Key Points
VRd is superior to Rd in veterans with myeloma, and the evidence of benefit is strongest in those with the highest levels of frailty.
More intensive treatment of a patient who is frail with myeloma may be a more effective treatment of frailty itself.
Abstract
Although randomized controlled trial data suggest that the more intensive triplet bortezomib-lenalidomide-dexamethasone (VRd) is superior to the less intensive doublet lenalidomide-dexamethasone (Rd) in patients newly diagnosed with multiple myeloma (MM), guidelines have historically recommended Rd over VRd for patients who are frail and may not tolerate a triplet. We identified 2573 patients (median age, 69.7 years) newly diagnosed with MM who were initiated on VRd (990) or Rd (1583) in the national US Veterans Affairs health care System from 2004 to 2020. We measured frailty using the Veterans Affairs Frailty Index. To reduce imbalance in confounding, we matched patients for MM stage and 1:1 based on a propensity score. Patients who were moderate-severely frail had a higher prevalence of stage III MM and myeloma-related frailty deficits than patients who were not frail. VRd vs Rd was associated with lower mortality (hazard ratio [HR], 0.81; 95% confidence interval [CI], 0.70-0.94) in the overall matched population. Patients who were moderate-severely frail demonstrated the strongest association (HR 0.74; 95% CI, 0.56-0.97), whereas the association weakened in those who were mildly frail (HR, 0.80; 95% CI, 0.61-1.05) and nonfrail (HR, 0.86; 95% CI, 0.67-1.10). VRd vs Rd was associated with a modestly higher incidence of hospitalizations in the overall population, but this association weakened in patients who were moderate-severely frail. Our findings confirm the benefit of VRd over Rd in US veterans and further suggest that this benefit is strongest in patients with the highest levels of frailty, arguing that more intensive treatment of myeloma may be more effective treatment of frailty itself.
Introduction
The optimal intensity of treatment in adults who are frail with multiple myeloma (MM) is often uncertain, given the higher risk of harm in patients who are frail on standard, intensive regimens.1,2 Randomized controlled trials (RCTs) directly or indirectly exclude patients who are frail, limiting the generalizability of results to a large portion of patients treated in practice.3,4 Although analyses from RCTs and observational studies consistently show that frailty is associated with higher risks of treatment toxicity, treatment discontinuation, and mortality,5 this evidence does little to inform the key clinical question, that is, whether to administer to patients who are frail, in practice, the same intensity of treatments evaluated in the younger, fitter patients in RCTs.
An illustrative example is Southwest Oncology Group (SWOG) S0777, the seminal randomized phase 3 trial that demonstrated an overall survival benefit associated with a more intensive triplet (bortezomib-lenalidomide-dexamethasone or VRd) vs a less intensive doublet (lenalidomide-dexamethasone or Rd) regimen in patients newly diagnosed with MM not intended for immediate hematopoietic stem cell transplant.6 These findings solidified the recommendation to favor initiation on the triplet VRd over the doublet Rd in most patients. However, many experts and guidelines have historically recommended Rd over VRd for patients who are frail and may not be able to tolerate a triplet.7,8 To date, there has been no evidence suggesting that patients who are frail treated with VRd experience either lesser benefits and/or greater harms than those treated with Rd. Although there are newer triplet regimens that are preferred in older patients (eg, daratumumab-lenalidomide-dexamethasone9), examining the effects of VRd vs Rd provides an opportunity to examine whether patients who are frail, in practice, fare worse on a more intensive initial regimen.
We designed a retrospective cohort study in US veterans, a population that is even more underrepresented in MM clinical trials than the general population, to evaluate for differences in survival associated with VRd vs Rd in newly diagnosed MM. We hypothesized that, similar to the results of SWOG S0777, we would observe a survival benefit associated with VRd over Rd in the overall population. Furthermore, we hypothesized that, similar to assumptions behind expert recommendations for clinical practice, the survival benefit associated with VRd would diminish in patients with high levels of frailty than in relatively fit patients, whereas the risk of hospitalization would increase (reflecting higher toxicity).
Methods
Study design and population
Emulating SWOG S0777,6 we designed a retrospective cohort study using a new-user design that uses data from the Veterans Affairs (VA) Corporate Data Warehouse (CDW), which collects clinical, billing, and electronic health record (EHR) information from veterans treated in VA facilities throughout the United States.10 We also used data from the national VA Cancer Registry, which collects information on cancer diagnosis and treatment compiled and submitted by local cancer registry staff at each of the 132 VA Medical Centers that diagnose and treat veterans with cancer.11,12 Using a selection algorithm validated against clinician chart review,13 we included all veterans newly diagnosed with MM who initiated treatment in VA Medical centers with either VRd or Rd from years 2004 (date of the first instance of using Rd) to 2020 (see supplemental Methods regarding our selection algorithm). We excluded veterans who received transplant within the first four months of treatment initiation. Although we could not measure a clinician’s intention to transplant at the time of initiation of VRd or Rd, as was measured in SWOG S0777,6 we measured the receipt of transplant at any point during follow-up. This study was approved by the VA Boston Health Care System Institutional Review Board. The study was conducted in accordance with the Declaration of Helsinki.
