Key Points
Treating severe malnutrition in older children with SCA was feasible with >94% enrollment, retention, and adherence.
Moderate-dose hydroxyurea therapy for severely malnourished children with SCA was not associated with myelosuppression over 3 months.
Abstract
Children with sickle cell anemia (SCA) living in Nigeria are at an increased risk of malnutrition, which contributes to increased morbidity and mortality. However, evidence-based guidelines for managing malnutrition in children with SCA are lacking. To address this gap, we conducted a multicenter, randomized controlled feasibility trial to assess the feasibility and safety of treating children with SCA aged from 5 to 12 years and having uncomplicated severe acute malnutrition (body mass index z score of <−3.0). Children with SCA and uncomplicated severe acute malnutrition were randomly allocated to receive supplemental ready-to-use therapeutic food (RUTF) with or without moderate-dose hydroxyurea therapy (20 mg/kg per day). Over a 6-month enrollment period, 3190 children aged from 5 to 12 years with SCA were evaluated for eligibility, and 110 of 111 children who were eligible were enrolled. During the 12-week trial, no participants withdrew or missed visits. One participant died of unrelated causes. Adherence was high for hydroxyurea (94%, based on pill counts) and RUTF (100%, based on the number of empty sachets returned). No refeeding syndrome event or hydroxyurea-related myelosuppression occurred. At the end of the trial, the mean change in body mass index z score was 0.49 (standard deviation = 0.53), and 39% of participants improved their body mass index z score to ≥−3.0. Our findings demonstrate the feasibility, safety, and potential of outpatient treatment for uncomplicated severe acute malnutrition in children with SCA aged from 5 to 12 years in a low-resource setting. However, RUTF sharing with household and community members potentially confounded the response to malnutrition treatment. This trial was registered at clinicaltrials.gov as #NCT03634488
Introduction
Sickle cell anemia (SCA) is one of the most common genetic disorders worldwide, with newborns in Nigeria accounting for 50% of all affected newborns, amounting to 150 000 cases annually.1,2 Children with SCA in sub-Saharan Africa face higher mortality rates compared with children in high-resource settings.3-5 Malnutrition in children with SCA further compounds this problem and increases the risk of hospitalization and death.6,7 Malnutrition is prevalent and likely one of the most common causes of death in children with SCA living in Africa.7-12 In Nigeria, only 20% of all children aged <5 years with severe acute malnutrition (SAM) receive treatment.13 Moreover, there are no evidence-based guidelines for treating malnutrition in children aged ≥5 years or children of any age with SCA,14,15 although the prevalence of growth faltering increases with age in children with SCA.6,16,17 The lack of evidence-based guidelines for managing SAM in children with SCA in Nigeria, and other sub-Saharan African regions where both malnutrition and SCA are prevalent,1,18 is a major public health challenge.
Furthermore, there is biological plausibility for improving nutritional status to decrease the morbidity and mortality due to SCA. Several preclinical studies in mice with sickle cell disease (SCD) have provided strong evidence of nutritional interventions to improve the health of the offspring, increase bone health, and decrease inflammatory vasculopathy.19-22 Furthermore, in a phase 3 randomized controlled trial, L-glutamine, an amino acid, compared with placebo, reduced the incidence of painful vaso-occlusive events in children and adults with SCA.23 These data demonstrate the potential importance of identifying and treating SAM in children with SCA.
Hydroxyurea therapy is a potentially unique addition to treating malnutrition in children with SCA. Hydroxyurea has multiple benefits for children with SCA, such as reducing morbidity from vaso-occlusive episodes, acute chest syndrome, and strokes while also improving hemoglobin (Hb) levels.24,25 Lower Hb levels are associated with decreased weight-for-age, height-for-age, and body mass index (BMI) z scores.26-28 Although some studies suggest that hydroxyurea has no adverse effects on growth and may even have a positive impact,26,29-32 there is currently no randomized controlled trial testing whether hydroxyurea can directly improve growth as a primary hypothesis.25
The absence of evidence-based malnutrition guidelines for children with SCA is a public health challenge in sub-Saharan Africa, where poverty, the high number of children with SCA, and the prevalence of SAM are pressing issues.1,18 To address this critical knowledge gap, we conducted the first National Institutes of Health (NIH)-funded trial to treat SAM (BMI z score of <−3.0) in children aged from 5 to 12 years with SCA in Nigeria (SAMS trial; NCT03634488).
Methods
Study design
The SAMS trial was a multicenter, open-label, feasibility randomized controlled trial (NCT03634488) conducted in Kano, Nigeria. The study protocol is presented in supplement 1. Participants who were eligible were assigned in a 1:1 ratio based on a permuted block allocation scheme, block sizes of 2 and 4, stratified based on the sex, within the clinical site, to receipt of ready-to-use therapeutic food (RUTF) alone or with hydroxyurea at a fixed dose (20 mg/kg per day).
