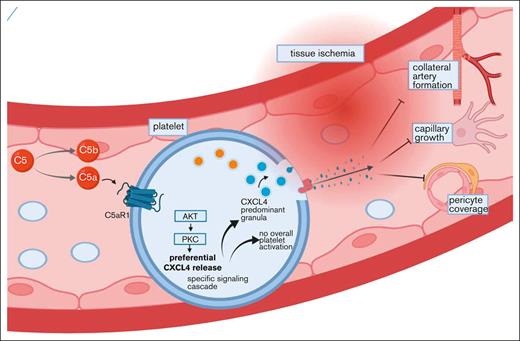
Key Points
Platelets modulate ischemia-induced revascularization by secretion of angiogenic factors.
The platelet C5a receptor 1 is a key mediator of the release of antiangiogenic CXCL4.
Abstract
In ischemic tissue, platelets can modulate angiogenesis. The specific factors influencing this function, however, are poorly understood. Here, we characterized the complement anaphylatoxin C5a-mediated activation of C5a receptor 1 (C5aR1) expressed on platelets as a potent regulator of ischemia-driven revascularization. We assessed the relevance of the anaphylatoxin receptor C5aR1 on platelets in patients with coronary artery disease as well as those with peripheral artery disease and used genetic mouse models to characterize its significance for ischemia and growth factor–driven revascularization. The presence of C5aR1-expressing platelets was increased in the hindlimb ischemia model. Ischemia-driven angiogenesis was significantly improved in C5aR1−/− mice but not in C5−/− mice, suggesting a specific role of C5aR1. Experiments using the supernatant of C5a-stimulated platelets suggested a paracrine mechanism of angiogenesis inhibition by platelets by means of antiangiogenic CXC chemokine ligand 4 (CXCL4, PF4). Lineage-specific C5aR1 deletion verified that the secretion of CXCL4 depends on C5aR1 ligation on platelets. Using C5aR1−/−CXCL4−/− mice, we observed no additional effect in the revascularization response, underscoring a strong dependence of CXCL4 secretion on the C5a-C5aR1-axis. We identified a novel mechanism for inhibition of neovascularization via platelet C5aR1, which was mediated by the release of antiangiogenic CXCL4.
Introduction
Recent efforts targeting platelet-associated mechanisms have focused on prothrombotic functions, which are distinct from the effect of platelets on hemostasis.1 For example, the glycoprotein VI-receptor mimetic peptide revacept inhibits platelet thrombus formation by interfering with its binding to collagen, whereas the hemostatic functions of platelets are not hampered.2 However, in a recent phase 3 study in patients undergoing percutaneous coronary intervention, the addition of revacept to standard antithrombotic therapy did not reduce the incidence of myocardial injury.3 Thus, modern drug development may head toward more directed therapies. After ischemic diseases, a major objective is the restoration of tissue perfusion. Inflammation not only contributes to tissue damage but also triggers the processes of angiogenesis and arteriogenesis and enhances repair mechanisms. An important system contributing to this inflammatory response is the complement cascade. It is one of the ontogenetically oldest plasmatic protein systems, which constitutes an important column of our innate immune response4 and interconnects various cell systems involved in immunity or other disease and tissue repair processes.5
During infections, complement components help to orchestrate the host’s immune defense; they opsonize microbial intruders, clear immune complexes, and induce or regulate the inflammatory response through the small cleavage fragments of C3 and C5, the so-called anaphylatoxins.6 Further downstream, it leads to the assembly of the membrane attack complex (C5b-C9), which can eliminate target cells.4 Besides this well-known function of complement, several of its components have been implicated in rather unexpected pathophysiological processes, including liver injury,7 organ regeneration,8 and angiogenesis.9,10 The underlying mechanisms, however, connecting it to processes such as angiogenesis, are largely unknown.
Platelets are of central importance in diseases featuring thrombus formation, mediate vascular occlusions, and thereby determine fatal events in the course of myocardial infarction or embolic stroke.11,12 Besides this classical function of platelets to mediate intravascular thrombosis, they are also involved in tissue remodeling processes such as inflammation13-15 or apoptosis.16 Recent evidence also highlights the role of platelets in different settings of angiogenesis,17 and different pro- and antiangiogenic factors could be visualized in distinct granules, where they are stored and can be secreted upon stimulation, if needed.18 How the release of these factors is regulated, however, and the specific factors orchestrating this release are not entirely understood.
To get insight into the regulation of angiogenesis-modulating factors released by platelets, we analyzed the interaction of complement components with platelets and the relevance of this cross talk for growth factor– and ischemia-induced revascularization in vivo, with particular focus on the C5a anaphylatoxin receptor 1 (C5aR1).
Methods
Please refer to the supplemental Information for detailed methods. Requests by researchers to access the data, analytic methods, and study materials for the purposes of reproducing the results or replicating procedures can be made to the corresponding author who manages the information.
Study approval
For experiments with human material, written informed consent was received from participants before inclusion in the study (approval numbers 270/2011B01 and 19-403).
All animal procedures were approved by the regional animal care and use committee of the District of Tübingen, Baden-Württemberg (Konrad-Adenauer-Straße 20, 72072 Tübingen, Germany) as well as the Ministry of Energy, Agriculture, the Environment, Nature and Digitalization of the State of Schleswig-Holstein (Mercatorstraβe, 324106 Kiel) and performed in accordance with German law and guidelines for animal care.
Data presentation and statistics
If not otherwise stated, the results are expressed as the mean ± standard error of the mean. All statistical analyses were performed using GraphPad Prism 9 or SPSS version 26.0 (SPSS Inc, Chicago, IL). Comparisons between the 2 groups were carried out using the Student t test. Comparisons between more than 2 groups were conducted using analysis of variance (ANOVA), and comparisons between 2 groups with several time points were performed using a 2-way ANOVA. An adequate post hoc test was carried out for all ANOVA analyses. P < .05 was considered statistically significant.
Results
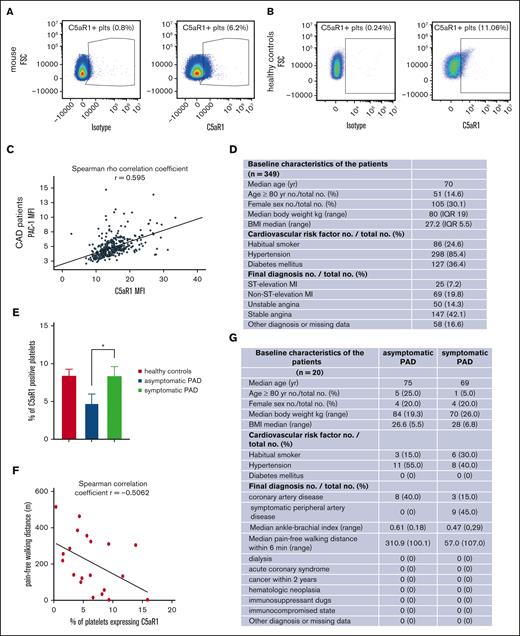
Compelling evidence highlights the role of pro- and antiangiogenic factors stored in platelets for endothelial functions.18,19 The relevance of platelets for angiogenesis in vivo and how their contribution is regulated, however, need to be more deeply characterized. Recently, a novel role of the anaphylatoxin receptor C3aR was discovered for platelet activation and thrombosis, whereas C5aR1−/− knockout mice had no phenotype of altered in vivo thrombus formation, suggesting other functions of this receptor on platelets.20 We could verify that murine platelets (Figure 1A; supplemental Figure 2A) and human platelets (Figure 1B, for gating; supplemental Figures 9 and 2B) express the anaphylatoxin receptor C5aR1 (CD88). Next, we assessed C5aR1 expression in patients suffering from atherosclerosis. In 349 patients with coronary artery disease, we observed a positive correlation of C5aR1 with activated GPIIbIIIa (detected by activation-specific antibody PAC-1, Figure 1C-D) measured in whole blood stained within 1 hour of blood collection. To get an impression how C5aR1 prevalence on the platelet surface changes in patients at different stages of coronary artery disease, we compared its expression in stable patients with coronary artery disease with those with acute coronary syndrome. Interestingly, we found that the expression of C5aR1 is specifically increased in stable coronary artery disease (supplemental Figure 3A) and is not influenced by antiplatelet medication (supplemental Figure 3B). Platelet C5aR1 expression did not correlate with platelet reactivity as assessed by aggregometry using adenosine diphosphate (ADP), collagen, or thrombin receptor activator peptide (TRAP) as platelet agonists (supplemental Figure 3C). Moreover, C5aR1 expression on platelets from patients with asymptomatic peripheral artery disease (PAD) was significantly lower than on those from patients with symptomatic PAD and healthy controls (Figure 1E) and correlated with pain-free walking distance (Figure 1F). Asymptomatic PAD is defined as PAD indicated by a pathological ankle-brachial index below 0.9 as well as atherosclerotic arterial stenosis assessed by sonography, the exclusion of diabetes mellitus, and no walking pain in the patient history or during a 6-minute walking test, respectively. Interestingly, the level of C5aR1-positive platelets was comparable between symptomatic patients with PAD and healthy controls (Figure 1E). However, the demographics and comorbidities of these healthy controls are very different from those of the patient group, which has to be considered a limitation of this study (Figure 1G; supplemental Figure 4C).