Frailty, MM stage, and covariates
We measured frailty using the Veterans Affairs Frailty Index (VA-FI), an electronic frailty index developed by geriatricians that carries both predictive and construct validity, including when validated against clinical measures of frailty assessed by geriatricians and the International Myeloma Working Group Frailty Score (supplemental Table 2).14-16 In brief, the VA-FI measures frailty based on the deficit-accumulation approach, which is one of the most widely-studied models of frailty.17 A total of 31 aging-related health deficits were measured from the EHR data and administrative claims, using diagnostic and procedural codes spanning the domains of morbidity (14 deficits), function (8), cognition (3), sensory (3), and other (3). These health deficits were measured within a 3-year assessment period leading up to the date of treatment initiation. A score was calculated, for each patient, as the proportion of possible health deficits that are present in an individual, ranging from 0 to 1, in which higher values indicate more severe frailty.18 Based on validated cut-points,14,15,19-22 veterans were classified as nonfrail (VA-FI < 0.2), mildly frail (VA-FI = 0.2-0.3), or moderate-severely frail (VA-FI ≥ 0.3).
We measured MM stage from both laboratory and EHR data within the CDW (supplemental Methods). We further measured pretreatment covariates spanning sociodemographic variables (age, sex, race, and rurality of the home address of the veteran23), MM risk24 (hemoglobin, platelets, calcium, and creatinine, measured within 90 days before initial treatment and imputing missing values as a function of all other covariates using multivariable regression), comorbidity burden (Charlson Comorbidity Index25), polypharmacy (having ≥ 5 chronic medications), and health care use (number of unplanned hospitalizations, emergency department visits, and skilled nursing facility stays in the year before initial treatment).
Outcomes
Our primary outcome was overall survival, measured using vital status information in the VA CDW (which provides 98.3% sensitivity and 97.6% exact agreement against the US National Death Index).26 Our secondary outcome was unplanned hospitalizations at the VA Health care System with a duration of stay ≥1 day.27 For mortality, veterans were followed up through 5 years (mimicking the SWOG S0777 median follow-up time) until death or the end of the study period, 31 December 2021, after which their data were censored. For hospitalizations, veterans were followed up for 1 year until death, the end of study, or until their last inpatient/outpatient record in the CDW, after which their data were censored.
Analysis
We compared baseline characteristics between VRd and Rd treatment groups. To reduce confounding within frailty categories, we matched patients based on the MM stage and estimated a propensity score (PS) using regularized logistic regression that modeled the probability of being initially treated with VRd vs Rd as a function of all covariates. We did not find differences in survival over increasing calendar years of treatment initiation (supplemental Figure 1), so we did not include initial treatment year as a covariate in the PS model. We performed a 1:1 nearest-neighbor matching with a caliper of 1% of the PS.
For our primary analysis, we evaluated the association between VRd vs Rd and overall survival within each matched frailty category and in the combined overall population, first by using Kaplan-Meier analyses to evaluate differences in survival between VRd and Rd groups and next by using Cox proportional hazards regression models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). We assessed for effect heterogeneity across frailty categories.28 For our secondary analysis, we evaluated the association between VRd vs Rd and the rates of unplanned hospitalizations. We conducted sensitivity analyses in multivariable Cox regression models, adjusting for any covariates that showed evidence of remaining imbalance (standardized mean difference [SMD] > 0.1) across those treated with VRd vs Rd after matching.29,30 We also repeated analyses with landmarking follow-up to begin on day 15 after the date of the first treatment with either VRd or Rd, accounting for potential immortal time bias.31 All analyses were consistent with an intent-to-treat approach, analyzing veterans according to their initial treatment assignment. Analyses were conducted in R 4.1.2 and Stata 17.0. We followed the STROBE guidelines in the design and reporting of observational studies.32
Results
Characteristics of the study population
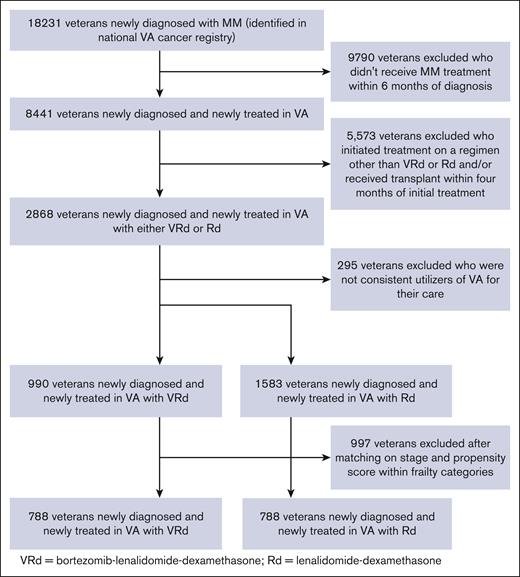
We identified 2573 patients with newly diagnosed MM (median age, 69.7 years; interquartile range, 74.2-75.8) who were initiated either on VRd (990) or Rd (1583) from 2004 to 2020 (Figure 1). After matching those within each frailty category based on the stage and PS, all covariates were adequately balanced between the VRd and Rd groups in the combined overall population and within each frailty category, except for minimal residual imbalance in prior emergency department use in patients who were nonfrail (SMD = 0.103) and prior skilled nursing facility use in patients who were moderate-severely frail (SMD == 0.111; Table 1; supplemental Tables 4-6). Compared with patients who were nonfrail, patients who were moderate-severely frail were older (median age, 67.6 years vs 71.1 years) and had a higher prevalence of stage III MM (19.5% vs 32.9%) and myeloma-related health deficits, such as anemia (47.2% vs 85%), kidney disease (11.2% vs 46.8%), and bone disease or pathologic skeletal fractures (4.6% vs 18.5%). A total of 170 patients (21.6%) in the VRd group and 134 patients (17%) in the Rd group underwent a transplant at some point in follow-up. In patients who were moderate-severely frail, 15 (8.7%) in the VRd group and 14 (8.1%) in the Rd group underwent transplants.