Ethical review of the study
Ethical approval was obtained from the institutional review boards of Aminu Kano Teaching Hospital, Murtala Mohammed Specialist Hospital, and the Vanderbilt University Medical Center. The National Agency for Food and Drug Administration and Control in Nigeria provided regulatory approval. The Data and Safety Monitoring Board (DSMB) reviewed the study progress and adverse events (AEs). Written informed consent and assent for children aged >7 years were obtained before screening and enrollment. Children whose caregivers declined enrollment in the study received standard care. The SAMS trial was conducted in accordance with the principles of the Declaration of Helsinki.
Study participants
Children receiving care at the 2 study sites, Aminu Kano Teaching Hospital and Murtala Mohammed Specialist Hospital, were evaluated for study eligibility. The inclusion criteria for children with SCA were as follows: (1) laboratory diagnosis of SCA (HbSS or HbSB0 thalassemia), (2) SAM defined as a BMI z score of <−3.0, (3) age between 5.00 and 12.99 years, and (4) residence within a 2-hour driving distance from the medical center. The exclusion criteria were as follows: (1) children with complicated SAM; (2) children with electrolyte disturbances at baseline; (3) children on disease-modifying therapy (hydroxyurea or regular blood transfusion therapy); (4) children enrolled in other studies; (5) children with diabetes and other chronic illnesses; (6) children with known HIV infection; and (7) children with known allergies to dairy or peanuts. Children with complicated acute SAM (including, but not limited to, poor appetite, altered mental status, fever, other signs of infection, bilateral edema of the extremities, or respiratory distress) were excluded and referred for inpatient treatment. To improve the adherence to the RUTF and decrease sharing among household members, siblings aged from 5 to 12 years with SAM but without SCA were eligible for screening and RUTF therapy.
Interventions
Hydroxyurea is an antimetabolite chemotherapy drug approved by National Agency for Food and Drug Administration and Control in Nigeria to prevent acute vaso-occlusive pain and other complications in SCA. Children with SCA randomly assigned to the treatment arm received moderate fixed-dose hydroxyurea therapy at 20 mg/kg per day.
Participants received Plumpy'Nut, a fortified peanut butter–like paste that contains essential macro- and micronutrients, classified as a RUTF used as an outpatient treatment for SAM.33,34 Plumpy'Nut is manufactured by Nutrik, a subsidiary of the Nutriset Group, based in Kano, Nigeria, the location of our randomized controlled trial. The nutritional objective was to supplement and not replace the calories derived from the habitual daily diet. Children aged from 5.00 to 8.99 years were given 1 daily sachet (500 calories), whereas those aged from 9.00 to 12.99 years received 2 daily (1000 calories) for 12 weeks.
Standardization of anthropometric evaluation
At each visit, participants underwent a complete anthropometric evaluation with research-quality equipment. Height was measured to the nearest 0.1 cm using a calibrated stadiometer (ShorrBoard Measuring Board, Weigh and Measure, Olney, MD), and weight was measured to the nearest 0.1 kg using a calibrated digital scale (Seca 874 doctor’s scale, Seca, CA). The BMI was calculated by dividing weight (kg) by height squared (m2). Using the World Health Organization’s growth reference, anthropometric measurements were converted to age- and sex-specific z scores.35 For children aged >10 years, we used Canadian Pediatric Endocrine Group growth charts to calculate weight-for-age z scores.36 SAM was defined as a BMI z score of <−3.0.37
All anthropometric measurements were performed in triplicates and averaged to ensure accuracy. If the smallest and largest values had a difference greater than the maximum allowable difference, the measurements were repeated (supplement 1). Before study initiation, the Nigerian team members completed training on proper anthropometric methods, including correct measurement techniques and documentation. To receive anthropometric certification, team members underwent a hands-on assessment, during which they recorded a series of anthropometric measurements of the participants and presented proper techniques and documentation.
Research visits
After randomization, study participants were closely monitored for 12 weeks. A visit was conducted within 3 or 5 days of starting the RUTF intervention to monitor for electrolyte abnormalities and refeeding syndrome. Every subsequent visit (conducted every 4 weeks) included a comprehensive clinical history, physical examination, anthropometric measurements, and a complete blood count. Between clinic visits, weekly phone calls were made to monitor for interim hospitalizations. As per standard care practices for SAM, all children received antibiotics (amoxicillin) and deworming treatment (albendazole). Children with SCA were prescribed folic acid, antimalarial prophylaxis (Proguanil), and penicillin up to 10 years of age as part of routine care. Parents received face-to-face education on nutrition and hygiene practices throughout the study period.
Participants with SCA who experienced weight loss or continued to have a BMI z score of < −3.0 after the 12-week period were eligible for a 12-week trial extension. During this extension period, eligible participants received moderate-dose hydroxyurea and 4or 5 daily RUTF sachets (2000-2500 calories per day). Results from the 12-week extension are part of a separate analysis.
Outcomes
The protocol-specified primary outcome was to evaluate the feasibility of a malnutrition randomized controlled trial in older children with SCA, via recruitment, retention, and adherence rates during the 12-week intervention period. The recruitment rate was the number of eligible participants who provided informed consent. The retention rate was the proportion of participants who completed the 12-week trial. Adherence to monthly visits was assessed based on the number of missed visits, whereas adherence to the RUTF was evaluated based on the proportion of empty food sachets returned at each visit. The adherence rate for hydroxyurea was determined based on the pill count and an increase in mean corpuscular volume and fetal Hb levels at study exit compared with the levels at baseline.
