In this issue of Blood Advances, Mannes et al1 address the mechanism of thrombosis in diseases marked by complement-driven hemolysis, of which there are abundant clinical examples, including paroxysmal nocturnal hemoglobinuria (PNH), atypical hemolytic uremic syndrome (aHUS), cold agglutinin disease and hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome. The authors find that hemolysis caused by membrane attack complex (MAC) formation leads to prothrombotic platelet activation through the release of adenosine diphosphate (ADP).
A complete understanding of the complement-coagulation link remains elusive despite the common evolutionary origins of these serine protease cascades.2 Nothing is more illustrative of their delicate homeostasis than pregnancy, in which upregulation of both is necessary to protect against hemorrhage and infection; however, imbalance can have devastating consequences.3 Thrombosis is a predominant manifestation and cause of morbidity and mortality in complementopathies, systemic disorders in which complement dysregulation drives disease pathogenesis and complement inhibitors mitigate end-organ damage.4 The prototypical complementopathy, PNH presents with complement-mediated intravascular hemolysis because of an acquired deficiency of the cell surface complement regulators, CD55 and CD59. Before the C5 inhibitor, eculizumab, thrombosis was the leading cause of death from PNH despite anticoagulation. Complement inhibitors have significantly reduced the risk of thrombosis and increased life expectancy to that of age-matched controls.5 In fact, our PNH experience suggests that discontinuation of anticoagulation for secondary prevention of thrombosis in patients well-controlled on terminal complement inhibition is safe.6 This underscores the concept that anticoagulation has a limited role in preventing complement-mediated thrombosis; an understanding that is potentially key to treating other deadly disorders of refractory thrombosis and complement amplification, such as catastrophic antiphospholipid syndrome.
In a comprehensive set of ex vivo whole blood experiments, Mannes et al demonstrate that ADP released after MAC–mediated erythrocyte lysis, rather than the direct effect of complement components, is a critical intermediary in prothrombotic platelet activation. First, the authors use a model of complement activation in whole blood triggered by artificial surfaces to show that in the absence of hemolysis, no change in the platelet activation profile is observed, despite generation of fluid-phase complement activation fragments. Next, adding purified complement components to whole blood or platelet-rich plasma, the authors present a corroborating piece of evidence that the proinflammatory anaphylatoxins, C3a and C5a, do not lead to platelet activation in the absence of other agonists (eg, ADP, thrombin receptor activating peptide [TRAP]). This is opposed to the direct effects of anaphylatoxins described on leukocytes; for example, C5a was shown to trigger tissue factor expression on neutrophils in models of antiphospholipid syndrome.7 Furthermore, the authors show quiescent platelets lack C3a and C5a receptors, further arguing against a role for anaphylatoxins in the initiation of platelet activation.
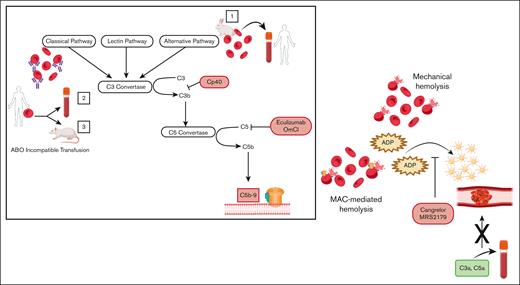
Turning to 2 ex vivo models of hemolysis, the authors use (1) rabbit erythrocytes, which are sensitive to lysis by the alternative pathway of complement, and (2) human erythrocytes, on which complement is activated by ABO incompatibility and measure CD62P (P-selectin) expression as a surrogate for platelet activation. They demonstrate that both hemolysis and platelet activation are abrogated by complement C3 and C5 inhibitors, indicating that blocking either proximal or terminal complement, and, therefore, MAC-mediated lysis, prevents the prothrombotic platelet phenotype. Furthermore, ADP receptor antagonists (Cangrelor and MRS2179) completely abolish platelet activation, suggesting that induction of complement leads to platelet activation through release of intracellular ADP. This effect is not specific to complement-driven hemolysis, as mechanical shearing of erythrocytes produces the same ADP–mediated platelet activation; however, complement inhibitors only block platelet activation when hemolysis is complement-triggered. Finally, in an in vivo rat model of mismatched transfusion (human AB red cells into Wistar rats containing antihuman AB antibodies), they attenuate hemolysis and the thrombotic phenotype, as assessed by platelet count and lung fibrin deposition, using a C5 inhibitor (OmCl) or by depleting complement with cobra venom factor.