Characteristics of veterans with newly diagnosed MM initially treated with either VRd or Rd before and after matching based on stage and PS within each frailty category
| . | Rd before matching (N = 1583) . | VRd before matching (N = 990) . | SMD before matching . | Rd after matching (N = 788) . | VRd after matching (N = 788) . | SMD after matching . |
|---|---|---|---|---|---|---|
| Age, median (IQR), y | 70.6 (65.1-78.1) | 68.7 (63.2-72.6) | 0.399 | 68.3 (62.9-74.3) | 69.7 (64.4-73.5) | 0.041 |
| Race/ethnicity, n (%) | 0.27 | 0.034 | ||||
| Non-Hispanic White | 1035 (65.4) | 526 (53.1) | 469 (59.5) | 467 (59.3) | ||
| Black | 362 (22.9) | 334 (33.7) | 215 (27.3) | 223 (28.3) | ||
| Hispanic | 70 (4.4) | 59 (6.0) | 42 (5.3) | 42 (5.3) | ||
| Other | 116 (7.3) | 71 (7.2) | 62 (7.9) | 56 (7.1) | ||
| Frailty, n (%) | 0.043 | < 0.001 | ||||
| Nonfrail (VA-FI < 0.2) | 760 (48.0) | 463 (46.8) | 389 (49.4) | 389 (49.4) | ||
| Mild frailty (VA-FI = 0.2-0.3) | 439 (27.7) | 294 (29.7) | 226 (28.7) | 226 (28.7) | ||
| Moderate-severe frailty (VA- FI ≥ 0.3) | 384 (24.3) | 233 (23.5) | 173 (22.0) | 173 (22.0) | ||
| Charlson Comorbidity Index, median (IQR) | 1.00 (0.00-2.00) | 1.00 (0.00-2.00) | 0.063 | 1.00 (0.00-2.00) | 1.00 (0.00-2.00) | 0.010 |
| Myeloma stage, n (%) | 0.361 | < 0.001 | ||||
| Stage I | 349 (22.0) | 91 (9.2) | 76 (9.6) | 76 (9.6) | ||
| Stage II | 350 (22.1) | 241 (24.3) | 200 (25.4) | 200 (25.4) | ||
| Stage III | 527 (33.3) | 392 (39.6) | 318 (40.4) | 318 (40.4) | ||
| Missing | 357 (22.6) | 266 (26.9) | 194 (24.6) | 194 (24.6) | ||
| Pretreatment laboratory levels, median (IQR) | ||||||
| Creatinine (mg/dL) | 1.20 (0.97-1.50) | 1.18 (0.96-1.51) | 0.013 | 1.15 (0.94-1.46) | 1.15 (0.94-1.50) | 0.026 |
| Calcium (mg/dL) | 9.10 (8.70-9.60) | 9.20 (8.80-9.70) | 0.168 | 9.20 (8.80-9.66) | 9.20 (8.80-9.70) | 0.002 |
| Hemoglobin (g/dL) | 11.10 (9.70-12.50) | 10.90 (9.30-12.47) | 0.066 | 11.01 (9.60-12.62) | 11.10 (9.50-12.60) | 0.010 |
| Platelets (n per mL) | 205.00 (158.50-250.00) | 202.00 (160.00-252.75) | 0.014 | 203.00 (158.00-250.00) | 204.42 (162.00-252.00) | 0.029 |
| Rurality of veteran, n (%) | 0.099 | 0.030 | ||||
| Rural | 561 (35.4) | 306 (30.9) | 263 (33.4) | 261 (33.1) | ||
| Urban | 1012 (63.9) | 679 (68.6) | 518 (65.7) | 522 (66.2) | ||
| Unknown | 10 (0.6) | 5 (0.5) | 7 (0.9) | 5 (0.6) | ||
| Polypharmacy (≥5 medications), n (%) | 522 (33.0) | 333 (33.6) | 0.014 | 265 (33.6) | 264 (33.5) | 0.003 |
| Healthcare utilization in the year before treatment, median (IQR) | ||||||
| Any hospital utilization | 0.00 (0.00-1.00) | 0.00 (0.00-1.00) | 0.017 | 0.00 (0.00-1.00) | 0.00 (0.00-1.00) | 0.040 |
| Any ED utilization | 0.00 (0.00-1.00) | 1.00 (0.00-2.00) | 0.309 | 0.00 (0.00-2.00) | 1.00 (0.00-2.00) | 0.060 |
| Any NH utilization | 0.00 (0.00-0.00) | 0.00 (0.00-0.00) | 0.096 | 0.00 (0.00-0.00) | 0.00 (0.00-0.00) | 0.019 |
