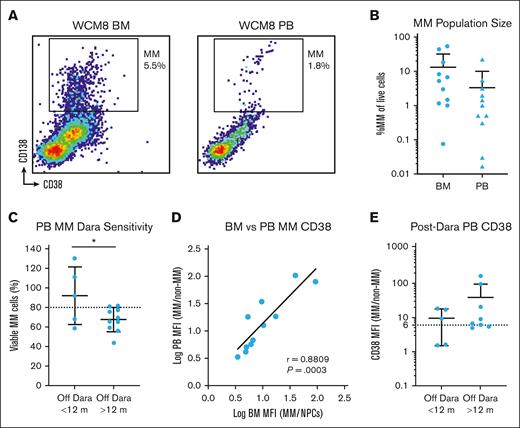
With our findings from bone marrow samples, we next sought to validate anti-CD38 antibody sensitivity and CD38 expression on circulating MM cells from peripheral blood samples from patients previously treated with daratumumab. We found MM in the blood simultaneously to that in the bone marrow, although these cells were nearly 1 order of magnitude less frequent (Figure 2A-B). We hypothesized that we could modify the My-DST format to measure anti-CD38 antibody sensitivity of rare circulating MM cells. By increasing cell numbers and culture volumes, we could identify MM cells and measure their ex vivo antibody sensitivity, finding, again, that most samples were less sensitive to daratumumab if the patient had been off for <12 months and better if >12 months had passed (Figure 2C). Through examining the MM cell populations in matched bone marrow and blood processed simultaneously, we found that MM cell CD38 expression was highly correlated across the 2 compartments (Figure 2D). Akin to the observations in the bone marrow sample cohort described earlier, CD38 expression on MM cells was lower than the sixfold overexpression threshold in 2 of 5 cases (40%) with ≤12 months since the last daratumumab dose and 2 of 8 cases (25%) with >12 months elapsed (Figure 2E). Thus, we found that My-DST was feasible using the peripheral blood from patients and validated the aforementioned findings that modest antibody sensitivity returns after >1 year elapsed since the last dose.
Peripheral blood (PB) measurement of Dara sensitivity. (A) Representative example of matched bone marrow (BM) and PB samples from a patient stained for MM cells analyzed by flow cytometry, showing a similar but smaller population in the blood. (B) Overall, the frequency of MM cells in the blood was nearly 1 order of magnitude less than that observed in the BM. (C) Similar to the BM My-DST, the PB sample showed that MM cells were more sensitive to Dara if the patient had been off that drug for >1 year. (D) The CD38 expression levels on MM cells in the PB were highly congruent with those in the BM from matched populations. Pearson r value is shown with its associated P value; the solid line represents linear regression. (E) CD38 levels on circulating MM cells in the blood are low <12 months from the last dose of Dara, then increase after >12 months. Statistical comparisons shown were performed using unpaired t tests.
Peripheral blood (PB) measurement of Dara sensitivity. (A) Representative example of matched bone marrow (BM) and PB samples from a patient stained for MM cells analyzed by flow cytometry, showing a similar but smaller population in the blood. (B) Overall, the frequency of MM cells in the blood was nearly 1 order of magnitude less than that observed in the BM. (C) Similar to the BM My-DST, the PB sample showed that MM cells were more sensitive to Dara if the patient had been off that drug for >1 year. (D) The CD38 expression levels on MM cells in the PB were highly congruent with those in the BM from matched populations. Pearson r value is shown with its associated P value; the solid line represents linear regression. (E) CD38 levels on circulating MM cells in the blood are low <12 months from the last dose of Dara, then increase after >12 months. Statistical comparisons shown were performed using unpaired t tests.
