Key Points
Baseline radiomics features accurately predict progression in aggressive B-cell lymphoma.
Radiomics features combined with MYC rearrangement status resulted in the most accurate selection of high-risk patients.
Abstract
We investigated whether the outcome prediction of patients with aggressive B-cell lymphoma can be improved by combining clinical, molecular genotype, and radiomics features. MYC, BCL2, and BCL6 rearrangements were assessed using fluorescence in situ hybridization. Seventeen radiomics features were extracted from the baseline positron emission tomography–computed tomography of 323 patients, which included maximum standardized uptake value (SUVmax), SUVpeak, SUVmean, metabolic tumor volume (MTV), total lesion glycolysis, and 12 dissemination features pertaining to distance, differences in uptake and volume between lesions, respectively. Logistic regression with backward feature selection was used to predict progression after 2 years. The predictive value of (1) International Prognostic Index (IPI); (2) IPI plus MYC; (3) IPI, MYC, and MTV; (4) radiomics; and (5) MYC plus radiomics models were tested using the cross-validated area under the curve (CV-AUC) and positive predictive values (PPVs). IPI yielded a CV-AUC of 0.65 ± 0.07 with a PPV of 29.6%. The IPI plus MYC model yielded a CV-AUC of 0.68 ± 0.08. IPI, MYC, and MTV yielded a CV-AUC of 0.74 ± 0.08. The highest model performance of the radiomics model was observed for MTV combined with the maximum distance between the largest lesion and another lesion, the maximum difference in SUVpeak between 2 lesions, and the sum of distances between all lesions, yielding an improved CV-AUC of 0.77 ± 0.07. The same radiomics features were retained when adding MYC (CV-AUC, 0.77 ± 0.07). PPV was highest for the MYC plus radiomics model (50.0%) and increased by 20% compared with the IPI (29.6%). Adding radiomics features improved model performance and PPV and can, therefore, aid in identifying poor prognosis patients.
Introduction
Patients with aggressive B-cell lymphoma have a large variation in outcome, which is partly explained by genetic abnormalities, such as MYC oncogene rearrangements (MYC-R).1MYC-R occur in ∼10% to 15% of patients.1-3 Thirty percent of the patients only have MYC-R and are often referred to as single-hit patients (MYC-SH). In 70% of these cases, MYC-R is accompanied by a translocation of the BCL2 and/or BCL6 genes, which is classified as high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangement, also called double/triple hit (DH/TH).4 For these patients, standard first-line therapy results in poor outcomes with a 2-year progression-free survival (PFS) of ∼60%.2,5,6 Therefore, patients with DH/TH are often treated with dose-intensification regimens, although no standard of care regimen has formally been established for them.7,8
18F-fluorodeoxyglucose (18F-FDG) positron emission tomography–computed tomography (PET/CT) is the current clinical standard for staging at baseline and response evaluation during or after treatment.9,10 18F-FDG PET/CT is also used to quantify the metabolic tumor volume (MTV) in patients11,12 as an estimate of the total tumor burden. Baseline MTV is an important predictor of outcome and is inversely related to overall survival (OS) and PFS.13,14 Moreover, we recently showed that MTV combined with Ann Arbor staging and age allows individual relapse prediction for de novo patients with diffuse large B-cell lymphoma (DLBCL) by applying the International Metabolic Prognostic Index.15 Besides MTV, additional quantitative parameters, often referred to as radiomics features, can be extracted from 18F-FDG PET/CT scans. Radiomics features provide detailed information on the distribution of 18F-FDG–tracer uptake, morphology, and spread and texture of lesions. Radiomics features extracted from baseline 18F-FDG PET/CT scans have shown to be predictive of relapse in patients with DLBCL beyond just MTV.16-20
Whether baseline radiomics features differ between patients with aggressive B-cell lymphoma with molecular high-risk features, such as MYC-R, and patients without these high-risk features is still unknown. Moreover, the added value of radiomics features on the predictive value of MYC-R status has not been studied yet. Therefore, this study aimed to analyze the relation between MYC-R status and baseline PET parameters and to investigate the added value of radiomics features to the predictive value of MYC-R status in aggressive B-cell lymphomas.