Upregulation of the complement anaphylatoxin receptor C5aR1 on platelets in patients with atherosclerotic disease and in ischemic tissue. (A) Dot plot depicting C5aR1 expression on murine platelets (right); the left side shows the dot plot obtained with an immunoglobulin G (IgG)-type matched isotype. The plot is representative of the analysis of 4 independent platelet samples. (B) Analyzing human platelets in whole blood by flow cytometry confirmed C5aR1 expression. Displayed is the staining with C5aR1 antibody (right), showing C5aR1+ platelets (CD41+CD45−C5aR1+) vs isotype control (left). (C) In patients with coronary artery disease, expression of C5aR1 and activated GPIIb/IIIa (activation-specific antibody PAC-1) on platelets was measured by flow cytometry. The correlation of C5aR1 with activated GPIIb/IIIa is depicted. Spearman rank coefficient, rs = 0.59, P < .001, n = 349 patients. (D) Baseline characteristics of the analyzed patients with coronary artery disease (CAD). (E) In patients with PAD, C5aR1 expression was decreased in asymptomatic disease compared with symptomatic patients and healthy controls. A total of 20 patients were included in the study, and 7 controls. Data are shown as the mean ± standard error of the mean (SEM) and are displayed as the fraction of C5aR1-expressing platelets (%), ∗P < .05 (F) Furthermore, we observed that the fraction of platelets expressing C5aR1 correlates with pain-free walking distance in patients with PAD. r = −0.5062, P < .05. (G) Baseline characteristics of the analyzed patients with PAD.
Upregulation of the complement anaphylatoxin receptor C5aR1 on platelets in patients with atherosclerotic disease and in ischemic tissue. (A) Dot plot depicting C5aR1 expression on murine platelets (right); the left side shows the dot plot obtained with an immunoglobulin G (IgG)-type matched isotype. The plot is representative of the analysis of 4 independent platelet samples. (B) Analyzing human platelets in whole blood by flow cytometry confirmed C5aR1 expression. Displayed is the staining with C5aR1 antibody (right), showing C5aR1+ platelets (CD41+CD45−C5aR1+) vs isotype control (left). (C) In patients with coronary artery disease, expression of C5aR1 and activated GPIIb/IIIa (activation-specific antibody PAC-1) on platelets was measured by flow cytometry. The correlation of C5aR1 with activated GPIIb/IIIa is depicted. Spearman rank coefficient, rs = 0.59, P < .001, n = 349 patients. (D) Baseline characteristics of the analyzed patients with coronary artery disease (CAD). (E) In patients with PAD, C5aR1 expression was decreased in asymptomatic disease compared with symptomatic patients and healthy controls. A total of 20 patients were included in the study, and 7 controls. Data are shown as the mean ± standard error of the mean (SEM) and are displayed as the fraction of C5aR1-expressing platelets (%), ∗P < .05 (F) Furthermore, we observed that the fraction of platelets expressing C5aR1 correlates with pain-free walking distance in patients with PAD. r = −0.5062, P < .05. (G) Baseline characteristics of the analyzed patients with PAD.
To generate more mechanistic insight, we isolated platelets from healthy donors and exposed them to an ex vivo atherosclerotic milieu (by adding acetylated low-density lipoprotein [acLDL]21). Along with the finding that C5aR1 on platelets may be linked to increased CXCL4 serum levels, we detected increased CXCL4 protein in the supernatant of platelets upon stimulation with acLDL (supplemental Figure 5B). Furthermore, we could show that acLDL stimulation increased C5aR1 expression on platelets (supplemental Figure 5A), and we found a difference in CXCL4 levels in the plasma of patients with PAD. For instance, there was a higher level of CXCL4 in patients with symptomatic PAD compared with those with asymptomatic PAD, corresponding to the differences found for C5aR1 expression. However, this difference was not significant, probably because of an insufficient cohort size (supplemental Figure 4A). When assessing the correlation of these parameters, we found a significant positive correlation of both parameters (r = 0.46, P = .046; supplemental Figure 4B).
In the in vivo model of hind limb ischemia (HLI), ischemia in the distal hind limb tissue is caused by femoral artery ligation, consecutively resulting in revascularization.22 This translational model is used to study the mechanisms of PAD. Previously, we detected expression of platelet C5aR1 in the gastrocnemius muscles of ischemic hind limbs, pointing toward a pathophysiological role in this translational disease model.23 The presence of platelets in the ischemia-induced angiogenic tissue and the expression of C5aR1 on platelets prompted us to investigate their role in revascularization.
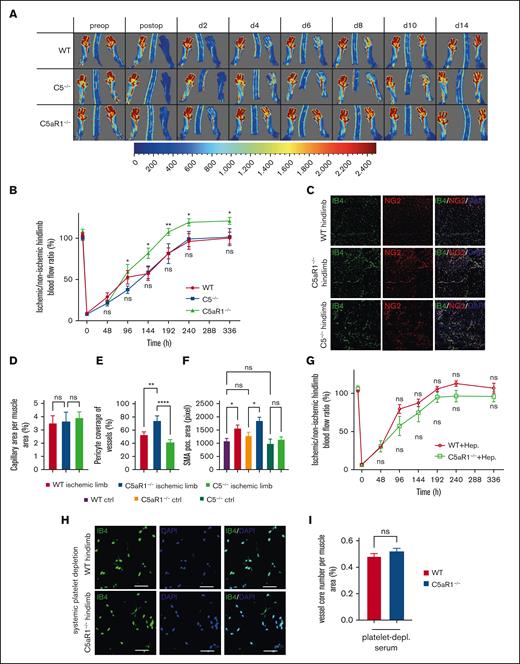
During complement activation, C5a is generated from C5.6 Thus, we applied C5−/− mice and C5aR1−/− mice in the HLI model to determine which cleavage fragment of C5 affects revascularization. Interestingly, we found that C5aR1−/− mice but not C5−/− mice have an increased level of ischemia-driven angiogenesis in comparison to wild-type (WT) mice (Figure 2A-B). We have quantified vessel density in WT, C5aR1−/−, and C5−/− animals at d4 and d14 after induction of ischemia. Vessels were stained with IB4, pericytes were visualized using NG2, and arteries were assessed by smooth muscle actin (SMA) staining. We analyzed capillary density by immunofluorescence in tissue sections at d4 (supplemental Figure 6A). We could show that at day 4, capillary density is increased in C5−/− mice compared with that in both WT and C5aR1−/− mice. Interestingly, pericyte coverage is already increased at day 4 in C5aR1−/− mice compared with that in both WT and C5−/− mice, providing a mechanistic explanation for our observations (supplemental Figure 6B). Finally, we measured an elevated arterial density in the distal tissue from ischemic hind limbs in C5aR1−/− mice, although this difference was not statistically significant (supplemental Figure 6C). At day 14, there was no significant increase in capillary density in C5aR1−/− mice (Figure 2C-D). Furthermore, a clear increase in pericyte coverage was observable in C5aR1−/− mice compared with WT and C5−/− mice at d14 (Figure 2C,E). Finally, arteriolar abundance was highest in C5aR1−/− mice. There was an increase in arteries in ischemic hind limb muscle tissue compared with control tissue, which was significant for WT and C5aR1−/− mice but not for C5−/− mice (Figure 2F). To demonstrate which changes take place in the HLI model in distal hind limb tissue, the representative images of vascular structures in operated vs control limbs are depicted (supplemental Figure 7).