Potential benefit from switching anti-CD38 monoclonal antibodies
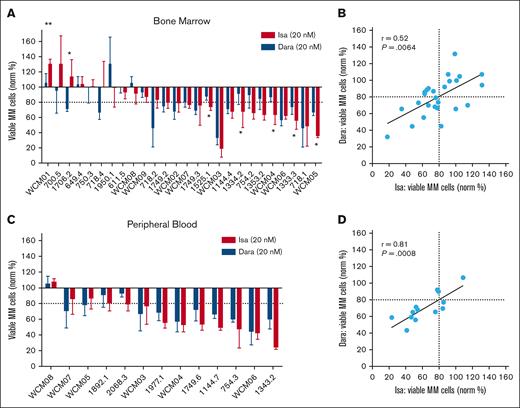
Isatuximab was the second anti-CD38 antibody to be approved by the Food and Drug Administration for patients with MM. Although it targets a different CD38 epitope than daratumumab, there have been no head-to-head clinical comparisons between the 2 agents.7 In addition, data are lacking about whether switching from 1 anti-CD38 antibody to the other is helpful in a later treatment line. Thus, we used My-DST to compare equimolar isatuximab and daratumumab directly. Daratumumab-exposed bone marrow samples showed overall similar sensitivity to daratumumab and isatuximab, with only 7 of 26 (27%) showing significantly different results between the 2 agents (Figure 3A). Interestingly, when sensitivity was present toward both drugs, all 5 of the significantly more sensitive results favored isatuximab. On comparing across these experiments, daratumumab and isatuximab results were highly correlated with each other (r = 0.52; P = .0064; Figure 3B). Similar results were observed in peripheral blood experiments; although no samples showed significantly different results, sensitivity to the agents was, again, highly correlated (r = 0.81; P = .0008; Figure 3C-D). These data support the precision of ex vivo measurement for 2 agents with common general mechanisms of action but show that there may be slightly improved ex vivo response to isatuximab in a subset of patients refractory to daratumumab.
Comparison of isatuximab and daratumumab sensitivity ex vivo in patients who were Dara refractory. (A) Waterfall plot showing the isatuximab (Isa) and daratumumab (Dara) sensitivity measured in BM samples from patients who were Dara refractory, with significantly different results between the 2 agents indicated. (B) Dara and isatuximab ex vivo sensitivity was correlated, with Pearson r and its associated P value shown. (C) Waterfall plot showing My-DST of PB samples with similar results for Dara and isatuximab. (D) PB samples sensitivity to anti-D38 antibodies compared with each other, again showing correlation between results for the 2 agents. Comparisons between the mean Dara and isatuximab sensitivities in panels A and C were performed using t tests.
Comparison of isatuximab and daratumumab sensitivity ex vivo in patients who were Dara refractory. (A) Waterfall plot showing the isatuximab (Isa) and daratumumab (Dara) sensitivity measured in BM samples from patients who were Dara refractory, with significantly different results between the 2 agents indicated. (B) Dara and isatuximab ex vivo sensitivity was correlated, with Pearson r and its associated P value shown. (C) Waterfall plot showing My-DST of PB samples with similar results for Dara and isatuximab. (D) PB samples sensitivity to anti-D38 antibodies compared with each other, again showing correlation between results for the 2 agents. Comparisons between the mean Dara and isatuximab sensitivities in panels A and C were performed using t tests.