| . | Rd before matching (N = 1583) . | VRd before matching (N = 990) . | SMD before matching . | Rd after matching (N = 788) . | VRd after matching (N = 788) . | SMD after matching . |
|---|---|---|---|---|---|---|
| Age, median (IQR), y | 70.6 (65.1-78.1) | 68.7 (63.2-72.6) | 0.399 | 68.3 (62.9-74.3) | 69.7 (64.4-73.5) | 0.041 |
| Race/ethnicity, n (%) | 0.27 | 0.034 | ||||
| Non-Hispanic White | 1035 (65.4) | 526 (53.1) | 469 (59.5) | 467 (59.3) | ||
| Black | 362 (22.9) | 334 (33.7) | 215 (27.3) | 223 (28.3) | ||
| Hispanic | 70 (4.4) | 59 (6.0) | 42 (5.3) | 42 (5.3) | ||
| Other | 116 (7.3) | 71 (7.2) | 62 (7.9) | 56 (7.1) | ||
| Frailty, n (%) | 0.043 | < 0.001 | ||||
| Nonfrail (VA-FI < 0.2) | 760 (48.0) | 463 (46.8) | 389 (49.4) | 389 (49.4) | ||
| Mild frailty (VA-FI = 0.2-0.3) | 439 (27.7) | 294 (29.7) | 226 (28.7) | 226 (28.7) | ||
| Moderate-severe frailty (VA- FI ≥ 0.3) | 384 (24.3) | 233 (23.5) | 173 (22.0) | 173 (22.0) | ||
| Charlson Comorbidity Index, median (IQR) | 1.00 (0.00-2.00) | 1.00 (0.00-2.00) | 0.063 | 1.00 (0.00-2.00) | 1.00 (0.00-2.00) | 0.010 |
| Myeloma stage, n (%) | 0.361 | < 0.001 | ||||
| Stage I | 349 (22.0) | 91 (9.2) | 76 (9.6) | 76 (9.6) | ||
| Stage II | 350 (22.1) | 241 (24.3) | 200 (25.4) | 200 (25.4) | ||
| Stage III | 527 (33.3) | 392 (39.6) | 318 (40.4) | 318 (40.4) | ||
| Missing | 357 (22.6) | 266 (26.9) | 194 (24.6) | 194 (24.6) | ||
| Pretreatment laboratory levels, median (IQR) | ||||||
| Creatinine (mg/dL) | 1.20 (0.97-1.50) | 1.18 (0.96-1.51) | 0.013 | 1.15 (0.94-1.46) | 1.15 (0.94-1.50) | 0.026 |
| Calcium (mg/dL) | 9.10 (8.70-9.60) | 9.20 (8.80-9.70) | 0.168 | 9.20 (8.80-9.66) | 9.20 (8.80-9.70) | 0.002 |
| Hemoglobin (g/dL) | 11.10 (9.70-12.50) | 10.90 (9.30-12.47) | 0.066 | 11.01 (9.60-12.62) | 11.10 (9.50-12.60) | 0.010 |
| Platelets (n per mL) | 205.00 (158.50-250.00) | 202.00 (160.00-252.75) | 0.014 | 203.00 (158.00-250.00) | 204.42 (162.00-252.00) | 0.029 |
| Rurality of veteran, n (%) | 0.099 | 0.030 | ||||
| Rural | 561 (35.4) | 306 (30.9) | 263 (33.4) | 261 (33.1) | ||
| Urban | 1012 (63.9) | 679 (68.6) | 518 (65.7) | 522 (66.2) | ||
| Unknown | 10 (0.6) | 5 (0.5) | 7 (0.9) | 5 (0.6) | ||
| Polypharmacy (≥5 medications), n (%) | 522 (33.0) | 333 (33.6) | 0.014 | 265 (33.6) | 264 (33.5) | 0.003 |
| Healthcare utilization in the year before treatment, median (IQR) | ||||||
| Any hospital utilization | 0.00 (0.00-1.00) | 0.00 (0.00-1.00) | 0.017 | 0.00 (0.00-1.00) | 0.00 (0.00-1.00) | 0.040 |
| Any ED utilization | 0.00 (0.00-1.00) | 1.00 (0.00-2.00) | 0.309 | 0.00 (0.00-2.00) | 1.00 (0.00-2.00) | 0.060 |
| Any NH utilization | 0.00 (0.00-0.00) | 0.00 (0.00-0.00) | 0.096 | 0.00 (0.00-0.00) | 0.00 (0.00-0.00) | 0.019 |
ED, emergency department; IQR, interquartile range; NH, nursing home utilization (including postacute care/rehabilitation).