To monitor the safety and AEs during each 4-week visit, we obtained an interval medical history to track any changes in their health status. In addition, a complete blood count was conducted at baseline and at every 4-week visit to monitor for hydroxyurea toxicity. To monitor for refeeding syndrome, we measured electrolytes twice during the first week: at baseline and 3 or 5 days after initiating the RUTF. We considered any electrolyte derangement during the study as a complication of treatment. As previously done in trials at our laboratory,38 hospitalizations were adjudicated for the primary reason for admission in the following hierarchal and mutually exclusive categories: stroke, acute chest syndrome, pain, or fever, as detailed in the study protocol (supplement 1). We followed the Common Toxicity Criteria for Adverse Events to evaluate the severity of the AEs. We classified severe AEs (SAEs) as those resulting in prolonged hospitalization (>7 days), admission to the intensive care unit, significant or persistent disability, near death, or death.
Randomization and masking
To randomize participants, the study statistician constructed allocation tables using a permuted block allocation scheme based on block sizes of 4, stratified based on sex, within the site. The tables were loaded into Research Electronic Data Capture (REDCap) and used to randomize the participants (1:1) by an unblinded study pharmacist after determining eligibility. The participants went to the trial pharmacists, who distributed the trial medication to their guardians. Allocation was concealed from all other study personnel, except statisticians, because of the requirement to prepare interim reports for the DSMB.
Sample size
Because our primary goal was to assess the feasibility of conducting a randomized controlled trial of this nature in northern Nigeria, we did not perform sample size calculations. Instead, the final sample size was determined based on budgetary constraints.
Statistical analysis
Data were collected and managed with REDCap hosted at Vanderbilt University Medical Center. We summarized continuous variables as means and standard deviations (SDs) or as medians and interquartile ranges (IQR) for variables not normally distributed. Categorical variables and prevalence were reported as numbers and percentages. For comparisons between groups, χ2 test or Fisher exact test was used for percentages, a t test for means, and a Mann-Whitney U test for medians.
We used 2 regression models to study the characteristics associated with weight gain for all participants with SCD. Per protocol, we used a logistic regression model to study whether participants achieved a BMI z score of at least −3.0 and whether they achieved at least a 15% weight gain. Finally, because of the evidence that weight-for-age z score is associated with mortality in children with SCA in the same age range living in northern Nigeria,7 we used the same covariates for the logistic mode for a linear regression model to study the change in weight-for-age z score from baseline.
Results
Enrollment, randomization, and follow-up
In August 2021, the study commenced its screening and enrollment process. At the end of November 2021 (at month 4 of 6), enrollment was paused to prepare for the DSMB meeting held in December. During the pause for the DSMB meeting, the study investigators elected to determine the reason for the low absolute weight gain in the entire cohort. Although the original protocol included a provision for increasing support to families, it was not until December 2021 that this measure was implemented to improve participant outcomes. Specifically, an extra daily sachet could be provided in addition to the existing sachet allocation for participants who were not gaining sufficient weight. Enrollment was restarted in March 2022 and completed in May 2022.
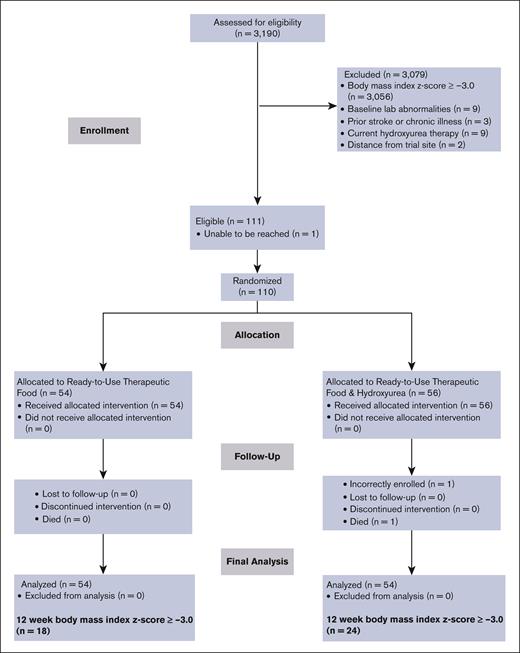
A total of 3190 children aged from 5 to 12 years with SCA were evaluated for eligibility during regularly scheduled clinic appointments. Of these children, 4.2% (n = 134) were found to have SAM (BMI z score of <−3.0; Figure 1; for specific site enrollment, see supplemental Table 1). One eligible child did not enroll in the trial, resulting in an overall enrollment rate of 99.1% (110 of 111). None of the participants in the treatment group withdrew voluntarily from the trial, indicating a retention rate of 100%. One participant died from an unrelated cause (motor vehicle accident). Another participant was enrolled based on the World Health Organization’s table of BMI z scores for sex and age but was administratively withdrawn because of a calculated BMI z score of >−3.0. This participant completed the 12-week trial but was not included in the analysis. The final analysis included 108 participants, with 54 participants from each arm (Figure 1).