Clinical benefit observed from CD38 antibody re-treatment
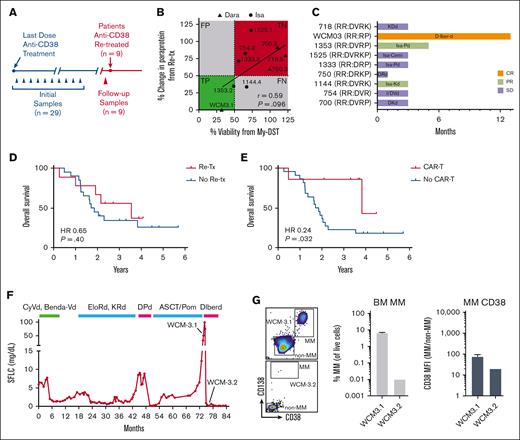
To check whether clinical re-treatment was beneficial and predicted by My-DST, we examined serial samples and clinical follow-up. The median overall survival of our cohort was 2.05 years after becoming daratumumab-refractory. Nine of the 29 patients were re-treated with a second anti-CD38 antibody–containing regimen, and follow-up samples were obtained before they started re-treatment (Figure 4A). Re-treated patients had a median washout from daratumumab of 1.88 years (range, 0.52-3.34 years), and none were re-treated in the next line of therapy. Only 1 of 9 of the re-treated patients had a washout from anti-CD38 treatment <1 year, and 4 of 9 were switched to isatuximab-based combinations. Ex vivo drug treatment results for the anti-CD38 antibody that were obtained clinically showed a trend toward a significant correlation with the re-treatment clinical response (R = 0.59; P = .09; Figure 4B) despite cell number limitations preventing analysis of the other drugs the patients received in their treatment combinations. Among the re-treated patients, the clinical overall response rate was 33.3%, and the clinical benefit rate was 88.8%. Patient WCM3 had a complete response, 2 patients had a partial response, 5 had SD, and 1 had progressive disease. In terms of response duration, the mean time on the re-treatment line of therapy was <6 months, with WCM3 achieving the longest remission at 13 months (Figure 4C). A more stringent ex vivo sensitivity threshold of 50% ex vivo sensitivity was 66.6% sensitive (2/3, true positive) and 100% specific (6/6, true negative) for a clinical partial response to CD38 antibody–based re-treatment. Compared with the rest of the cohort, receiving re-treatment with anti-CD38 antibody–based therapy did not significantly benefit overall survival (Figure 4D). By contrast, a new class of therapy of chimeric antigen receptors (CARs) did show a statistically significant benefit in this group (Figure 4E). Thus, although the clinical benefits of anti-CD38 re-treatment were limited, the best responses were usually predicted by My-DST. Sensitivity profiling including the other drugs used in combination regimens may further refine this approach.
Patients who were Dara refractory with ex vivo sensitivity to CD38 antibodies benefit from clinical re-treatment. (A) Schematic timeline of the acquisition of follow-up samples before re-treatment with an anti-CD38 monoclonal antibody. (B) Ex vivo drug sensitivity as determined by normalized viability with My-DST vs depth of clinical response at re-treatment. Drug sensitivity was defined as <80% normalized viability, and PR as a 50% change in paraprotein from baseline after initiating treatment. (C) Swimmers plot showing the clinical benefit of anti-CD38 antibody–based therapy in the 8 patients who were Dara refractory and received re-treatment. Orange bar indicates complete response (CR), light green bars indicate partial response(PR), and purple bars indicate stable disease (SD). (D) Among patients who were Dara refractory, there was no significant difference in the overall survival between those re-treated with anti-CD38 antibodies and those who were not re-treated. (E) The probability of survival between those patients who were Dara refractory treated with anti-BCMA CAR-T was significantly higher than that of those who were not. (F) The disease course for patient WCM3, shown by serum free light chain, who relapsed after prior daratumumab but was not refractory and achieved complete response to daratumumab-based treatment. Before re-treatment with anti-CD38 antibody–based treatment, a BM sample WCM3.1 was obtained 26 months after the last dose of daratumumab. (G) After re-treatment started, a BM sample WCM3.2 was obtained, in which the MM cell population drastically decreased and the CD38 level on MM cells decreased as well. Cemi, cemiplimab; D, daratumumab; d, dexamethasone; FN, false negative; FP, false positive; Iber, iberdomide; Isa, isatuximab; K, carfilzomib;; P, pomalidomide; R, lenalidomide; Re-tx, re-treatment; RR, relapsed and refractory; TN, true negative; TP, true positive; V, bortezomib.