The platelet C5aR1 modulates ischemia-driven vessel growth. (A) WT, C5−/− or C5aR1−/− mice were subjected to HLI and analyzed after 2 weeks, and revascularization of the hindlimbs after femoral artery ligation was visualized by laser doppler fluximetry (LDI) under standardized conditions with the scale adapted to the experiment. Representative LDI images of mouse hind limbs after femoral artery ligation illustrate increased revascularization in C5aR1−/− animals compared with WT controls or C5−/− mice. (B) We found increased revascularization in C5aR1−/− animals in comparison to WT or C5−/− mice. Data are shown as the mean ± SEM (n = 5-8 animals per group) and are displayed as % of the perfusion in the contralateral control limb. ∗P < .05. (C) Representative images depicting capillaries (stained by IB4) and pericyte coverage (colocalization of IB4 and NG2) in the analyzed genotypes 14 days after induction of ischemia. (D) At day 14, there was no significant increase in capillary density in C5aR1−/− mice. Data represent the mean ± SEM. n = 9 to 12. (E) An increase in pericyte coverage was observable in C5aR1−/− mice compared with WT and C5−/− mice at day 14. Data represent mean ± SEM. n = 9 to 15. ∗∗P < .01, ∗∗∗P < .001. (F) Arteriolar abundance was highest in C5aR1−/− mice. There was an increase in arteries in ischemic hind limb muscle tissue compared with control tissue, which was significant for WT and C5aR1−/− mice but not for C5−/− mice. Data represent mean ± SEM. n = 6 to 9. ∗P < .05. (G) When WT or C5aR1−/− mice subjected to HLI were additionally treated with heparin, we observed no difference in revascularization anymore. Data are shown as the mean ± SEM (n = 6-8 animals per group) and are displayed as % of the perfusion in the contralateral control limb. (H) Furthermore, WT or C5aR1−/− mice were subjected to HLI, and platelets were depleted systemically by injection of platelet-depleting serum starting on the first day after induction of ischemia. This scheme induces persistent and pronounced platelet depletion, as verified previously.23 Vessel density in the gastrocnemius muscle of the ischemic limbs was quantified by immunofluorescence staining. The vessels were visualized by isolectin B4 (IB4 in green, nuclei in blue), and images of whole muscle sections were acquired as tile scans and analyzed. Fourteen days after the induction of ischemia, C5aR1−/− mice additionally treated with platelet-depleting serum exhibited no significant difference in comparison to WT mice. Images 400× original magnification, scale bars represent 10 μm. (I) Quantification of vessel core numbers per area at day 14 in WT and C5aR1−/− mice, which received platelet-depleting serum over 2 weeks, revealed no significant difference. Data represent the mean ± SEM. n = 7 to 8. n.s. = no statistically significant difference was observed.
The platelet C5aR1 modulates ischemia-driven vessel growth. (A) WT, C5−/− or C5aR1−/− mice were subjected to HLI and analyzed after 2 weeks, and revascularization of the hindlimbs after femoral artery ligation was visualized by laser doppler fluximetry (LDI) under standardized conditions with the scale adapted to the experiment. Representative LDI images of mouse hind limbs after femoral artery ligation illustrate increased revascularization in C5aR1−/− animals compared with WT controls or C5−/− mice. (B) We found increased revascularization in C5aR1−/− animals in comparison to WT or C5−/− mice. Data are shown as the mean ± SEM (n = 5-8 animals per group) and are displayed as % of the perfusion in the contralateral control limb. ∗P < .05. (C) Representative images depicting capillaries (stained by IB4) and pericyte coverage (colocalization of IB4 and NG2) in the analyzed genotypes 14 days after induction of ischemia. (D) At day 14, there was no significant increase in capillary density in C5aR1−/− mice. Data represent the mean ± SEM. n = 9 to 12. (E) An increase in pericyte coverage was observable in C5aR1−/− mice compared with WT and C5−/− mice at day 14. Data represent mean ± SEM. n = 9 to 15. ∗∗P < .01, ∗∗∗P < .001. (F) Arteriolar abundance was highest in C5aR1−/− mice. There was an increase in arteries in ischemic hind limb muscle tissue compared with control tissue, which was significant for WT and C5aR1−/− mice but not for C5−/− mice. Data represent mean ± SEM. n = 6 to 9. ∗P < .05. (G) When WT or C5aR1−/− mice subjected to HLI were additionally treated with heparin, we observed no difference in revascularization anymore. Data are shown as the mean ± SEM (n = 6-8 animals per group) and are displayed as % of the perfusion in the contralateral control limb. (H) Furthermore, WT or C5aR1−/− mice were subjected to HLI, and platelets were depleted systemically by injection of platelet-depleting serum starting on the first day after induction of ischemia. This scheme induces persistent and pronounced platelet depletion, as verified previously.23 Vessel density in the gastrocnemius muscle of the ischemic limbs was quantified by immunofluorescence staining. The vessels were visualized by isolectin B4 (IB4 in green, nuclei in blue), and images of whole muscle sections were acquired as tile scans and analyzed. Fourteen days after the induction of ischemia, C5aR1−/− mice additionally treated with platelet-depleting serum exhibited no significant difference in comparison to WT mice. Images 400× original magnification, scale bars represent 10 μm. (I) Quantification of vessel core numbers per area at day 14 in WT and C5aR1−/− mice, which received platelet-depleting serum over 2 weeks, revealed no significant difference. Data represent the mean ± SEM. n = 7 to 8. n.s. = no statistically significant difference was observed.
Cross talk between the complement and coagulation pathways was described as a noncanonical mechanism of complement activation.24 To question this hypothesis in our context, we treated WT and C5aR1−/− mice with heparin and subjected them to the HLI model. There was no significant difference in revascularization, when the coagulation system was blocked (Figure 2G), whereas heparin treatment resulted in increased tail bleeding times, as expected (supplemental Figure 8). To check for the relevance of platelet C5aR1 for the modulation of revascularization, we applied platelet-depleting serum, which is very effective in causing immediate thrombocytopenia.20,23,25 Interestingly, the phenotype of increased revascularization in C5aR1−/− mice was virtually abolished in the absence of platelets, indicating an important role of the platelet C5aR1 for the modulation of vascular growth in this experimental setting (Figure 2H-I).
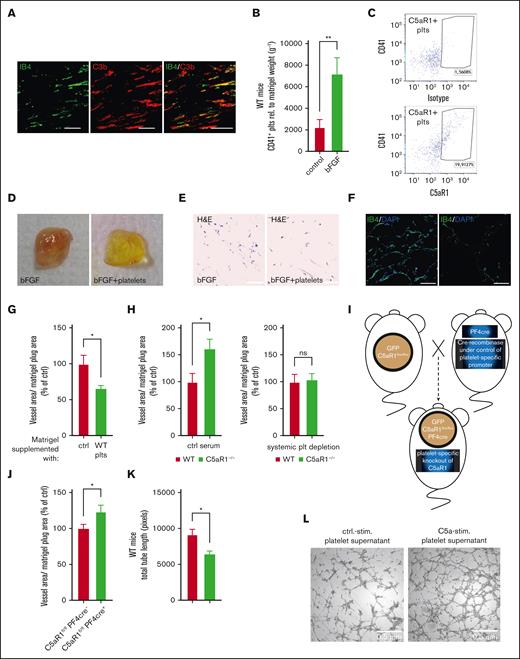
To apply a further experimental in vivo setting of angiogenesis, we used the growth factor–driven Matrigel plug assay.9 When we analyzed the plugs for the presence of complement, we found C3b colocalization with vascular structures, indicating increased complement activation upon vessel formation (Figure 3A). Then, we analyzed the presence of platelets during the process of angiogenesis induced by supplementation of the Matrigel with the growth factor bFGF (basic fibroblast growth factor). Using the platelet marker CD41, we detected platelets by flow cytometry (Figure 3B). Moreover, isotype vs C5aR1 antibody staining showed that platelets isolated from Matrigel plugs express C5aR1 (Figure 3C; supplemental Figure 10). When platelets were supplied to the plugs in addition to bFGF, vessel formation was decreased, suggesting an inhibitory function of platelets on angiogenesis (Figure 3D-G). Using platelets isolated from complement receptor–deficient mice, we found that C5aR1−/− platelets triggered significantly more growth factor–induced angiogenesis compared with WT mice (Figure 3H). However, when we applied platelet-depleting serum, the phenotype of increased angiogenesis in C5aR1−/− mice was reversed, pointing toward a platelet-specific role for this receptor (Figure 3H). To further underscore this observation, we generated lineage-specific knockout mice and crossed floxed GFP-C5aR1fl/fl mice26 with PF4-cre+ mice,27 generating PF4-cre+C5aR1fl/fl mice, which lack C5aR1 in platelets but not in other analyzed cells (Figure 3I; supplemental Figure 11). Using these mice, we found improved vascularization in the Matrigel plugs of platelet-specific C5aR1-knockout mice compared with those of PF4-cre−C5aR1fl/fl littermate controls (Figure 3J). In line with these in vivo observations using the Matrigel plug assay, in vitro tube formation of endothelial cells could be significantly inhibited by platelet supernatant stimulated with C5a (Figure 3K-L), thus suggesting a paracrine mechanism by which platelets inhibit angiogenesis.