Flow diagram for recruitment, screening, enrollment, and follow-up of RUTF and RUTF plus moderate-dose hydroxyurea group participants in the SAMS trial for treating SAM in children with SCA.
Flow diagram for recruitment, screening, enrollment, and follow-up of RUTF and RUTF plus moderate-dose hydroxyurea group participants in the SAMS trial for treating SAM in children with SCA.
If a participant enrolled, siblings aged from 5 to 12 years without SCA were asked to present to the clinic for screening for possible study enrollment. A total of 73 siblings were screened, with 30% classified as having SAM (n = 22) and otherwise eligible for the trial. All eligible siblings elected to enroll (enrollment rate of 100%) and completed the trial (retention rate of 100%).
Baseline characteristics
Baseline characteristics were balanced across the randomization groups (Table 1). The median age of participants in the RUTF-only and RUTF-plus-hydroxyurea groups was 10.5 and 10.2 years, respectively (P = .232; Table 1), and the baseline BMI z scores for the RUTF-only and RUTF-plus-hydroxyurea arms were not statistically different (−3.66 vs −3.64; P = .821). No significant differences were found between the baseline demographics and clinical parameters of those randomly assigned to RUTF only or RUTF plus hydroxyurea (supplemental Table 2).
Baseline characteristics of participants with SCA and SAM based on randomization status in the SAMS trial
| Variable . | Total cohort (N = 109) . | RUTF only (n = 54) . | RUTF plus hydroxyurea (n = 55) . | P value∗ . |
|---|---|---|---|---|
| Age, y, median (IQR) | 10.3 (8.7-11.5) | 10.5 (9.4-11.7) | 10.2 (8.2-11.4) | .232 |
| Sex, female, n (%) | 53 (48.6) | 27 (50.0) | 26 (47.3) | .776 |
| Head of household education, n (%) (n = 107) | .627 | |||
| None/Primary/Junior secondary | 34 (32.1) | 19 (36.5) | 15 (27.8) | |
| Senior secondary/OND | 59 (55.7) | 27 (51.9) | 32 (59.3) | |
| University/professional | 13 (12.3) | 6 (11.5) | 7 (13.0) | |
| Height, cm, mean (SD) | 123.8 (9.8) | 124.1 (9.5) | 123.5 (10.1) | .747 |
| Weight, kg, mean (SD) | 18.6 (3.5) | 18.7 (3.5) | 18.4 (3.5) | .617 |
| BMI, kg/m2, mean (SD) | 12.0 (0.6) | 12.1 (0.6) | 12.0 (0.6) | .387 |
| BMI, z score, mean (SD) | −3.66 (0.46) | −3.66 (0.44) | −3.64 (0.49) | .821 |
| Hb, g/dl, mean (SD) | 7.3 (1.0) | 7.3 (1.1) | 7.4 (1.0) | .646 |
| Variable . | Total cohort (N = 109) . | RUTF only (n = 54) . | RUTF plus hydroxyurea (n = 55) . | P value∗ . |
|---|---|---|---|---|
| Age, y, median (IQR) | 10.3 (8.7-11.5) | 10.5 (9.4-11.7) | 10.2 (8.2-11.4) | .232 |
| Sex, female, n (%) | 53 (48.6) | 27 (50.0) | 26 (47.3) | .776 |
| Head of household education, n (%) (n = 107) | .627 | |||
| None/Primary/Junior secondary | 34 (32.1) | 19 (36.5) | 15 (27.8) | |
| Senior secondary/OND | 59 (55.7) | 27 (51.9) | 32 (59.3) | |
| University/professional | 13 (12.3) | 6 (11.5) | 7 (13.0) | |
| Height, cm, mean (SD) | 123.8 (9.8) | 124.1 (9.5) | 123.5 (10.1) | .747 |
| Weight, kg, mean (SD) | 18.6 (3.5) | 18.7 (3.5) | 18.4 (3.5) | .617 |
| BMI, kg/m2, mean (SD) | 12.0 (0.6) | 12.1 (0.6) | 12.0 (0.6) | .387 |
| BMI, z score, mean (SD) | −3.66 (0.46) | −3.66 (0.44) | −3.64 (0.49) | .821 |
| Hb, g/dl, mean (SD) | 7.3 (1.0) | 7.3 (1.1) | 7.4 (1.0) | .646 |
OND, ordinary national diploma.
χ2 test for categorical variables, t test for means, and Mann-Whitney U test for medians.
Adherence
Per protocol, all participants were expected to attend research visits every 4 weeks for interval history, physical examination, anthropometric measurements, and laboratory surveillance. None of the scheduled visits were missed.
The median percentage of RUTF sachets returned empty was 100% (IQR, 99.98-100). After the DSMB meeting in December 2022, questions on food sharing were added to the study because the aggregated group results indicated that many participants had a BMI z score of <−3.0 at the study end point and indirect evidence from interviews that the participants were sharing the RUTF. Of the 70 participants with available data on sharing, the majority (62.9%; n = 44) reported at least sometimes sharing the RUTF (Table 2).