Patients who were Dara refractory with ex vivo sensitivity to CD38 antibodies benefit from clinical re-treatment. (A) Schematic timeline of the acquisition of follow-up samples before re-treatment with an anti-CD38 monoclonal antibody. (B) Ex vivo drug sensitivity as determined by normalized viability with My-DST vs depth of clinical response at re-treatment. Drug sensitivity was defined as <80% normalized viability, and PR as a 50% change in paraprotein from baseline after initiating treatment. (C) Swimmers plot showing the clinical benefit of anti-CD38 antibody–based therapy in the 8 patients who were Dara refractory and received re-treatment. Orange bar indicates complete response (CR), light green bars indicate partial response(PR), and purple bars indicate stable disease (SD). (D) Among patients who were Dara refractory, there was no significant difference in the overall survival between those re-treated with anti-CD38 antibodies and those who were not re-treated. (E) The probability of survival between those patients who were Dara refractory treated with anti-BCMA CAR-T was significantly higher than that of those who were not. (F) The disease course for patient WCM3, shown by serum free light chain, who relapsed after prior daratumumab but was not refractory and achieved complete response to daratumumab-based treatment. Before re-treatment with anti-CD38 antibody–based treatment, a BM sample WCM3.1 was obtained 26 months after the last dose of daratumumab. (G) After re-treatment started, a BM sample WCM3.2 was obtained, in which the MM cell population drastically decreased and the CD38 level on MM cells decreased as well. Cemi, cemiplimab; D, daratumumab; d, dexamethasone; FN, false negative; FP, false positive; Iber, iberdomide; Isa, isatuximab; K, carfilzomib;; P, pomalidomide; R, lenalidomide; Re-tx, re-treatment; RR, relapsed and refractory; TN, true negative; TP, true positive; V, bortezomib.
Interestingly, patient WCM3 with a markedly better response was unique in that she had relapsed after daratumumab treatment but was not refractory. This contrasts with the other patients, who relapsed and were refractory, which is to say they had previous progression of disease at or within 60 days of daratumumab. However, patient WCM3 was treated with daratumumab-based therapy, followed by autologous stem cell transplant and pomalidomide maintenance. After 26 months, the patient progressed on pomalidomide, and My-DST showed sensitivity to both daratumumab and isatuximab. The patient then received daratumumab, iberdomide, and dexamethasone on a clinical trial, achieving a complete response (Figure 4F). Flow cytometry was performed before and after starting re-treatment to measure the dynamics of the therapeutic window in CD38 expression. Two weeks after starting re-treatment, sample WCM3.2 showed a reduction of MM cells to near undetectable levels, and the residual MM cells already showed a decreased level of CD38 (Figure 4G). This patient shows a stark contrast from the responses of those who were re-treatment relapsed/refractory. Although it should be noted that iberdomide might have been responsible, this case does suggest that better clinical benefit of CD38 antibody re-treatment will occur in patients who are daratumumab exposed but not refractory.
CD38 expression is not elevated on MM cells at maximum response to daratumumab
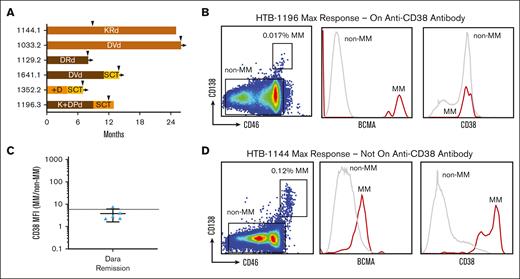
Considering the finding that clinical benefit was much better when antibody treatment had not been administered after transplant, we hypothesized that CD38 levels may be low during the maintenance phase. To answer this question, we studied samples obtained from patients with residual disease during maintenance treatment, including daratumumab- or isatuximab-based therapies. In total, we were successful in detecting MM cells by flow cytometry in 6 patient bone marrow samples obtained during maintenance treatment (Figure 5A). Using CD138 and BCMA to identify residual MM cells, we found that CD38 levels were not above the background levels present on normal bone marrow cells (Figure 5B). Using the MFI ratio of MM cells to non-MM cells, the CD38 level was found to be at or below the sixfold threshold described earlier for antibody drug sensitivity in most samples (Figure 5C). By comparison, a daratumumab-naïve patient not on anti-CD38 antibody during maintenance after maximum response had high levels of CD38 on their MM cells based on the same methods (Figure 5D). Based on these results, it appears that CD38 is not expressed at targetable levels on detectable MM cells in most cases during maintenance treatment from anti-CD38 antibodies. It should be noted that the CD38 expression level was not measurable in patients who tested negative for minimal residual disease, so it is possible that these results do not apply to that population.