The platelet C5aR1 contributes to growth factor-mediated angiogenesis. In vivo Matrigel plug assay experiments were carried out as described in the “Materials and methods.” (A) We detected increased complement activation (C3b, red) in the angiogenic tissue, which colocalizes with vascular structures (IB4, green). (B-C) After 7 days, matrigels were explanted, and a single-cell suspension generated from the plugs was analyzed by flow cytometry. We observed increased presence of platelets (CD41+; B) and the presence of C5aR1 on platelets (CD41+CD45−C5aR1+; C). n = 7 to 8 ∗P < .05, ∗∗P < .01. (D-G) Isolated platelets were coinjected with bFGF into the Matrigel solution before implantation into mice. We observed an inhibition of neovascularization by platelet addition. (D) Panel D shows reduced macroscopic vascularization in explanted plugs, which was associated with reduced cellularity (E) (H and E staining) and reduced green signal (F) (IB4 staining for vessels). (G) n = 7 to 8 plugs were analyzed. Matrigels injected with vehicle control represent 100%. ∗P < .05 in comparison to control. (H) WT or C5aR1−/− mice were applied in the Matrigel plug in vivo assay. We observed increased angiogenesis in the absence of C5aR1. n = 8 to 11 plugs were analyzed. The group with WT mice represents 100%. ∗P < .05. When platelets were depleted systemically by injection of platelet-depleting serum, we could not detect significant differences in angiogenesis. Data are shown as the mean ± SEM (n = 7 animals per group) and are displayed as % of WT mice. ns = no statistically significant difference could be observed. (I) PF4-cre+C5aR1fl/fl mice were generated as described in “Methods.” (J) Platelet-specific C5a receptor 1 knockout mice showed increased revascularization. Data are presented as the mean ± SEM (n = 9) and are displayed as % of the perfusion in the contralateral control limb. ∗P < .05 in comparison to cre-negative WT mice. (K-L) MHEC-5T were coincubated with platelet releasate after C5a stimulation. C5a-stimulated WT platelet supernatant inhibited endothelial tube formation. Data are displayed as the mean ± SEM (n = 5). ∗P < .05. (L) Panel L shows representative images of these in vitro experiments.
The platelet C5aR1 contributes to growth factor-mediated angiogenesis. In vivo Matrigel plug assay experiments were carried out as described in the “Materials and methods.” (A) We detected increased complement activation (C3b, red) in the angiogenic tissue, which colocalizes with vascular structures (IB4, green). (B-C) After 7 days, matrigels were explanted, and a single-cell suspension generated from the plugs was analyzed by flow cytometry. We observed increased presence of platelets (CD41+; B) and the presence of C5aR1 on platelets (CD41+CD45−C5aR1+; C). n = 7 to 8 ∗P < .05, ∗∗P < .01. (D-G) Isolated platelets were coinjected with bFGF into the Matrigel solution before implantation into mice. We observed an inhibition of neovascularization by platelet addition. (D) Panel D shows reduced macroscopic vascularization in explanted plugs, which was associated with reduced cellularity (E) (H and E staining) and reduced green signal (F) (IB4 staining for vessels). (G) n = 7 to 8 plugs were analyzed. Matrigels injected with vehicle control represent 100%. ∗P < .05 in comparison to control. (H) WT or C5aR1−/− mice were applied in the Matrigel plug in vivo assay. We observed increased angiogenesis in the absence of C5aR1. n = 8 to 11 plugs were analyzed. The group with WT mice represents 100%. ∗P < .05. When platelets were depleted systemically by injection of platelet-depleting serum, we could not detect significant differences in angiogenesis. Data are shown as the mean ± SEM (n = 7 animals per group) and are displayed as % of WT mice. ns = no statistically significant difference could be observed. (I) PF4-cre+C5aR1fl/fl mice were generated as described in “Methods.” (J) Platelet-specific C5a receptor 1 knockout mice showed increased revascularization. Data are presented as the mean ± SEM (n = 9) and are displayed as % of the perfusion in the contralateral control limb. ∗P < .05 in comparison to cre-negative WT mice. (K-L) MHEC-5T were coincubated with platelet releasate after C5a stimulation. C5a-stimulated WT platelet supernatant inhibited endothelial tube formation. Data are displayed as the mean ± SEM (n = 5). ∗P < .05. (L) Panel L shows representative images of these in vitro experiments.
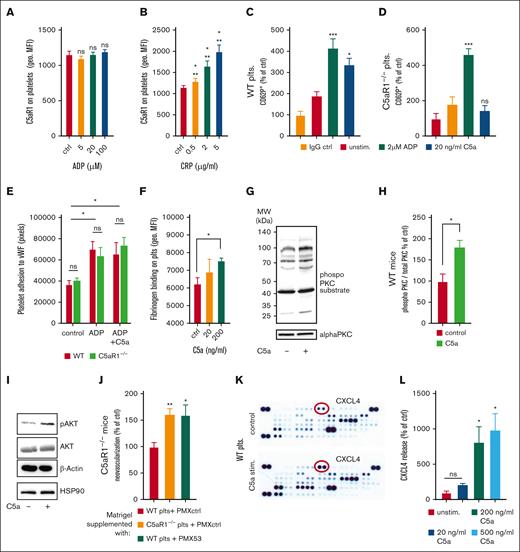
Next, we investigated the underlying mechanisms of how platelets inhibit revascularization or endothelial functions upon activation of the C5a-C5aR1 axis. First, we checked whether C5aR1 on platelets is subject to regulation by platelet activators. Indeed, we found that its expression is upregulated upon stimulation using the glycoprotein VI agonist CRP (collagen-related peptide), whereas there is no upregulation by ADP stimulation (Figure 4A-B). Furthermore, we observed that C5a was capable of activating platelets. When isolated WT platelets were stimulated with C5a, we observed upregulation of P-selectin, indicating a functional relevance of C5aR1 for platelet activation, which was absent when C5aR1−/− platelets were used (Figure 4C-D). In contrast, ADP caused platelet activation in both WT and C5aR1−/− platelets (Figure 4C-D). However, we found no significant change in ex vivo aggregation induced by classical agonists (supplemental Figure 12) upon C5a stimulation. Furthermore, C5a caused no change in adhesion to von Willebrand factor under static conditions (Figure 4E). However, C5a induced a dose-dependent increase in fibrinogen binding both in murine as well as in human platelets (Figure 4F; supplemental Figure 13A), as well as in the binding of activation-specific antibody PAC-1, which detects activated GPIIbIIIa (supplemental Figure 13B). These results are in line with our observations of C5aR1 correlating positively with PAC-1 in patients with atherosclerotic disease (Figure 1C). Furthermore, application of the small-molecule C5aR1 antagonist PMX205 could reduce the C5a-induced increase in fibrinogen binding (supplemental Figure 14). To analyze a potential pathway connecting C5aR1 ligation on platelets by C5a with intracellular signaling, we measured phosphorylated protein kinase C (PKC), because PKC has been implicated in C5aR1-mediated signaling previously.23,28 We found that stimulation with C5a results in increased PKC phosphorylation (Figure 4G-H). We also found phosphorylation of Akt upon exposure to C5a, indicating a regulatory mechanism for platelets after anaphylatoxin receptor ligation (Figure 4I). To further underscore the hypothesis of an involved paracrine mechanism, we used the in vivo Matrigel assay and supplemented the plugs with platelets from WT or C5aR1−/− mice, and additionally applied PMX53, a cyclic hexapeptide C5aR-inhibitor. Supplementation of the Matrigel with WT platelets was able to rescue the WT phenotype even when Matrigel was implanted into KO mice, whereas the application of C5aR1−/− or WT platelets treated with the C5a inhibitor PMX53 resulted in increased angiogenesis (Figure 4J), similarly to C5aR1−/− mice without specific treatment (Figure 3H). To identify paracrine antiangiogenic factors released from platelets, we stimulated isolated platelets with C5a and carried out a proteome profiler analysis of the harvested platelet supernatant. Indeed, this screening approach revealed an increased secretion of some antiangiogenic factors upon C5a exposure (Figure 4K; supplemental Figure 15). The platelet α granule component CXCL4 exhibited the strongest increase in secretion after C5a stimulation (Figure 4K; supplemental Figure 15), which could be confirmed by a conventional enzyme-linked immunosorbent assay demonstrating dose-dependent CXCL4 secretion after C5a-stimulation of platelets (Figure 4L). Furthermore, we have stimulated murine platelets with classical agonists such as ADP, CRP, or thrombin and C5a. In some groups, we blocked C5aR1 using PMX205 preincubation (supplemental Figure 16B); in the other, we used a control peptide for preincubation (supplemental Figure 16A). Blockade of C5aR1 did not result in obvious changes in platelet secretion behavior, whereas C5a-induced CXCL4 release was blocked. However, we observed no significant change in vascular endothelial growth factor A (VEGF-A), thrombospondin-1, endostatin, or TIMP-1 secretion upon C5a stimulation (supplemental Figure 17).