VRd vs Rd and survival: overall and based on frailty severity
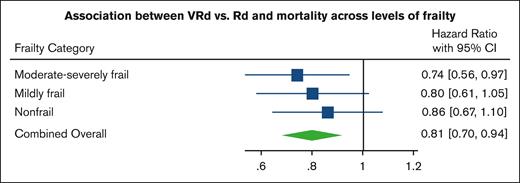
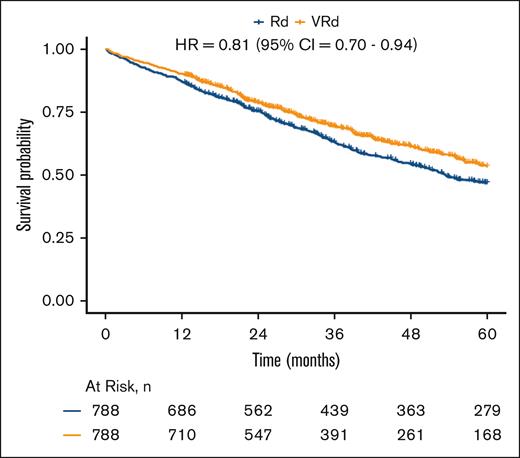
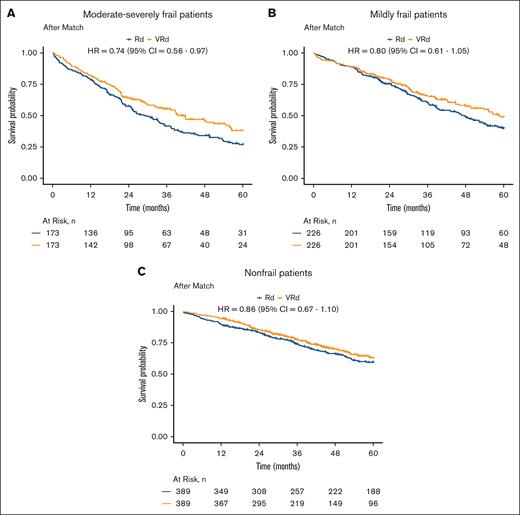
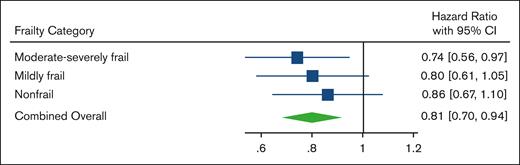
A total of 1213 patients died during the study period, with a median follow-up time of 3.1 years. In the combined overall population, after matching within each frailty category based on the stage and PS, VRd was associated with improved survival vs Rd (log-rank P = .007; Figure 2) and lower mortality (HR, 0.81; 95% CI, 0.70-0.94;). When viewing each matched frailty category separately, patients who were moderate-severely frail demonstrated the strongest association between VRd vs Rd and lower mortality (HR, 0.74; 95% CI, 0.56-0.97; Figures 3A and 4), whereas the association was weaker in those who were mildly frail (HR, 0.80; 95% CI, 0.61-1.05; Figures 3B and 4) and nonfrail (HR, 0.86; 95% CI, 0.67-1.10; Figures 3C and 4). There was no evidence of the effect of heterogeneity across frailty categories (P = .73). In our exploratory analysis stratifying based on both frailty and stage, the largest magnitude of benefit associated with VRd vs Rd was seen in patients who were moderate-severely frail with stage III disease (HR, 0.57; 95% CI, 0.36-0.90; supplemental Figure 2).
Survival of VRd vs. Rd in combined overall population. Kaplan-Meier analyses of patients with newly diagnosed MM, evaluating differences in survival between those initially treated with VRd (orange) and those initially treated with Rd (blue), after matching based on stage and PS within each frailty category and combining these for the overall population.
Survival of VRd vs. Rd in combined overall population. Kaplan-Meier analyses of patients with newly diagnosed MM, evaluating differences in survival between those initially treated with VRd (orange) and those initially treated with Rd (blue), after matching based on stage and PS within each frailty category and combining these for the overall population.