Patients at maximum response on anti-CD38 antibodies express background level CD38. (A) Swimmers plot for samples from patients who were at maximum response on or within 6 months from last dose on anti-CD38 antibody therapy. Tan brown bar indicates complete response, dark brown bar indicates very good partial response, yellow bars indicate partial response, and orange bars indicate stable disease (SD). (B) Example of a patient on Dara-based therapy who had achieved maximum response, with flow cytometry showing that CD38 expression on the residual MM cells is not distinguishable from the background expression on normal BM MNCs (non-MM). Residual MM cells were identified from BM samples as double-positive for CD138 and CD46, then verified to have BCMA. (C) For patients tested at maximum response, the ratio of CD38 expression on MM vs non-MM was below the sixfold threshold correlated to ex vivo sensitivity for most of the patients tested. (D) In contrast, in a patient who was Dara-naïve on maintenance therapy, residual MM cells gated in the same manner of greatly overexpressed CD38 expression.
Patients at maximum response on anti-CD38 antibodies express background level CD38. (A) Swimmers plot for samples from patients who were at maximum response on or within 6 months from last dose on anti-CD38 antibody therapy. Tan brown bar indicates complete response, dark brown bar indicates very good partial response, yellow bars indicate partial response, and orange bars indicate stable disease (SD). (B) Example of a patient on Dara-based therapy who had achieved maximum response, with flow cytometry showing that CD38 expression on the residual MM cells is not distinguishable from the background expression on normal BM MNCs (non-MM). Residual MM cells were identified from BM samples as double-positive for CD138 and CD46, then verified to have BCMA. (C) For patients tested at maximum response, the ratio of CD38 expression on MM vs non-MM was below the sixfold threshold correlated to ex vivo sensitivity for most of the patients tested. (D) In contrast, in a patient who was Dara-naïve on maintenance therapy, residual MM cells gated in the same manner of greatly overexpressed CD38 expression.
Anti-CD38 antibody re-treatment at relapse after BCMA CAR-T
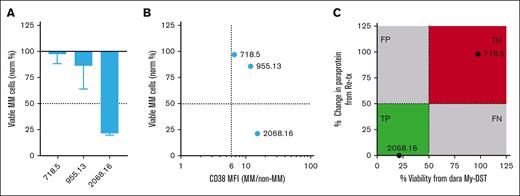
CAR T-cell therapy (CAR-T) targeting BCMA has emerged as a new treatment option for patients refractory to daratumumab who have received 4 prior lines of therapy, but limited options are available when patients relapse after CAR-T. Thus, we examined the effects of daratumumab re-treatment and CD38 expression in 3 available samples from patients in the post–CAR-T setting. MM cells from 1 of 3 patients were sensitive by the more stringent 50% cutoff (Figure 6A). In all 3, MM cell CD38 expression was above the sixfold threshold associated with resistance to daratumumab ex vivo (Figure 6B). To evaluate whether clinical re-treatment was beneficial and predicted by My-DST, we compared ex vivo daratumumab sensitivity with that of 2 patients who were re-treated with a daratumumab-based regimen after CAR-T (Figure 6C). Patient 718 initiated daratumumab re-treatment 40 months after the last dose of anti-CD38 antibody and 37 months after infusion of CAR-T. As suggested by My-DST, patient 718 did not respond but did have 3 months SD on a 4-drug regimen that included daratumumab re-treatment. Patient 2068 began daratumumab re-treatment with selinexor 12 months after the last dose of anti-CD38 antibody and 9 months after CAR-T infusion. Also similar to My-DST results, patient 2068 responded to the re-treatment and remains in complete response 11 months later. Thus, although more patients will need to be studied with comprehensive drug-sensitivity profiling, anti-CD38 antibody re-treatment in the post–CAR-T setting may be effective in select patients.