C5a treatment of platelets results in activation and mediator release. (A-B) Human whole blood was stimulated with ADP (A) or CRP (B), and the expression of C5aR1 on platelets was analyzed by flow cytometry. Data are shown as the mean ± SEM (n = 5) and are displayed as geometric mean fluorescence intensity (geo. MFI). (C-D) Citrated whole blood from WT and C5aR1−/− mice was stimulated using ADP, C5a, or vehicle control and assessed for the platelet activation marker P-selectin by flow cytometry. C5a-induced platelet activation in WT platelets (C) but not in C5aR1−/− platelets (D). Data are shown as the mean ± SEM (n = 5) and are displayed as % of control. The % gated CD62+ platelets in the vehicle-stimulated group represents 100%. ∗∗P < .001, ∗P < .05 in comparison to the IgG control. (E) Human isolated platelets were incubated on von Willebrand factor (vWF)-coated wells. After careful washing, adherent platelets were quantified. We observed an increase in adhesion upon ADP stimulation; however, no additional effect by C5a stimulation. There was no significant difference between WT and C5aR1−/− platelets. Data represent mean ± SEM. n = 4 to 8. ∗P < .05. ns = no statistically significant difference could be observed in between WT compared with C5aR1−/− platelets. (F) Murine platelets were isolated and stimulated using different concentrations of C5a. C5a induced a significant increase in platelet fibrinogen binding. Data are shown as the mean ± SEM (n = 5) and are displayed as geometric mean fluorescence intensity. ∗P < .05. (G) To uncover a potential signaling mechanism downstream of C5aR1 ligation, lysates of WT platelets were generated after vehicle control or C5a stimulation and samples were probed at equal protein concentrations for phosphoproteins as well as nonphosphorylated controls. C5a induced reproducible PKC phosphorylation as detected by a phospho-PKC substrate antibody, which detects phosphorylation of various PKC isoforms when phosphorylated at serine residues surrounded by Arg or Lys at the −2 and +2 positions and a hydrophobic residue at the +1 position.82 As control, we used PKC α. Displayed are representative images of at least 4 independent experiments. (H) Quantification of the phosphorylated signal relative to PKC α control signal is displaed. n = 4. The group of control-stimulated supernatant of WT platelets represents 100%. ∗P < .05. (I) Analysis of phosphorylated Akt revealed involvement in C5a-induced signaling in platelets. Displayed are representative images of at least 4 independent experiments. (J) C5aR1−/− mice were applied in the Matrigel plug in vivo assay, and platelets isolated from WT or C5aR1−/− mice were coinjected with the Matrigel solution. We observed a reversed phenotype, when WT platelets were used, but not when C5aR1−/− platelets or WT platelets treated with the small-molecule inhibitor PMX53 were applied. n = 5 to 10 plugs were analyzed. The group of C5aR1−/− mice reinjected with WT platelets represents 100%. ∗∗P < .01, ∗P < .05. (K) Washed murine WT platelets were stimulated with C5a. The supernatant was analyzed by a membrane-based antibody array. Specific mediators such as CXCL4 (red circles), were upregulated after stimulation with C5a (bottom) compared with the vehicle control (top). The strongest increase was observed for CXCL4. (L) Conventional enzyme-linked immunosorbent assay (ELISA) confirmed a significant dose-dependent increase in CXCL4 secretion from platelets after C5a stimulation. Maximum CXCL4 release is reached at a C5a concentration of 200 ng/mL, which was similar at 500 ng/mL. Data are shown as the mean ± SEM (n = 4-9) and are displayed as percentage of control. The CXCL4 protein level of unstimulated platelet supernatant represents 100%. ∗P < .05.
C5a treatment of platelets results in activation and mediator release. (A-B) Human whole blood was stimulated with ADP (A) or CRP (B), and the expression of C5aR1 on platelets was analyzed by flow cytometry. Data are shown as the mean ± SEM (n = 5) and are displayed as geometric mean fluorescence intensity (geo. MFI). (C-D) Citrated whole blood from WT and C5aR1−/− mice was stimulated using ADP, C5a, or vehicle control and assessed for the platelet activation marker P-selectin by flow cytometry. C5a-induced platelet activation in WT platelets (C) but not in C5aR1−/− platelets (D). Data are shown as the mean ± SEM (n = 5) and are displayed as % of control. The % gated CD62+ platelets in the vehicle-stimulated group represents 100%. ∗∗P < .001, ∗P < .05 in comparison to the IgG control. (E) Human isolated platelets were incubated on von Willebrand factor (vWF)-coated wells. After careful washing, adherent platelets were quantified. We observed an increase in adhesion upon ADP stimulation; however, no additional effect by C5a stimulation. There was no significant difference between WT and C5aR1−/− platelets. Data represent mean ± SEM. n = 4 to 8. ∗P < .05. ns = no statistically significant difference could be observed in between WT compared with C5aR1−/− platelets. (F) Murine platelets were isolated and stimulated using different concentrations of C5a. C5a induced a significant increase in platelet fibrinogen binding. Data are shown as the mean ± SEM (n = 5) and are displayed as geometric mean fluorescence intensity. ∗P < .05. (G) To uncover a potential signaling mechanism downstream of C5aR1 ligation, lysates of WT platelets were generated after vehicle control or C5a stimulation and samples were probed at equal protein concentrations for phosphoproteins as well as nonphosphorylated controls. C5a induced reproducible PKC phosphorylation as detected by a phospho-PKC substrate antibody, which detects phosphorylation of various PKC isoforms when phosphorylated at serine residues surrounded by Arg or Lys at the −2 and +2 positions and a hydrophobic residue at the +1 position.82 As control, we used PKC α. Displayed are representative images of at least 4 independent experiments. (H) Quantification of the phosphorylated signal relative to PKC α control signal is displaed. n = 4. The group of control-stimulated supernatant of WT platelets represents 100%. ∗P < .05. (I) Analysis of phosphorylated Akt revealed involvement in C5a-induced signaling in platelets. Displayed are representative images of at least 4 independent experiments. (J) C5aR1−/− mice were applied in the Matrigel plug in vivo assay, and platelets isolated from WT or C5aR1−/− mice were coinjected with the Matrigel solution. We observed a reversed phenotype, when WT platelets were used, but not when C5aR1−/− platelets or WT platelets treated with the small-molecule inhibitor PMX53 were applied. n = 5 to 10 plugs were analyzed. The group of C5aR1−/− mice reinjected with WT platelets represents 100%. ∗∗P < .01, ∗P < .05. (K) Washed murine WT platelets were stimulated with C5a. The supernatant was analyzed by a membrane-based antibody array. Specific mediators such as CXCL4 (red circles), were upregulated after stimulation with C5a (bottom) compared with the vehicle control (top). The strongest increase was observed for CXCL4. (L) Conventional enzyme-linked immunosorbent assay (ELISA) confirmed a significant dose-dependent increase in CXCL4 secretion from platelets after C5a stimulation. Maximum CXCL4 release is reached at a C5a concentration of 200 ng/mL, which was similar at 500 ng/mL. Data are shown as the mean ± SEM (n = 4-9) and are displayed as percentage of control. The CXCL4 protein level of unstimulated platelet supernatant represents 100%. ∗P < .05.
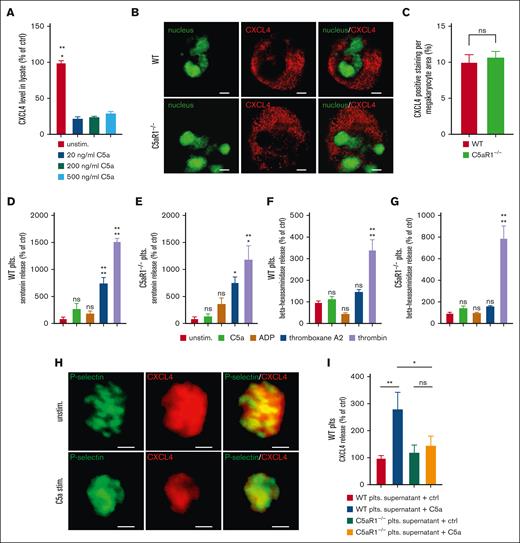
Analyzing lysates of the pellet of platelets stimulated with C5a, we found that a significant amount of platelet CXCL4 content is secreted in response to C5a stimulation (Figure 5A). Questioning the possibility that C5aR1 also affects CXCL4 contents in platelets, we assessed its regulation at the megakaryocyte level. However, when we analyzed WT or C5aR1−/− bone marrow–derived megakaryocytes by immunofluorescence, we observed no difference in CXCL4 content (Figure 5B-C). Moreover, real-time polymerase chain reaction showed no difference in CXCL4 over P-selectin levels between WT and C5aR1−/− megakaryocytes (supplemental Figure 18). Interestingly, analyzing serotonin levels (from dense granules) or β-hexosaminidase levels (from lysosomes), we observed no difference in the secretion of other platelet granule types between WT and C5aR1−/− mice (Figure 5D-G). Thus, C5a seems to induce a specific α granule secretion response. Finally, on the single-cell level, we could confirm a specific release of CXCL4 upon stimulation with C5a, analyzing platelet granule secretion. Although P-selectin displays an upregulation upon C5a stimulation, indicating platelet activation, the CXCL4 intensity decreases (Figure 5H).