Hemolysis caused by either MAC formation or mechanical shearing of erythrocytes leads to prothrombotic platelet activation, through the release of ADP, which is blocked by the ADP-receptor antagonists, Cangrelor and MRS2179. Addition of isolated complement anaphylatoxins (C3a, C5a) to blood does not lead to platelet activation in the absence of hemolysis. Box: activation of (1) the alternative pathway of complement on rabbit erythrocytes or (2,3) the classical pathway on human erythrocytes through ABO incompatible transfusion was used to induce MAC-mediated lysis. ABO incompatible transfusion was stimulated (2) ex vivo by addition of human type AB erythrocytes into type O blood and (3) in vivo through transfusion of human type AB erythrocytes into rats possessing antihuman AB antibodies. Proximal (CP40) and terminal (eculizumab, OmCl) complement inhibitors block MAC-mediated hemolysis and downstream platelet activation.
Hemolysis caused by either MAC formation or mechanical shearing of erythrocytes leads to prothrombotic platelet activation, through the release of ADP, which is blocked by the ADP-receptor antagonists, Cangrelor and MRS2179. Addition of isolated complement anaphylatoxins (C3a, C5a) to blood does not lead to platelet activation in the absence of hemolysis. Box: activation of (1) the alternative pathway of complement on rabbit erythrocytes or (2,3) the classical pathway on human erythrocytes through ABO incompatible transfusion was used to induce MAC-mediated lysis. ABO incompatible transfusion was stimulated (2) ex vivo by addition of human type AB erythrocytes into type O blood and (3) in vivo through transfusion of human type AB erythrocytes into rats possessing antihuman AB antibodies. Proximal (CP40) and terminal (eculizumab, OmCl) complement inhibitors block MAC-mediated hemolysis and downstream platelet activation.
This work offers an exciting approach to linking innate immunity, platelets, and thrombosis and provides a potential explanation as to why complement is a promising target in a host of complement-mediated diseases with prominent thrombotic manifestations. It also suggests that MAC formation, and not upstream complement activation products, is necessary to induce thrombosis. Importantly, this mechanism supports a crucial distinction between cell surface and fluid-phase complement activation. For example, thrombosis is not observed in C3 glomerulopathies, in which C3 nephritic factor increases complement activation in the fluid-phase only. Although these findings unequivocally add a missing link connecting complement and coagulation, they raise additional questions as to whether this tells the whole story. Even in PNH, there are rare patients who have large PNH granulocytes clones but few PNH erythrocytes or predominantly type 2 PNH red cells (partial deficiency of glycosylphosphatidylinositol [GPI]–anchored proteins protective against hemolysis), normal or near-normal hemoglobin, and minimal elevation in lactate dehydrogenase. However, many of these patients have severe thrombotic tendencies that are mitigated by C5 inhibitors.8 Moreover, patients with congenital CD55 deficiency present with protein losing enteropathy and a severe thrombotic phenotype without intravascular hemolysis.9 Additionally, germline mutations in the GPI biosynthesis pathway, which lead to only partial GPI–anchored protein deficiency without hemolysis, produce a clotting phenotype alongside intellectual disability and seizures.10 Although low level hemolysis cannot be excluded in these conditions, the model used by the authors is one of larger scale complement amplification and frank intravascular hemolysis. Furthermore, clotting characteristic of PNH (eg, cerebral venous sinus thrombosis, splanchnic vein thrombosis) presents differently from the typical thrombosis seen in aHUS and autoimmune hemolytic anemias. These cases suggest that additional mechanisms are at play. The authors acknowledge that the endothelium may be an important regulator of thrombosis and this was not a factor outside the rat model, in which it was not specifically investigated. Activated endothelial cells release ADP, so this may further extend the mechanism elucidated by the authors. Although cytolysis of the target cell in each disease state, for example, the renal endothelium in aHUS, and release of danger signals may mediate local thrombosis, this is yet to be elucidated and synergistic in vivo mechanisms, such as free hemoglobin, may also be implicated. This work is an important advance in our understanding of platelet activation mediated by intravascular hemolysis and likely explains a large component of thrombosis in complement-driven diseases; future studies on the contributions of leukocytes, platelets, and endothelial cells will further unravel mechanisms of thrombosis in complement disorders.
Conflict-of-interest disclosure: G.F.G. served on advisory boards for Apellis Pharmaceuticals and Alexion Pharmaceuticals, and contributed to the Merck Manual, for which she received an honorarium. The spouse of G.F.G. is an employee of and holds stock in Pfizer. R.A.B. receives research funding from Alexion.