Patients relapsed after CAR-T can display sensitivity to CD38 antibody re-treatment. (A) Waterfall plot showing the Dara sensitivity ex vivo measured in BM samples from patients refractory to Dara who had also relapsed after anti-BCMA CAR-T. (B) CD38 overexpression ratio compared with ex vivo Dara sensitivity in the same 3 patients with Dara-refractory MM relapsed after anti-BCMA CAR-T. (C) Ex vivo drug sensitivity as determined by normalized viability with My-DST vs depth of clinical response in 2 patients who received Dara-based re-treatment after CAR-T. Clinically predictive drug sensitivity was defined as <50% normalized viability and PR as a 50% change in paraprotein from baseline after initiating treatment.
Patients relapsed after CAR-T can display sensitivity to CD38 antibody re-treatment. (A) Waterfall plot showing the Dara sensitivity ex vivo measured in BM samples from patients refractory to Dara who had also relapsed after anti-BCMA CAR-T. (B) CD38 overexpression ratio compared with ex vivo Dara sensitivity in the same 3 patients with Dara-refractory MM relapsed after anti-BCMA CAR-T. (C) Ex vivo drug sensitivity as determined by normalized viability with My-DST vs depth of clinical response in 2 patients who received Dara-based re-treatment after CAR-T. Clinically predictive drug sensitivity was defined as <50% normalized viability and PR as a 50% change in paraprotein from baseline after initiating treatment.
Discussion
Anti-CD38 monoclonal antibodies are a vital component of MM treatment, harnessing the power of a patient’s immune system to attack malignant cells. Put together with treatment advances from the PIs and IMiDs, the outlook for patients with MM has steadily improved. Next-generation IMiDs and PIs have extended disease control for patients, enabling sequential drug combinations. Still, the problem of drug resistance remains. In the triple-class refractory setting, promising new agents are emerging, including CAR-Ts and bispecific antibodies.32,33 Interestingly, survival in our cohort of mostly patients refractory to daratumumab was better than what has been previously reported in this setting.3 This is likely because of the culmination of recent treatment advances. However, patients who relapse after CAR-T will continue to need efficacious treatment lines, and clinicians will continue to face the question of whether to attempt re-treatment with another anti-CD38 antibody.
We hypothesized that patients may regain sensitivity after enough time had elapsed after the last dose of daratumumab. Indeed, MM cells from daratumumab-exposed patients regained sensitivity after ≥1 year, and isatuximab led to slightly better ex vivo results for some patients. Levels of the CD38 also partially recovered after 1 year off, extending results of prior studies.21,26 We also observed that a threshold of approximately sixfold CD38 overexpression compared with that of normal cells was necessary for recovery of antibody sensitivity. When receiving daratumumab-based maintenance for patients with detectable residual disease, CD38 levels were nearly indistinguishable from background levels. Work with cocultured cell lines have suggested that trogocytosis, with uptake of antigen/antibody complexes by immune cells, may play a role in reducing MM cell CD38.34 CD38 usually recovered after 1 year off daratumumab, but many showed continued resistance consistent with other mechanisms, which may include complement-inhibitory proteins upregulation, Fcγ receptor polymorphisms, and CD47 overexpression.23 Going forward, clinical trials could investigate the optimal timing for re-treatment, and ex vivo drug-sensitivity testing with My-DST could be used to identify resistance encompassing both CD38-dependent and -independent types of resistance. Further characterization of the immune cell–mediated and complement mechanisms of resistance may shed light on ways to overcome resistance.
We followed up patients after My-DST to check whether our predictions held up in patients’ clinical treatment outcomes. Among patients tested, a subset received CD38 antibody in a later treatment line, most after at least a year off. The clinical benefit rate was favorable, but periods of control were short lived. This extends what had been shown previously in prospective and retrospective clinical studies.26-30 Based on these results, re-treatment with anti-CD38 antibodies in patients refractory to daratumumab before CAR-T is likely to be of limited benefit and may be restricted to bridging therapy to a more definitive option, such as CAR-T or bispecific antibodies. Interestingly, My-DST with a more stringent cutoff for sensitivity was predictive of clinical partial response. Furthermore, in a small number of samples available from patients who relapsed after CAR-T, re-treatment showed potential for good efficacy in select patients. Development of My-DST as a clinical diagnostic test may be helpful to identify these patients.