Regulation of CXCL4 secretion from platelets by C5a. (A) To determine the secretion of CXCL4 from platelets upon exposure to different C5a concentrations (20-500 ng/mL), lysates were prepared from the stimulated platelets, and levels of remaining intracellular CXCL4 were determined by ELISA. Data are shown as the mean ± SEM (n = 4-7) and are displayed as % of control. The CXCL4 protein level of unstimulated platelet supernatant represents 100%. ∗P < .05 in comparison to control. (B) Murine megakaryocytes from WT vs C5aR1−/− mice were assessed for the content of CXCL4-predominant granules (red). Displayed are images representative of 10 to 12 single megakaryocytes analyzed; nuclei are shown in green. 630× original magnification, scale bars represent 10 μm. (C) We quantified CXCL4 staining in 11 vs 14 images of megakaryocytes from WT or C5aR1−/− mice. This quantification showed no difference in the area of the CXCL4 signal in relation to the area of the whole cell between both genotypes. Data represent mean ± SEM. n = 11 to 14. n.s. = no statistically significant difference was observed. (D-G) Serotonin levels (indicating dense granules) or β-hexosaminidase levels (indicating lysosomes) were analyzed in the supernatant of isolated murine platelets after stimulation with activators, as indicated in the figure. We observed no relevant secretion after C5a stimulation, in contrast to classical platelet activators. Data are shown as the mean ± SEM (n = 4-9) and are displayed as % of control. The respective protein level of unstimulated platelet supernatant represents 100%. ∗∗∗P < .001, ∗∗P < .01, ∗P < .05 in comparison to control. ns = no significant difference was measured. (H) Single platelets from WT mice were stimulated with C5a or vehicle control. Granules were stained green for P-selectin and red for CXCL4, yellow areas represent overlay, that is, P-selectin-CXCL4 double-positive α granules. 630× original magnification, scale bars represent 1 μm. Images are representative of >100 analyzed single platelets. (I) Washed murine platelets from WT or C5aR1−/− mice were stimulated with C5a. The supernatant was analyzed by ELISA for the level of CXCL4. C5a stimulation yielded significant CXCL4 secretion only in WT platelets but not in C5aR1−/− platelets. Data are shown as the mean ± SEM. n = 8. ns = no significant difference was measured; ∗P < .05; ∗∗P < .01.
Regulation of CXCL4 secretion from platelets by C5a. (A) To determine the secretion of CXCL4 from platelets upon exposure to different C5a concentrations (20-500 ng/mL), lysates were prepared from the stimulated platelets, and levels of remaining intracellular CXCL4 were determined by ELISA. Data are shown as the mean ± SEM (n = 4-7) and are displayed as % of control. The CXCL4 protein level of unstimulated platelet supernatant represents 100%. ∗P < .05 in comparison to control. (B) Murine megakaryocytes from WT vs C5aR1−/− mice were assessed for the content of CXCL4-predominant granules (red). Displayed are images representative of 10 to 12 single megakaryocytes analyzed; nuclei are shown in green. 630× original magnification, scale bars represent 10 μm. (C) We quantified CXCL4 staining in 11 vs 14 images of megakaryocytes from WT or C5aR1−/− mice. This quantification showed no difference in the area of the CXCL4 signal in relation to the area of the whole cell between both genotypes. Data represent mean ± SEM. n = 11 to 14. n.s. = no statistically significant difference was observed. (D-G) Serotonin levels (indicating dense granules) or β-hexosaminidase levels (indicating lysosomes) were analyzed in the supernatant of isolated murine platelets after stimulation with activators, as indicated in the figure. We observed no relevant secretion after C5a stimulation, in contrast to classical platelet activators. Data are shown as the mean ± SEM (n = 4-9) and are displayed as % of control. The respective protein level of unstimulated platelet supernatant represents 100%. ∗∗∗P < .001, ∗∗P < .01, ∗P < .05 in comparison to control. ns = no significant difference was measured. (H) Single platelets from WT mice were stimulated with C5a or vehicle control. Granules were stained green for P-selectin and red for CXCL4, yellow areas represent overlay, that is, P-selectin-CXCL4 double-positive α granules. 630× original magnification, scale bars represent 1 μm. Images are representative of >100 analyzed single platelets. (I) Washed murine platelets from WT or C5aR1−/− mice were stimulated with C5a. The supernatant was analyzed by ELISA for the level of CXCL4. C5a stimulation yielded significant CXCL4 secretion only in WT platelets but not in C5aR1−/− platelets. Data are shown as the mean ± SEM. n = 8. ns = no significant difference was measured; ∗P < .05; ∗∗P < .01.
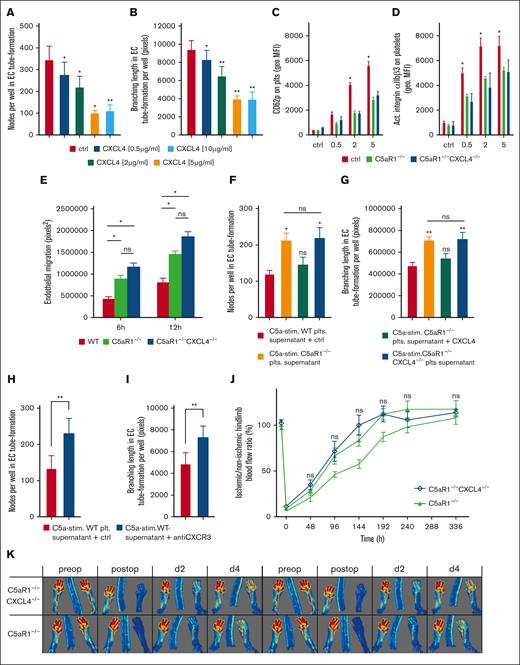
To further underscore this paracrine inhibitory mechanism, we treated endothelial cells with increasing concentrations of CXCL4 and analyzed in vitro tube formation. We observed a dose-dependent decrease in branching nodes and reduced mean branching lengths (Figure 6A-B; supplemental Figure 19). To demonstrate the relevance of a C5aR1-CXCL4 axis in vivo, we generated C5aR1−/−CXCL4−/− double knockout mice. After stimulation with CRP, platelet activation as assessed by CD62P and activated integrin αIIbβ3 upregulation was lower in C5aR1−/− as well as C5aR1−/−CXCL4−/− platelets compared with WT (Figure 6C-D). Furthermore, CRP-induced upregulation of CD61 and CD63 was lower both in C5aR1−/− as well as C5aR1−/−CXCL4−/− platelets (supplemental Figure 20B-C). Spiking in recombinant CXCL4 had no significant effect on platelet activation status (supplemental Figure 21). CRP-induced fibrinogen binding was significantly lower in C5aR1−/−CXCL4−/− platelets compared with that in both WT and C5aR1−/− platelets (supplemental Figure 20A).
C5aR1-induced inhibition of neovascularization by platelets depends on CXCL4 secretion and is distinct from platelet activation. (A-B) Murine endothelial cells (MHEC-5T) were incubated in wells on Matrigel with increasing concentrations of CXCL4 (0.5-10 μg/mL), and branching points of forming tubes as well as branching lengths were determined as indicated in “Materials and methods” (also refer to supplemental Figure 19). Data are displayed as the mean ± SEM (n = 7). ∗P < .05. (C-D) We generated C5aR1−/−CXCL4−/− double knockout mice. Platelets isolated from these animals were stimulated with ADP, and the expression of platelet activation markers CD62P or activated GPIIbIIIa were analyzed by flow cytometry. Data are shown as the mean ± SEM (n = 5) and are displayed as geometric MFI. ∗P < .05 in comparison to WT control. ns = no significant difference was measured. (E) Platelets from WT, C5aR1−/−, or C5aR1−/−CXCL4−/− mice were stimulated with C5a, and the supernatant was added to endothelial cells (MHEC-5T). In comparison to C5aR1−/− and C5aR1−/−CXCL4−/− platelet supernatant, coincubation with C5a-conditioned WT platelet supernatant led to lower endothelial migration. Double-deficient platelet supernatant did not differ significantly from C5aR1−/− platelet supernatant. Data represent mean ± SEM. n = 6. ∗P < .05. (F-G) MHEC-5T cells were incubated with the C5a-stimulated supernatant of platelets isolated from WT, C5aR1−/−, and C5aR1−/−CXCL4−/− mice. In 1 group, the supernatant of C5aR1−/− platelets was additionally supplemented with CXCL4 (2 μg/mL). C5a-stimulated WT platelet supernatant inhibited endothelial tube formation, which was not detectable in C5aR1−/− and C5aR1−/−CXCL4−/− platelets. Reconstitution with CXCL4 in the C5aR1−/− group led to a similar level of tube formation as in the control WT group. Data are displayed as the mean ± SEM (n = 4-5). ∗∗P < .01, ∗P < .05. (H-I) Similarly, MHEC-5T cells were incubated with the C5a-stimulated supernatant of platelets isolated from WT mice but additionally treated with a blocking antibody to the CXCL4 receptor, CXCR3. Data are displayed as the mean ± SEM (n = 7). ∗P < .05 in comparison to IgG control. (J-K) HLI was induced in C5aR1−/− mice or C5aR1−/−CXCL4−/− mice and a WT control group, which were generated as described in the “Methods.” Double knockout mice showed no increased revascularization in comparison to C5aR1−/− mice. Data are presented as the mean ± SEM (n = 5-7) and are displayed as a percentage of the perfusion in the contralateral control limb. n.s. = no significant difference could be observed compared with C5aR1−/− animals. (M) Shows representative LDI images of mouse hind limbs after femoral artery ligation and during revascularization over 14 days.