Material and methods
Study population
In this posthoc analysis, we included all patients with de novo aggressive B-cell lymphoma whose tumor data on MYC, BCL2, and BCL6 rearrangements by fluorescence in situ hybridization (FISH)21 and baseline 18F-FDG PET scans were available from the PETRA database. 18F-FDG PET/CT scans and patient-level clinical and genetic data were collated and harmonized by the PETRA consortium.22 All patients were originally included in the multicenter phase 2 HOVON-130 trial (https://eudract.ema.europa.eu/, #2014-002654-39),23 the multicenter randomized phase 3 HOVON-84 trial, (https://eudract.ema.europa.eu/, #2006-005174-42)24 and the multicenter randomized phase 3 PETAL trial (https://eudract.ema.europa.eu/, #2006-001641-33).25 Individual trials were approved by institutional review boards and all the patients included provided informed consent. The use of all data within the PETRA imaging database has been approved by the institutional review board of the Vrije Universiteit University Medical Center (JR/20140414). Patients with wild-type MYC (hereafter referred to as patients with MYC-WT DLBCL) were treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP); R-CHOP intensified with rituximab (RR-CHOP); or 2 cycles of R-CHOP after a Burkitt protocol consisting of high-dose methotrexate, cytarabine, hyperfractionated cyclophosphamide and ifosfamide, split-dose doxorubicin and etoposide, vincristine, vindesine, and dexamethasone. Patients with MYC-SH and DH/TH were treated with R-CHOP combined with lenalidomide (R2-CHOP), R-CHOP, RR-CHOP, or the Burkitt protocol.
Pathology review
For all patients, MYC, BCL2, and BCL6 rearrangement statuses were assessed using FISH.21 The FISH analysis was performed according to routine procedures with the following standard commercial probes as part of the diagnostic workup: MYC break-apart, BCL2 break-apart, and BCL6 break-apart probes (Vysis/Abbott, DAKO, and Kreatech). In selected cases, FISH data were completed as part of the central pathology review process using Vysis/Abbott break-apart probes for only BCL2 and BCL6.23 Patients were classified as DH/TH according to the World Health Organization 2016 classification.4
PET/CT analysis
For PET/CT quality control (QC), we used the ranges as suggested by the guidelines of the European Association of Nuclear Medicine for the hepatic mean standardized uptake value (SUVmean) and plasma glucose.26 When the hepatic SUVmean fell outside the suggested ranges, but the total image activity was 50% to 80% of the total injected activity, scans were still included. Moreover, QC rejected scans if (1) scans were incomplete, (2) essential Digital Imaging and Communications in Medicine information was missing, and (3) scans were from a PET-only system. Quantitative PET/CT analysis of all tumor lesions was performed using the ACCURATE tool. MTV was calculated at baseline using the fixed SUV ≥4.0 segmentation method.27 Nontumor 18F-FDG avid regions (eg, brain, kidney, and bladder) adjacent to lesions were manually removed. All scans were reviewed by a nuclear medicine physician, and delineations were performed under the supervision of a nuclear medicine physician who was blinded to the outcome.
Radiomics feature extraction
MTV, SUVmax, SUVpeak, SUVmean, and total lesion glycolysis (TLG) were extracted at patient level for all patients included. Furthermore, the following 12 dissemination features were extracted: the number of lesions, 4 features quantifying distance between lesions,28 5 features quantifying the differences in SUVpeak between lesions, and 3 features quantifying the differences in MTV between lesions. All image processing and feature calculations were performed using RaCaT software,29 which complies with the imaging biomarker standardization initiative criteria.30
Statistical analysis
Differences in radiomics features between MYC subgroups
Differences in radiomics features between patients with MYC-WT DLBCL, MYC-SH DLBCL, and DH/TH were assessed using the Kruskal-Wallis test by ranks. In the case of significant differences in radiomics features, Dunn's test of multiple comparisons with Benjamini-Hochberg correction for multiple testing was used as a post hoc test. Correlations between radiomics features stratified for MYC-R status were calculated using Spearman correlation coefficients.