In considering other ways for how re-treatment could be improved, 1 patient in our study who had relapsed after daratumumab but was not refractory responded considerably better to re-treatment ex vivo and in clinical care. This raises the question of whether discontinuing anti-CD38 antibodies after remission could lead to improved responses upon re-treatment. Continuous dosing of anti-CD38 antibodies may not be beneficial after maximum response is achieved and instead lead to drug resistant residual disease. Analysis from the CASSIOPEIA trial was consistent with this, comparing the inclusion of daratumumab either with induction, maintenance, or both. Daratumumab addition was beneficial during maintenance only if patients had not received it during induction.35 In contrast, the GRIFFIN study found minimal residual disease–negative rates improved over time with extended daratumumab, indicating that some patients do benefit from longer CD38 antibody.17 The ongoing phase 3 GMMG-HD7 trial will compare isatuximab maintenance plus lenalidomide with lenalidomide alone. In addition, My-DST and measurement of CD38 expression in serial samples from patients who are anti-CD38 antibody exposed but not refractory could validate whether these patients are better candidates for re-treatment.
The results from this study of the daratumumab-refractory setting provided insights that have been hard to obtain from pure clinical studies without laboratory correlatives. First, re-treatment with anti-CD38 antibodies is most likely to benefit patients with MM after they have been off treatment with that class for at least a year. We also obtained better results from switching between agents, albeit in a small subset of samples. Because MM treatment line sequencing is highly situation- and physician-dependent, these points can be considered for patients in the relapsed/refractory setting. Currently, patients receiving daratumumab in the first line setting need treatment decisions on their maintenance and first relapse therapies, further increasing the need to understand anti-CD38 antibody resistance. Although we focused on patients who were both relapsed and refractory to daratumumab, not continuing treatment until drug resistance develops may produce substantially better re-treatment results. In patients refractory to daratumumab, re-treatment had only modest effects, and anti-CD38 antibody resistance appeared to rapidly redevelop in most patients. Thus, further clinical trials are needed to test whether fixed CD38 antibody dosing is as effective as the conventional continuous dosing.
Acknowledgments
The authors dedicate this manuscript to the memory of Mary Jo Dougherty. The authors thank the Hematology Clinical Trials Unit at the University of Colorado for tissue bank and regulatory support. The authors appreciate the mentorship and project feedback provided by Craig Jordan and Clay Smith. They also thank Shelby Bearrows, Michael VanWyngarden, and Sarah Parzych for technical assistance.
This work was supported by a grant from the National Cancer Institute (K08CA222704) (D.W.S.) and a philanthropic donation from David Kessenich.
Authorship
Contribution: O.P.d.A., L.R., and D.W.S. designed experiments; O.P.d.A., L.R., Z.J.W., A.L.K., B.M.I., and S.E.P. performed experiments and data analysis; D.S.J., D.R., R.N., T.M.M., P.A.F., and D.W.S. contributed patient samples and provided clinical correlations; G.B. performed statistical analyses, with supervision from D.A.; O.P.d.A. and D.W.S. wrote the manuscript; D.W.S. supervised the research and finalized the manuscript; and all authors contributed to manuscript revision.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel W. Sherbenou, Division of Hematology, Department of Medicine, University of Colorado Anschutz Medical Campus, 12700 E 19th Ave, Box B170, Aurora, CO 80045; e-mail: daniel.sherbenou@cuanschutz.edu.
References
Author notes
∗O.P.d.A. and L.R. contributed equally to this study.
Data are available on request from the corresponding author, Daniel W. Sherbenou (daniel.sherbenou@cuanschutz.edu).
The full-text version of this article contains a data supplement.