C5aR1-induced inhibition of neovascularization by platelets depends on CXCL4 secretion and is distinct from platelet activation. (A-B) Murine endothelial cells (MHEC-5T) were incubated in wells on Matrigel with increasing concentrations of CXCL4 (0.5-10 μg/mL), and branching points of forming tubes as well as branching lengths were determined as indicated in “Materials and methods” (also refer to supplemental Figure 19). Data are displayed as the mean ± SEM (n = 7). ∗P < .05. (C-D) We generated C5aR1−/−CXCL4−/− double knockout mice. Platelets isolated from these animals were stimulated with ADP, and the expression of platelet activation markers CD62P or activated GPIIbIIIa were analyzed by flow cytometry. Data are shown as the mean ± SEM (n = 5) and are displayed as geometric MFI. ∗P < .05 in comparison to WT control. ns = no significant difference was measured. (E) Platelets from WT, C5aR1−/−, or C5aR1−/−CXCL4−/− mice were stimulated with C5a, and the supernatant was added to endothelial cells (MHEC-5T). In comparison to C5aR1−/− and C5aR1−/−CXCL4−/− platelet supernatant, coincubation with C5a-conditioned WT platelet supernatant led to lower endothelial migration. Double-deficient platelet supernatant did not differ significantly from C5aR1−/− platelet supernatant. Data represent mean ± SEM. n = 6. ∗P < .05. (F-G) MHEC-5T cells were incubated with the C5a-stimulated supernatant of platelets isolated from WT, C5aR1−/−, and C5aR1−/−CXCL4−/− mice. In 1 group, the supernatant of C5aR1−/− platelets was additionally supplemented with CXCL4 (2 μg/mL). C5a-stimulated WT platelet supernatant inhibited endothelial tube formation, which was not detectable in C5aR1−/− and C5aR1−/−CXCL4−/− platelets. Reconstitution with CXCL4 in the C5aR1−/− group led to a similar level of tube formation as in the control WT group. Data are displayed as the mean ± SEM (n = 4-5). ∗∗P < .01, ∗P < .05. (H-I) Similarly, MHEC-5T cells were incubated with the C5a-stimulated supernatant of platelets isolated from WT mice but additionally treated with a blocking antibody to the CXCL4 receptor, CXCR3. Data are displayed as the mean ± SEM (n = 7). ∗P < .05 in comparison to IgG control. (J-K) HLI was induced in C5aR1−/− mice or C5aR1−/−CXCL4−/− mice and a WT control group, which were generated as described in the “Methods.” Double knockout mice showed no increased revascularization in comparison to C5aR1−/− mice. Data are presented as the mean ± SEM (n = 5-7) and are displayed as a percentage of the perfusion in the contralateral control limb. n.s. = no significant difference could be observed compared with C5aR1−/− animals. (M) Shows representative LDI images of mouse hind limbs after femoral artery ligation and during revascularization over 14 days.
We also assessed the possibility that platelet C5aR1-mediated CXCL4 secretion could be mediated in a paracrine or autocrine fashion. For instance, we stimulated resting platelets with C5a and used the supernatant to stimulate other resting platelets. As a readout, we assessed platelet activation measured by flow cytometry. By this approach, we found that the supernatant of platelets alone could not induce platelet activation in an autocrine fashion (supplemental Figure 22A). Furthermore, we have probed the idea of a paracrine activation loop. Most leukocytes express C5aR1,26 and it is possible that activation of C5aR1 on other immune cells triggers CXCL4 release from platelets in a paracrine fashion. Therefore, we stimulated whole blood as well as isolated platelets adjusted to equal platelet concentrations with C5a. Interestingly, in whole blood, only mild secretion could be observed, which did not reach statistical significance, whereas C5a stimulation of isolated platelets resulted in significant CXCL4 secretion (supplemental Figure 22B). This observation suggests that a direct mechanism (stimulation of platelets) rather than an indirect mechanism (via leukocytes) is responsible for the observed phenotype.
Next, we applied these double-deficient mice to in vitro assays testing endothelial functions. We observed a significantly higher amount of endothelial cell migration when we treated them with the supernatant of C5aR1−/− and C5aR1−/−CXCL4−/− platelets (Figure 6E). When we analyzed in vitro tube formation, we found that the supernatant of C5aR1−/− platelets stimulated tube formation. The resupplementation of the supernatant with CXCL4 restored the effect to that of WT platelet supernatant. Interestingly, the addition of supernatant from C5aR1−/−CXCL4−/− platelets did not lead to an increase in tube formation compared with C5aR1−/− platelet supernatant pointing toward a C5aR1-CXCL4 axis (Figure 6F-G). Using a pharmacological approach with a blocking antibody to CXCR3 (the endothelial receptor for CXCL429), we observed increased in vitro tube formation (Figure 6H-I). To further scrutinize the in vivo relevance of C5a-induced CXCL4 secretion for ischemia-driven angiogenesis, we tested the C5aR1−/−CXCL4−/− double-deficient mice using the HLI model. In line with our previous results, both C5aR1−/− and C5aR1−/−CXCL4−/− mice showed a similarly increased level of revascularization without any statistically significant difference between single and double knockout mice compared with a WT control group (Figure 6J-K), pointing toward a C5a-C5aR1-CXCL4 axis mediating the angiogenesis-inhibiting effect of platelets.
In conclusion, we describe a novel intersection point between complement and platelet activation in ischemia and growth factor–driven vessel formation, as the anaphylatoxin receptor C5aR1 expressed on platelets modulates experimental angiogenesis by CXCL4 release.
Discussion
To uncover mechanisms of thromboinflammation, linking innate immunity with tissue recovery may open new avenues for drug development in cardiovascular diseases. Here, we describe a novel mechanism by which the anaphylatoxin receptor C5aR1 inhibits revascularization and may therefore be a promising target in ischemic diseases. We could show that platelets, which are abundant in ischemic tissue and express C5aR1, have an antiangiogenic effect on growth factor and ischemia-induced angiogenesis in vivo. Genetic deficiency for C5aR1 but not for C5 or genetic ablation of C5aR1 in platelets results in significantly increased revascularization. Upon C5a stimulation, platelets are activated and release the antiangiogenic factor CXCL4.
We were able to measure a positive correlation of C5aR1 on platelets with an activation-specific platelet marker. Interestingly, C5aR1 was specifically elevated in stable patients with coronary artery disease. This observation might indicate that platelet C5aR1 is particularly important for chronic processes (rather than acute coronary events) in ischemic heart disease. Targeting complement components in cardiovascular diseases has been suggested before.30,31 In a porcine model of myocardial infarction, blockade of C5a cleavage by coversin significantly reduced myocardial infarction in the area at risk by 39% as analyzed with triphenyl tetrazolium chloride staining and by 19% using magnetic resonance imaging.32 For ischemic stroke, C5aR1-deficient mice are protected against cerebral ischemia-reperfusion injury (IRI) using the transient middle cerebral artery occlusion (MCAO) model.33 In patients with percutaneous coronary intervention, however, administration of an anti-C5 antibody during the procedure had no significant effect on myocardial infarction or mortality.34 As complement activation, determined by soluble C5b-9 (sC5b-9), the final activation product that should be completely blocked by the antibody, was increased in the treatment and placebo groups, respectively,35 it was suggested that the applied drug dose was too low. Another possibility is that C5a instead of C5 has more relevance in this clinical setting. Our results further suggest another possible time point to administer drugs targeting the complement system at later stages of the healing process when revascularization takes place.
Interestingly, there is no increased capillary density in the distal hind limb tissue (gastrocnemius muscle) in C5aR1−/− mice. This is likely because hind limb revascularization is largely driven by arteriogenesis, which is sprouting into the ischemic tissue.36
Besides obvious diseases with direct relation to complement activation, such as paroxysmal nocturnal hemoglobinuria,37,38 arthritis,39 sepsis,40 more recently diseases with ischemia/reperfusion injury have been investigated for the relevance of complement activation for disease progression.41-43 Moreover, it has been shown that many thromboinflammatory disorders, such as antiphospholipid syndrome and thrombotic thrombocytopenic purpura (TTP) are complement-mediated,44,45 and complement-directed drugs are used in these disorders.31 Thus, it is possible that there is a role for complement-directed therapy targeting receptors on platelets and other cells in modulating the angiogenic response and the progression of disease.