Survival of VRd vs. Rd within each frailty category. Kaplan-Meier analyses of patients who were (A) moderate-severely frail, (B) mildly frail, and (C) nonfrail with newly diagnosed MM, evaluating differences in survival between those initially treated with VRd (orange) and those initially treated with Rd (blue), after matching for stage and PS within each frailty category.
Survival of VRd vs. Rd within each frailty category. Kaplan-Meier analyses of patients who were (A) moderate-severely frail, (B) mildly frail, and (C) nonfrail with newly diagnosed MM, evaluating differences in survival between those initially treated with VRd (orange) and those initially treated with Rd (blue), after matching for stage and PS within each frailty category.
Mortality of VRd vs. Rd within each frailty category and in combined overall population. Forest plot of HRs and 95% CIs from Cox proportional hazards models evaluating association of VRd vs Rd with mortality, based on frailty severity and in the combined overall population after matching or stage and PS within frailty categories.
Mortality of VRd vs. Rd within each frailty category and in combined overall population. Forest plot of HRs and 95% CIs from Cox proportional hazards models evaluating association of VRd vs Rd with mortality, based on frailty severity and in the combined overall population after matching or stage and PS within frailty categories.
VRd vs Rd and hospitalizations: overall and based on frailty severity
There were 2440 unplanned VA hospitalizations in the year after treatment initiation. In the combined overall population after matching within each frailty category based on stage and PS, veterans initiated on VRd had a modestly higher incidence of hospitalizations than those initiated on Rd (VRd, 1.44 hospitalizations per 5 person-years [95% CI, 1.34-1.54]; Rd, 1.18 hospitalizations per 5 person-years [95% CI, 1.10-1.27]); incidence rate ratio [IRR], 1.22; 95% CI, 1.03-1.34; Table 2). When viewing each frailty category separately, there was evidence that the association between VRd vs Rd and increased incidence of hospitalizations was present in patients who were nonfrail (IRR, 1.24; 95% CI, 1.06-1.46) and mildly frail (IRR, 1.27; 95% CI, 1.06-1.54), but this association weakened in patients who were moderate-severely frail (IRR, 1.14; 95% CI, 0.96-1.36).
Incidence rate of unplanned hospitalizations in patients newly diagnosed with MM treated with VRd vs Rd, based on frailty severity and in combined overall population after matching for stage and PS within frailty categories
| Population . | Incidence rate per 5 person-years (95% CI) . | Incidence rate ratio (95% CI) . | |
|---|---|---|---|
| VRd . | Rd . | ||
| Moderate-severe frailty (VA-FI ≥ 0.3) | 1.36 (1.21-1.54) | 1.19 (1.05-1.35) | 1.14 (0.96-1.36) |
| Mild frailty (VA-FI = 0.2-0.3) | 1.44 (1.26-1.65) | 1.13 (0.99-1.29) | 1.27 (1.06-1.54) |
| Nonfrail (VA-FI < 0.2) | 1.51 (1.34-1.69) | 1.21 (1.08-1.35) | 1.24 (1.06-1.46) |
| Overall | 1.44 (1.34-1.54) | 1.18 (1.1-1.27) | 1.22 (1.10-1.34) |
| Population . | Incidence rate per 5 person-years (95% CI) . | Incidence rate ratio (95% CI) . | |
|---|---|---|---|
| VRd . | Rd . | ||
| Moderate-severe frailty (VA-FI ≥ 0.3) | 1.36 (1.21-1.54) | 1.19 (1.05-1.35) | 1.14 (0.96-1.36) |
| Mild frailty (VA-FI = 0.2-0.3) | 1.44 (1.26-1.65) | 1.13 (0.99-1.29) | 1.27 (1.06-1.54) |
| Nonfrail (VA-FI < 0.2) | 1.51 (1.34-1.69) | 1.21 (1.08-1.35) | 1.24 (1.06-1.46) |
| Overall | 1.44 (1.34-1.54) | 1.18 (1.1-1.27) | 1.22 (1.10-1.34) |
Sensitivity analyses
Results were similar in our sensitivity analyses, which showed that VRd vs Rd was associated with a reduction in mortality in the combined overall population and that patients who were moderate-severely frail demonstrated the strongest evidence of mortality reduction (supplemental Table 7).