Anthropometric indicators at baseline and at 12 weeks for participants of the SAMS trial based on the randomization status
| Variable . | Total cohort (N = 108) . | RUTF only (n = 54) . | RUTF plus hydroxyurea (n = 54) . | P value∗ . |
|---|---|---|---|---|
| Time from baseline to 12-wk visit, d, mean (SD) | 91.2 (4.6) | 91.0 (4.9) | 91.3 (4.3) | .737 |
| Height-for-age z score at baseline, mean (SD) | −2.3 (1.0) | −2.4 (1.1) | −2.1 (0.8) | .119 |
| Height-for-age z score at 12 wk, mean (SD) | −2.3 (1.0) | −2.4 (1.1) | −2.1 (0.8) | .082 |
| Weight-for-age z score at baseline, mean (SD) | −3.6 (0.7) | −3.6 (0.7) | −3.5 (0.6) | .262 |
| Weight-for-age z score at 12 wk, mean (SD) | −3.3 (0.7) | −3.4 (0.8) | −3.2 (−0.7) | .130 |
| BMI z score at baseline, mean (SD) | −3.7 (0.5) | −3.7 (0.4) | −3.7 (0.5) | .905 |
| BMI z score at 12 wk, mean (SD) | −3.2 (0.6) | −3.2 (0.5) | −3.2 (0.7) | .740 |
| Sharing food at home, (n = 70), n (%) | 44 (62.9) | 22 (59.5) (n = 37) | 22 (66.7) (n = 33) | .533 |
| Variable . | Total cohort (N = 108) . | RUTF only (n = 54) . | RUTF plus hydroxyurea (n = 54) . | P value∗ . |
|---|---|---|---|---|
| Time from baseline to 12-wk visit, d, mean (SD) | 91.2 (4.6) | 91.0 (4.9) | 91.3 (4.3) | .737 |
| Height-for-age z score at baseline, mean (SD) | −2.3 (1.0) | −2.4 (1.1) | −2.1 (0.8) | .119 |
| Height-for-age z score at 12 wk, mean (SD) | −2.3 (1.0) | −2.4 (1.1) | −2.1 (0.8) | .082 |
| Weight-for-age z score at baseline, mean (SD) | −3.6 (0.7) | −3.6 (0.7) | −3.5 (0.6) | .262 |
| Weight-for-age z score at 12 wk, mean (SD) | −3.3 (0.7) | −3.4 (0.8) | −3.2 (−0.7) | .130 |
| BMI z score at baseline, mean (SD) | −3.7 (0.5) | −3.7 (0.4) | −3.7 (0.5) | .905 |
| BMI z score at 12 wk, mean (SD) | −3.2 (0.6) | −3.2 (0.5) | −3.2 (0.7) | .740 |
| Sharing food at home, (n = 70), n (%) | 44 (62.9) | 22 (59.5) (n = 37) | 22 (66.7) (n = 33) | .533 |
χ2 test for categorical variables and t test for means. The P value represents the comparison between RUTF only and RUTF plus hydroxyurea.
Multiple hydroxyurea adherence measurements were performed. The median percentage of pills returned was 4.4% (IQR, 0.00-8.27; n = 54). The mean increase in mean corpuscular volume was higher in the group receiving RUTF plus hydroxyurea than in that with only RUTF (5.4 vs −1.0; P = .007; supplemental Table 3). The median HbF level increased by 2.2% (IQR, 0.5-4.9) and 3.9% (IQR, 1.7-6.8) from baseline to study exit in the RUTF-only and RUTF-plus-hydroxyurea groups, respectively (supplemental Table 3). Additional laboratory values demonstrating the anticipated hydroxyurea response from baseline to study exit are shown in supplemental Table 4.
Safety
During the 12-week trial, there were 4 hospitalizations in the RUTF-only group and 2 hospitalizations in the RUTF-plus-hydroxyurea group (P = .678). Hospitalization diagnoses included pain (2 hospitalizations), acute chest syndrome, fever, and other (elective surgery for tonsillectomy and adenoidectomy; anemia with an inadequate investigation to determine etiology, splenic sequestration, parvovirus B19, or malaria). One hospitalization was prolonged and met the criteria for an SAE but was deemed unrelated to the study treatment. One participant died from a motor vehicle accident unrelated to the study treatment before completing the trial.
No participant had hydroxyurea discontinued due to myelosuppression or drug-related toxicity, and no participant required dose reductions. The baseline and study-exit hematology laboratory values per treatment group are displayed in supplemental Table 4.
Electrolytes were evaluated at baseline in 100% of participants (110 of 110) and repeated 3 or 5 days after starting RUTF in 98% of the participants (108 of 110 participants; supplemental Table 5). No participant developed hypophosphatemia (supplemental Table 5). There were no clinical cases of refeeding syndrome.
Efficacy results
The mean baseline BMI z score was −3.7 (SD = 0.5) and increased to −3.2 (SD = 0.5) at the study end point, for a change of 0.49 (SD = 0.53; 95% confidence interval [CI], 0.39-0.59; n = 108; Figure 2), and 61.1% of participants continued to have SAM (Figure 1).