The formation of new vessels after injury or ischemia is a key process for organ restoration and recovery of function.46 The complement system is a proteolytic cascade of proteins. C5a is formed when C5 is cleaved into C5a and C5b.5 The necessary precondition for this is the assembly of the C5-convertase, which is formed when C3 is cleaved into C3a and C3b. The main source of complement proteins is the liver.47 However, many immune cells, such as macrophages, have been shown to be able to produce complement components as well.48 In the context of IRI, it is well documented that complement activation occurs in ischemic conditions.49 Although reactive oxygen species production is regarded as the main driver of complement activation in IRI by the classical pathway, the lectin pathway is also involved.50 In addition, activated endothelium in ischemic tissues may bind gC1qR, thereby activating the classical complement pathway.51,52
Importantly, this study involves both C5aR1-deficient mice as well as C5-deficient animals. In this way, it is possible to delineate the effect mediated by C5a as opposed to C5. In C5−/− mice, also C5b is missing, and therefore, TCC activation cannot occur. Interestingly, we found that C5aR1−/− mice but not C5−/− mice have an increased level of ischemia-driven angiogenesis in comparison to WT mice. It is known that the TCC (C5b-9, terminal complement cascade) has a proangiogenic role.53,54 This might explain our observation that increased angiogenesis is specifically observed in C5aR1−/− mice. As we have witnessed that the velocity of revascularization in mice in response to femoral artery occlusion is subject to considerable variability, all our experimental groups included in this study were always treated in parallel.
Apart from complement components, platelet-derived mediators have also been implicated in the regulation of angiogenesis.9,10,14,17-19,55-64 A functional cross talk of platelet activation with factors of the complement cascade for revascularization, however, has not been extensively studied so far.
Platelets can both promote and inhibit angiogenesis. For instance, platelet preparations are applied for the promotion of healing,65 as platelets are known to contain a number of proangiogenic factors.58,66 In addition, in conditions such as ovarian cancer, platelets have been shown to promote tumor angiogenesis.67 Furthermore, platelets have been demonstrated to modulate angiogenesis and prevent excessive hemorrhage.17,68 We found that the addition of platelets to Matrigel plugs reduces growth factor–driven neovascularization. It is, however, likely that in a milieu of low levels of stimulating angiogenic factors, platelets may contribute to proangiogenic processes. In fact, it was demonstrated before that pro- and antiangiogenic factors are stored in platelet α granules, which can be released in response to specific stimuli.18,19 Very limited information, however, exists on how the release of these factors is regulated. It was suggested that thrombospondin-1 can serve as a negative regulator of angiogenesis.55,57 Furthermore, it was demonstrated that angiostatin release from platelets follows different kinetics than VEGF secretion during thrombus formation69 and that proteinase-activated receptors 1 and 4 can counter-regulate endostatin and VEGF release from human platelets.70 Here, we describe a novel mechanism underlying the release of antiangiogenic factors from platelets tailored by the anaphylatoxin receptor C5aR1. Interestingly, the antiangiogenic factor CXCL4 was released from platelets after stimulation by complement, a paracrine mechanism that was dependent on the C5a-C5aR1 axis and resulted in inhibition of neovascularization in vitro and in vivo. Chemokines are well-recognized factors that can significantly contribute to platelet activation.71 To uncover the effects of C5a-mediated release of CXCL4 on angiogenesis, we have generated C5aR1−/−CXCL4−/− double knockout mice. Using the double knockout strain, we wanted to make sure that only effects related to the C5aR1-CXCL4 axis were investigated, because CXCL4-deficient mice display a number of phenotypes related to platelets and thrombosis,72 as well as immune functions,73,74 and further functions75 such as tissue remodeling.76,77 When characterizing these mice, we observed distinct effects of these molecules. Regarding platelet activation as assessed by platelet activation markers (fibrinogen binding or adhesion capacity), the absence of C5aR1 and CXCL4 resulted in reduced platelet reactivity. Previous reports already demonstrated that CXCL4 is capable of mediating platelet functions and that it can activate platelets in concert with other factors such as Galectin-1.78,79 When we used these mice to study in vitro endothelial functions or in vivo ischemia-driven revascularization, we observed no additive effect for double-deficient mice in comparison to C5aR1−/− animals, demonstrating that CXCL4 is released upon ligation of C5aR1. However, given the large number of identified angiogenesis-modulating factors stored in platelets,14,59,80 future studies should also cover these proteins and how stimulation with complement components influences their release. As platelets contain both pro- and antiangiogenic factors, their net effect on the promotion of neovascularization may depend on the surrounding microenvironment. For future studies, it will be central to understand how this balance is exactly regulated. Furthermore, it will be interesting to see how targeting platelet C5aR1, for example, with small-molecule inhibitors would influence the formation of new vessels. Previously, we have shown that the C5aR1 antagonist PMX205 induces a phenotype of improved revascularization in a mouse model of HLI in WT mice.23 In previous studies, Ma et al have shown that proteinase-activated receptors 1 and 4 differentially control endostatin or VEGF release from platelets.70 Furthermore, Italiano et al have demonstrated in vitro that platelets are able to specifically release either pro- or antiangiogenic factors depending on the stimulus.18 In a recent study, we were able to confirm that platelets secrete a specific subtype of α granules containing predominantly CXCL4 upon stimulation with C5a.23
Compared with this previous report,23 there are several new aspects with mechanistic and translational significance in this work. We demonstrate that human (vs mouse) platelets express C5aR1 (Figure 1B). Furthermore, C5aR1 was assessed in 2 different patient collectives (coronary artery disease and PAD). From the literature, it is known that the “upstream” complement component C5 (which forms the terminal complement lytic complex with other factors and from which the anaphylatoxin C5a originates after cleavage) has proangiogenic effects itself.53,54 Here, an additional C5 knockout mouse is applied to differentiate the angiogenesis-modulating function of C5 vs C5a (Figure 2). Furthermore, the effects on the soluble coagulation cascade are addressed by experiments using heparin (Figure 1G), and the effect of platelet-specific C5aR1 knockout is assessed in growth factor–driven angiogenesis (vs ischemia-induced revascularization), and the specific effect of the C5aR1-CXCL-4 axis vs CXCL4, which has angiogenesis-modulating effects itself, is addressed in vivo by applying double knockout mice (Figure 6). Finally, the effect of in vitro atherosclerotic conditions on C5aR1 expression is checked (supplemental Figure 5), the role of C5a on previously not investigated α granule cargo is evaluated (supplemental Figure 17), and we excluded a paracrine or an autocrine platelet self-activation loop mediated by C5a (supplemental Figure 22).
Importantly, the recent development of many new therapeutics targeting complement and the approval of inhibitors of complement components for therapy in various diseases renders this field promising for further translational and clinical research31,32,81
Taken together, our data suggest that targeting the complement system is also a promising approach in ischemic diseases featuring platelet activation and the need to revascularize the tissue. Shifting the platelet-mediated response toward the promotion of vessel growth may be achieved with drugs inhibiting the C5a/C5aR1 axis.
Acknowledgments
The authors thank Sarah Gekeler, Birgit Fehrenbacher, and Anke Constantz for excellent technical assistance.
This work was supported by the Volkswagen Foundation (Lichtenberg program) and the DZHK (German Research Centre for Cardiovascular Research), partner site Hamburg/Lübeck/Kiel (STO Projekt F280404; H.F.L. and H.N.). H.N. and E.R. are supported by the Clinician Scientist Program of the DZHK (German Research Centre for Cardiovascular Research), partner site Hamburg/Lübeck/Kiel. H.N. is also supported by the Clinician Scientist Program of the University of Lübeck. H.N. and H.F.L. were supported by an ERA PerMed 2020 JTC grant “PROGRESS.” H.F.L. and D.D. were supported by the Deutsche Forschungsgemeinschaft (DFG), project number 394046768 - SFB1366, and the DZHK partner site Mannheim/Heidelberg.
Authorship
Contribution: H.N. and H.F.L. designed the research study; H.N., L.B., M.S., A.L., J.v.E., Y.S., H.E., and S.P.G. conducted the experiments; H.N., M.S., M.M., T.G., P.v.H., D.D., R. Szepanowski, C.K., and H.F.L. analyzed the data; P.v.H. provided reagents; and H.N., T.G., N.S., P.v.H., N.v.B., K.S., J.M., D.D., C.K., R. Saraei, E.T., R.S., J.K., C.D.S., and H.F.L. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Harald F. Langer, Medical Clinic I, Medical Faculty Mannheim, Heidelberg University, Theodor-Kutzer-Ufer 1.3, 68167 Mannheim, Germany; e-mail: harald.langer@umm.de; and Henry Nording, Cardioimmunology Group, Medical Clinic II, University Heart Center Lübeck, 23538 Lübeck, Germany; e-mail: henry.nording@uksh.de.
References
Author notes
Data are available on request from the corresponding author, Harald F. Langer (harald.langer@umm.de).
The full-text version of this article contains a data supplement.