Discussion
Similar to the results of the SWOG S0777 trial, we found in US veterans newly diagnosed and treated for MM a mortality benefit associated with the more intensive triplet VRd over the less intensive doublet Rd. However, the evidence of benefit was strongest in veterans with the highest levels of frailty, contrary to our hypothesis that the benefit would diminish. Moreover, although there was a modestly higher incidence of hospitalizations associated with VRd vs Rd, this association was diminished, rather than strengthened, among veterans with the highest levels of frailty. Our findings suggest that, on average, veterans with the highest levels of frailty derive an even greater net benefit from VRd over Rd than fit veterans, challenging historical expert opinion-based recommendations to consider Rd over VRd in patients who are frail.7,8
To date, to our knowledge, our study is one of the largest designed to evaluate benefits and harms of more intensive vs less intensive therapy in patients with high levels of frailty newly diagnosed with and treated for MM. Within the SWOG S0777 trial itself, the authors found that the overall mortality benefit conferred by VRd was maintained in subgroup analyses stratified based on the intent to undergo transplant and actual receipt of transplant.6,33 In a post hoc analysis recently presented as a conference abstract, the magnitude of mortality benefit was weaker in patients aged ≥65 years (n = 269) vs patients aged <65 years (n = 202).34 However, frailty was not measured in SWOG S0777, and only 14% of trial participants had an Eastern Cooperative Oncology Group performance status > 1, making it difficult to compare results across the fitter trial population and our frailer veteran population. Investigators have used a simplified frailty score in post hoc analyses of other RCTs to find that the benefit of more intensive MM regimens is generally preserved in frail subgroups.35-37 Similar to most RCTs, however, exclusion criteria of these trials restricted the enrollment of patients with poorer performance status and multimorbidity, limiting both the number of patients who are frail available for subgroup analyses and the generalizability of results (ie, patients who are frail as defined in the trial may not correspond to those treated in practice).35,36,38 Differences in frailty measures and the categorization of frailty severity may also hinder comparisons of outcomes between different trials as well as between trials and real-world studies.39
Although observational studies other than ours have investigated outcomes of VRd and Rd in real-world clinical populations with newly diagnosed MM, many in descriptive analyses do not allow for valid head-to-head comparisons.40-43 A conference abstract reporting on a cohort study comparing VRd with Rd suggested that VRd showed survival benefit in patients age <75 years but not in patients age ≥75 years; however, these analyses were not adjusted for any confounders, with substantial missingness in covariates.44 Better-powered multivariable analyses of large clinical and administrative databases have compared triplet and doublet regimens, but they either lacked data on survival45 or did not control for important confounders such as MM stage and/or frailty.46,47 Our analysis leveraged several features that reduce bias in observational studies examining treatments. Emulating a specific target trial in SWOG S0777, we used a new user, active comparator design with validated, highly specific selection algorithms to reduce misclassification, selection, and immortal time biases.48,49
We also leveraged both the structured and unstructured data within VA to control for a wide range of pretreatment confounders. Multiple pretreatment factors in our cohort could have driven the individual decisions to initiate treatment with either VRd or Rd, including MM aggressiveness, concerns regarding triplet regimens used in patients who were frail or regarding bortezomib-specific side effects in patients with multiple comorbidities, and logistical challenges/patient preferences concerning travel to oncology clinics to receive bortezomib injections (vs oral-only Rd). We controlled for frailty by stratifying based on the levels of severity and balanced these within each level across VRd vs Rd treatment groups and for many other pretreatment covariates, including MM stage, MM-specific laboratory variables (eg, creatinine), rurality, and comorbidity burden. Moreover, evolving treatment practices and greater awareness of trial data (with the main analysis of SWOG S0777 published in 2017) could have influenced treatment decisions over the time period of our study, but we did not find any association between the calendar year of treatment initiation and survival. Although residual unmeasured confounding could still remain, as with any observational study, the control for measured confounding and the other study features noted above mitigate the common threats to internal validity of observational analyses while maintaining good external validity in our frail veteran population.
Our findings call for viewing frailty not merely as a measure of treatment tolerability but more broadly as a summary measure of a patient’s health status that reflects both cancer and noncancer contributors. The electronic VA-FI we used to measure frailty is based on one of the most widely-accepted constructs of frailty in deficit accumulation,50 which carries an advantage over other frailty constructs used in MM51 in its ability to evaluate the individual deficits contributing to the overall frailty score. When looking at the frailty deficits in our veteran population, myeloma-related deficits and aggressive (stage III) MM were more prevalent in patients who were moderate-severely frail than in those who were nonfrail. Moreover, these myeloma-related deficits were more prevalent in patients who were frail in this study than in those identified through the VA-FI in a more general veteran population.15,52 MM and its complications likely drive this higher prevalence of frailty, and as frailty increases, MM and its complications contribute disproportionately more to frailty than other, nononcologic frailty deficits. Therefore, more intensive (eg, VRd) vs less intensive (eg, Rd) treatment for a veteran who is frail and having MM may actually deliver more effective treatment of frailty itself. Further supporting this interpretation is our exploratory analysis that found that the frailest patients with the most aggressive disease (stage III MM) derived the greatest mortality benefit associated with VRd compared with all other frailty/stage subgroups.