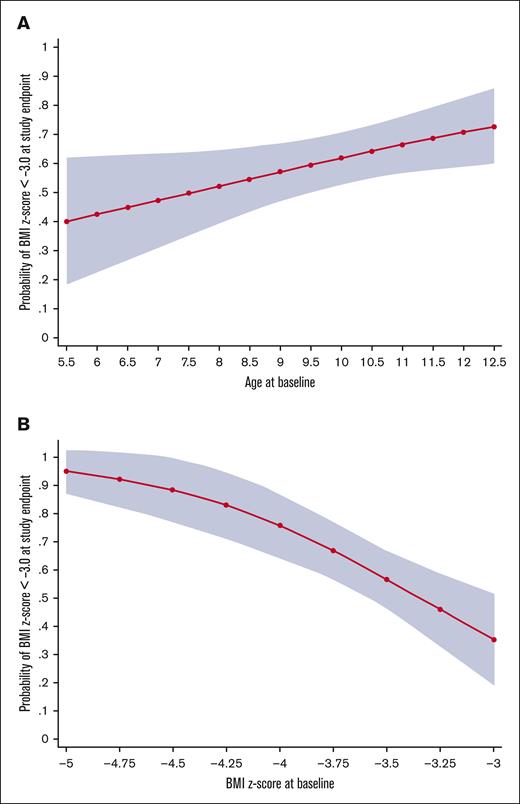
Probability of continued SAM (BMI z score < −3.0) at the study end point. (A-B) The predicted probability of continued SAM (BMI z score < −3.0), with 95% CIs based on age and baseline BMI z score, respectively, from a logistic regression model of the participants of the SAMS Trial.
Probability of continued SAM (BMI z score < −3.0) at the study end point. (A-B) The predicted probability of continued SAM (BMI z score < −3.0), with 95% CIs based on age and baseline BMI z score, respectively, from a logistic regression model of the participants of the SAMS Trial.
Factors postulated to be associated with treatment response were analyzed in a multivariable linear regression model. A higher baseline BMI z score predicted a higher end point BMI z score (β = 0.64; P ≤ .001; Table 3), whereas older age predicted a lower BMI z score at 12 weeks (β = −0.06; P ≤ .023; Table 3). The treatment group was not significantly associated with the BMI z score at 12 weeks (β = 0.01; P = .928). We, then, constructed a multivariable logistic regression with the same covariates to assess covariates associated with continued SAM (BMI z score of <−3.0) after 12 weeks (treatment nonresponse). Baseline BMI z score and age remained significant in the logistic model to predict continued SAM (Table 4). Among all participants, for every additional year of age, the odds of remaining severely malnourished increased (odds ratio [OR], 1.26; 95% CI, 1.01-1.57; P = .041; Table 4; Figure 3A). Increasing baseline BMI z score level up to <−3.0 was associated with decreased odds of remaining severely malnourished (OR, 0.16; 95% CI, 0.05-0.47; P = .001; Table 4; Figure 3B). For participants who did and who did not achieve a study-exit BMI z score ≥ −3.0, the change in BMI z score was greater for children with lower baseline BMI z scores (supplemental Figure 1). No participant with a baseline BMI z score <−4.4 improved their BMI to ≥−3.0 during the study.
Multivariable linear regression to assess independent covariates for the BMI z score at the study end point for the SAMS trial participants with SCA and SAM who completed the trial (n = 108)
| Variable . | β . | 95% CI . | P value . |
|---|---|---|---|
| Age, y | −0.059 | From −0.110 to −0.008 | .023 |
| Sex (female) | −0.105 | From −0.301 to – 0.090 | .287 |
| Hb (g/dl) | −0.047 | From −0.143 to – 0.050 | .339 |
| Baseline BMI z score | 0.643 | From 0.424 to – 0.862 | < .001 |
| Group (RUTF plus hydroxyurea) | 0.009 | From −0.187 to – 0.205 | .928 |
| Variable . | β . | 95% CI . | P value . |
|---|---|---|---|
| Age, y | −0.059 | From −0.110 to −0.008 | .023 |
| Sex (female) | −0.105 | From −0.301 to – 0.090 | .287 |
| Hb (g/dl) | −0.047 | From −0.143 to – 0.050 | .339 |
| Baseline BMI z score | 0.643 | From 0.424 to – 0.862 | < .001 |
| Group (RUTF plus hydroxyurea) | 0.009 | From −0.187 to – 0.205 | .928 |
Multivariable logistic regression to assess covariates for continued SAM (BMI z score less than −3.0) at the study end point in participants with SCA and SAM in the SAMS trial allocated to receive RUTF with or without moderate-dose hydroxyurea (20 mg/kg per day) for 12 weeks
| Variable . | OR . | 95% CI . | P value . |
|---|---|---|---|
| Age, y | 1.258 | 1.009-1.568 | .041 |
| Sex (female) | 1.310 | 0.551-3.110 | .541 |
| Hb | 1.174 | 0.769-1.792 | .458 |
| Baseline BMI z score | 0.155 | 0.051-0.474 | .001 |
| Group (RUTF plus hydroxyurea) | 0.666 | 0.281-1.582 | .357 |
| Variable . | OR . | 95% CI . | P value . |
|---|---|---|---|
| Age, y | 1.258 | 1.009-1.568 | .041 |
| Sex (female) | 1.310 | 0.551-3.110 | .541 |
| Hb | 1.174 | 0.769-1.792 | .458 |
| Baseline BMI z score | 0.155 | 0.051-0.474 | .001 |
| Group (RUTF plus hydroxyurea) | 0.666 | 0.281-1.582 | .357 |
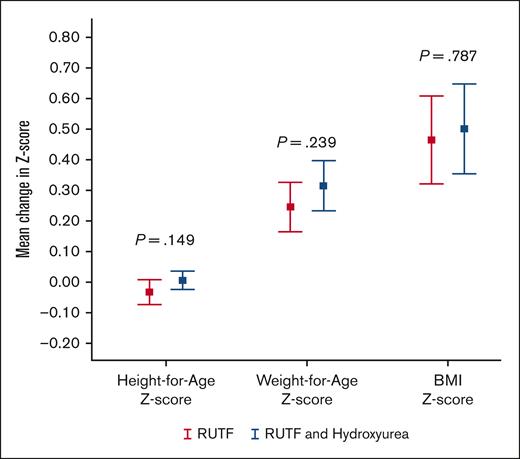
Mean change in height-for-age, weight-for-age and BMI z scores. Error bar chart displaying the mean and 95% CIs for change in z scores from study start for height-for-age, weight-for-age, and BMI for participants who completed the SAMS trial (n = 108), comparing the RUTF and RUTF-plus-hydroxyurea groups.