However, the added benefit of a more intensive regimen must outweigh its added risk of toxicity, which increases with frailty.2,53,54 Indeed, the recommendation to consider Rd in patients who are frail stems from the concern that the added toxicity of triplets in such patients outweighs their treatment benefit. We did observe a slightly higher incidence of unplanned hospitalizations associated with VRd thanwith Rd, consistent with the higher rate of adverse events associated with VRd in SWOG S0777,6 but this association weakened in patients who were moderate-severely frail. If it is true that MM and its complications are underlying a large portion of the frailty of a veteran, then more effective MM treatment may lower the risk of myeloma-associated hospitalizations to balance out the higher risk of treatment toxicity–associated hospitalizations. Other harms must be considered in association with VRd vs Rd, including more trips to the clinic to receive bortezomib injections that could be burdensome to patients who are frail and considered as not worth the survival benefit. Moreover, patients who are frail may not accept a survival benefit if treatment with VRd leads to severe peripheral neuropathy, especially if the neuropathy is disabling. Future prospective studies should measure peripheral neuropathy and other frailty deficits using validated and responsive measures to determine what aspects of frailty may be changing in response to VRd. Nonetheless, our findings argue that for patients with high levels of frailty who prioritize prolongation of survival, the more intensive triplet VRd is on average superior to the doublet Rd and should be recommended over the doublet, not vice versa.
There are limitations to our study. First, we did not measure the dose of lenalidomide and bortezomib used at treatment initiation, nor did we measure postprotocol regimens used after VRd or Rd. Our goal in this study was to compare the triplet vs the doublet regimens to address the question of whether patients who are frail fare less well on 3 drugs rather than 2, and our intention-to-treat analysis mirrored SWOG S0777 in primarily evaluating the effect of the initial assignment to VRd or Rd. Second, unmeasured confounding, for example, with respect to cytogenetics, could be present in our study. However, we did measure disease aggressiveness via the main MM staging systems and achieved a balance across VRd vs Rd with respect to stage and other measures of MM risk. Moreover, although we modeled our population after SWOG S0777, because patients initiating therapy were not eligible for immediate stem cell transplantation, we could not measure and, thus, balance at treatment initiation the intention of an oncologist to prescribe a transplant at some point in the future. Although, in the overall combined population, a modestly higher percentage (21.6%) of patients in the VRd group underwent transplantation at some point in our follow-up period compared with the Rd group (17%), this could have been in part due to subsequent improvement in health and disease status with more effective initial therapy rather than differences in baseline confounding. Of note, transplantation rates during the follow-up period were nearly equal across VRd and Rd in patients who were moderate-severely frail. Lastly, the competing risk of mortality could have affected our measurement of the incidence rates of unplanned hospitalizations.
In conclusion, our findings not only confirm the net benefit of VRd over Rd in US veterans newly diagnosed with MM but also suggest that this benefit is strongest in patients with the highest levels of frailty, countering historical recommendations to consider doublets in this population. Although there are newer triplet regimens that are preferred in older patients, our findings argue that frailty is not merely a measure of treatment tolerability and a patient who is frail with MM should be considered treatable cause of their frailty, wherein more intensive treatment may be more effective. Rigorous observational studies of real-world practice should be used to determine how the benefits and harms of novel regimens tested in RCTs translate to frail, underrepresented populations in practice.
Acknowledgments
This work was supported by the VA Office of Research and Development, Cooperative Studies Program (N.R.F, N.V.D., and M.B.); HCSRN-OAICs AGING Initiative Pilot (NIAR33-AG057806) (C.D. and N.R.F.); VA Career Development Award IK2CX002218 (C.D.); VA Merit Review Award 1I01BX001584 (N.C.M.); National Institutes of Health grants P01-155258-07 and P50-100707 (N.C.M.); American Heart Association 857078 (N.R.F.); and NIA K24-AG073527 (D.H.K.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The views expressed are those of the authors and do not represent the views of VA or the US Government.
Authorship
Contribution: C.D., J.L., D.H.K., N.C.M., N.R.F., and J.A.D. conceptualized and designed the study; J.L., J.B., J.C., and N.R.F. performed data curation and quality control; C.D., J.L., J.B., J.C., and N.R.F. performed data analysis; and all authors contributed to the interpretation of results and manuscript writing.
Conflict-of-interest disclosure: N.C.M. reported receiving personal fees from Bristol Myers Squibb, Janssen, Amgen, Takeda, OncoPep, AbbVie, Karyopharm, Novartis, Legend, Raqia, Adaptive Biotechnolology, and Pfizer outside submitted work; and IP licensed to OncoPep and held stocks in C4 Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Clark DuMontier, Division of Aging Dana-Farber Cancer Institute, Division of Population Sciences Assistant Professor of Medicine, Geriatrician and Clinical Investigator VA Boston Healthcare System, New England GRECC Brigham and Women’s Hospital, Harvard Medical School, 150 S Huntington Ave, Boston, MA 02130; e-mail: cdumontier@bwh.harvard.edu.
References
Author notes
∗C.D. and J.L. are joint first authors.
†J.A.D. and N.R.F. are joint senior authors.
The derived data generated in this research are available, as permitted by VA policy, on reasonable request from the corresponding author, Clark DuMontier (cdumontier@bwh.harvard.edu).
The full-text version of this article contains a data supplement.