Mean change in height-for-age, weight-for-age and BMI z scores. Error bar chart displaying the mean and 95% CIs for change in z scores from study start for height-for-age, weight-for-age, and BMI for participants who completed the SAMS trial (n = 108), comparing the RUTF and RUTF-plus-hydroxyurea groups.
Weight gain of at least 15% was uncommon; only 9.3% (10 of 110) had a 15% weight gain. Given the low percentage of participants with 15% weight gain, a limited model demonstrated that participants with higher baseline BMI z score were less likely to increase weight by 15% (OR, 0.19; 95% CI, 0.04-0.88; P = .034; supplemental Table 6). Participants in the RUTF-plus-hydroxyurea group had 4.6 higher odds of achieving a 15% weight gain but did not reach statistical significance (95% CI, 0.99-21.3; P = .052). The mean change in weight-for-age z score was 0.28 (SD = 0.298). Male sex and older age predicted a lower weight-for-age z score at 12 weeks (sex: β = −0.12; P = .039; age β = −0.03; P = .044; supplemental Table 7).
Discussion
Malnutrition and SCD are common comorbidities in sub-Saharan Africa.1,18 However, no evidence-based guidelines have developed the optimal management when both comorbidities occur simultaneously.15 To our knowledge, the SAMS trial is the first randomized controlled trial to demonstrate the feasibility and safety of outpatient treatment of severely malnourished children aged from 5 to 12 years with SCA. An accurate diagnosis of SAM was made in 99% of children (109 of 110), with no participant defaulting on treatment and no SAEs associated with malnutrition treatment. Weight and BMI increased during the 12-week intervention with supplemental RUTF, with or without hydroxyurea.
A significant public health finding was the lower rate of SAMS in our malnutrition trial (4.2%) than in our primary stroke prevention trial (20.9%) in the same region.7 Most likely, this discrepancy was due to the enhanced fidelity of anthropometric assessment after rigorous training and measurement protocols for the research in the malnutrition trial. However, the SAM prevalence in children aged >5 years with SCA in this study is higher than in children aged <5 years in the same region (Kano, Nigeria; 1.5%; n = 754),39 emphasizing that SAM remains a public health concern in older children with SCA.
All the feasibility and safety measures were met during the 12-week intervention period. Notably, we had no missed visits; the acceptable default rate (≥2 missed weighing visits) is 15% for children aged <5 years, as set by the Sphere Association’s Humanitarian Charter and Minimum Standards in Humanitarian Response,40 with default rates in the practice ranging from 5% to 40%.41 Furthermore, the data suggest that hydroxyurea therapy is safe for severely malnourished children, a population previously excluded from an important study on the safety of hydroxyurea therapy in sub-Saharan Africa42 but not excluded from other clinical trials with hydroxyurea.38,43-45 Based on our data, and data from other studies, we believe the risk-benefit ratio should not exclude children with SAM from hydroxyurea treatment unless definitive evidence indicates direct harm to the child.
An unexpected important finding was that >50% of the children shared their RUTF. The adherence rates to the RUTF were overall very high, as was the rate of sharing, which may explain the variability in response to treatment. Sharing supplements with other household members has been reported in other studies and highlights cultural imperatives and moral obligations to feed all family members, which can hinder the strategy of deliberative targeting of food supplements.46,47 In children with HIV aged from 5 to 18 years, 38% of caregivers and participants reported sharing ready-to-use food (RUF), with caregivers reporting that they felt other adults or children needed the RUF.48 In a study for outpatient treatment of severe and moderate malnutrition in children with HIV, 32% of caregivers admitted to consuming the RUF themselves.49 Strategies to reduce sharing, such as the rationalization of RUF prescription, the enrollment of other undernourished family members in RUF therapy, and food complements for the household, have not been systematically evaluated.48,49
As a secondary outcome, we assessed the anthropometric measures. Our main conclusion from the anthropometric measures during the 12 weeks of the trial was that providing 500 or 1000 additional calories may not be sufficient for SAM recovery, particularly in those in food sharing settings and with very low baseline BMI z scores. A study of older children with HIV and a median age of 11 years treated for moderate or SAM in Ethiopia reported that only 65% recovered from malnutrition during a 6-month period.50 Possible reasons for the lower magnitude of our treatment response could be attributable to the use of only supplemental calories from RUTF, the common practice of food sharing, and the length of the intervention.
As a feasibility study, the sample size was not based on determining the difference in efficacy of the 2 treatment arms.51 We could not analyze daily calories and nutrient intake from the habitual diet because of limited food composition databases and difficulty quantifying the amount of food consumed as part of shared meals. A limitation of the malnutrition trial was its failure to assess pubertal status, which could have influenced the study outcomes; however, there was not a significant change in absolute height for females during the trial, indicating the pubertal growth spurt was an unlikely confounder for our trial. These limitations do not affect the primary trial outcome measures, demonstrating the feasibility of our treatment strategy. The results will guide future definitive trials on treating SAM in older children with SCA.
In the first NIH-funded malnutrition trial for SCA, outpatient treatment of SAM with RUTF and hydroxyurea was safe and feasible for children aged from 5 to 12 years in a low-income setting. This trial supports the safety of hydroxyurea therapy in low-income settings, even with concomitant SAM. The results indicate promise for the effects of RUTF and hydroxyurea on improved nutritional status. Future studies should consider more intensive interventions (ie, higher target daily calories from RUTF), with longer durations and the inclusion of the cultural aspect of food sharing in the trial design. Our team is planning a larger scale randomized controlled phase 3 trial to evaluate the effectiveness of hydroxyurea and RUTF for SAM treatment in children aged from 5 to 12 years in northern Nigeria.
Acknowledgments
The authors are grateful to the research coordinators and study personnel who tirelessly coordinated the trial in Kano, Nigeria. The authors appreciate Bilya Musa, Awwal Gambo, Saifuddeen Sani, Leshana Saint-Jean, Mustafa Nateqi, Jamil Galadanci, and Jennifer Beck-Smith for facilitating the administrative tasks required for the successful conduct of this study. The work would not have been possible without the philanthropy of the Aaron Ardoin Foundation and the David and Mary Phillips Foundation. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under award number R21HD097992. L.J.K. was supported by the Fogarty International Center and the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number D43TW009337, and the National Institute of Diabetes & Digestive & Kidney Diseases of the National Institutes of Health under award number T32DK007673. Data collection and storage were supported through a grant from the Vanderbilt Institute for Clinical and Translational Research NCATS/NIH UL1 TR000445.
The sponsors did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The findings and conclusions in this paper are those of the authors and do not represent the official position of the National Institutes of Health.
Authorship
Contribution: S.U.A., S.G., G.G., S.A., V.A.S., M.R., L.J.K., and M.R.D. conceptualized and designed the study; M.R.D., M.R., and L.J.K. had full access to all the data in the study and took responsibility for data integrity and the data analyses accuracy; S.U.A., S.G., H.A.M., K.A.S., and H.K. conducted the trial; L.J.K. and M.R. performed the analyses; L.J.K., M.R., and M.R.D. wrote the initial manuscript; and before submission, all authors reviewed the manuscript and approved its submission.
Conflict-of-interest disclosure: M.R.D. reports that he and his institution sponsor 2 externally funded research investigator-initiated projects. Global Blood Therapeutics will provide funding for the cost of the clinical studies but was not a cosponsor of either study. M.R.D. did not receive any compensation for the conduct of these 2 investigator-initiated observational studies; is a member of the Global Blood Therapeutics advisory board for a proposed randomized controlled trial for which he receives compensation; serves on the steering committee for a Novartis-sponsored phase 2 trial to prevent priapism in men; was a medical adviser in developing the CTX001 Early Economic Model; provided medical input on the economic model as part of an expert reference group for the Vertex/CRISPR CTX001 Early Economic Model in 2020; and consulted for the Formal Pharmaceutical company about sickle cell disease in 2021 and 2022. The remaining authors declare no competing financial interests.
Correspondence: Lauren J. Klein, Department of Pediatrics, D. Brent Polk Division of Pediatric Gastroenterology, Hepatology, and Nutrition at Monroe Carell Jr. Children's Hospital at Vanderbilt and Vanderbilt Institute for Global Health, 2525 West End Ave, Suite 725, Nashville, TN 37203-1738; e-mail: lauren.klein@vumc.org.
References
Author notes
The trial protocol and deidentified individual participant data for the primary analysis will be available on request from the corresponding author, Lauren J. Klein (lauren.klein@vumc.org), until 2028, ∼5 years after publication. Requestors will need to prepare and sign a data transfer agreement between Vanderbilt University Medical Center and their respective institutions. After 2028, institutional resources may not be available to provide the data.
The full-text version of this article contains a data supplement.