Prediction models
We tested the predictive value of the following models:
IPI: the International Prognostic Index (IPI) using low, low-intermediate, high-intermediate, and high-risk groups.31
MYC: MYC-R status (categorical: MYC-WT, MYC-SH, and DH/TH).
IPI + MYC: a combination of IPI and MYC-R status (categorical).
IPI + MYC + MTV: a combination of IPI, MYC-R status (categorical), and MTV.
Radiomics: MTV, SUVmax, SUVpeak, SUVmean, TLG, and 12 dissemination features.
Radiomics + MYC: MTV, SUVmax, SUVpeak, SUVmean, TLG, 12 dissemination features, and MYC-R status.
Combined: MTV, SUVmax, SUVpeak, SUVmean, TLG, 12 dissemination features, IPI, and MYC-R status.
Multivariate logistic regression with backward feature selection was used to predict the risk of progression or relapse after 2 years. Follow-up started at the time of baseline 18F-FDG PET/CT scan. We started with all potential predictors in the model and at every turn, the predictor with the highest P value was excluded from the model until all remaining predictors were significant. Patients who died without progression or were lost to follow-up within 2 years were excluded. Before feature selection, continuous variables that had a skewness of >0.5 were log-transformed using the natural logarithm. Model performance was assessed using repeated cross-validation (fivefold, 2000 repeats) yielding the cross-validated area under the curve of the receiver operating characteristics curve (CV-AUC). To match the prevalence of patients with MYC-SH and DH/TH with real-world prevalence,1 for each repeat all 245 patients with MYC-WT DLBCL were included, and 10 patients with MYC-SH DLBCL and 20 with DH/TH were selected using random stratified sampling. Within the same cross-validation loop, we determined overfitting in the regression coefficients of the best model by applying the train linear predictor (calibration slope) in the test data sets and determined its Akaike information criterion (AIC).
The cell of origin (COO) was available for 298 patients,32 for whom we also tested the predictive value of a prediction model with all features from the model that included IPI and MYC-R status (model 3) and the combined model (model 7) and COO (categorical: germinal center B cell, nongerminal center B cell, unclassified).
Relative feature importance
z scores of individual predictors were calculated to compare the relative effects of predictors that were measured on different scales for all multivariate logistic models. z scores were calculated by subtracting the mean and dividing by the standard deviation. These standardized features were used as predictors in logistic regression. The absolute values of the regression coefficients quantify the relative importance of the predictors.
Diagnostic performance
For all multivariate models, the sum of individual predictors, weighted by the regression coefficients, together with the intercept of the model resulted in the predicted probability (expressed as log odds) of progression for each patient. To calculate the diagnostic performance of the models, high- and low-risk groups were defined based on prior probability (ie, prevalence) of events.33 For the IPI prediction model, patients with 4 or 5 adverse factors were considered high risk. For the MYC prediction model, patients with DH/TH were considered high risk. The diagnostic performance of the prediction models was assessed using sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). Survival curves for time to progression (TTP), PFS, and OS were obtained with Kaplan-Meier analyses and compared with log-rank tests.
Statistical analysis was performed using R (version 4.0.3). A P value of < .05 was considered statistically significant.
Results
Patient characteristics
There were 458 patients with DLBCL with MYC, BCL2, and BCL6 rearrangement status available, of whom 323 were included in this analysis. A total of 135 patients were excluded based on the following criteria/reasons: (1) no whole-body PET/CT scan available (n = 59), (2) PET/CT scan outside QC (n = 13), (3) essential Digital Imaging and Communications in Medicine information missing (n = 12), (4) no 18F-FDG avid disease (n = 6), (5) BCL2 and BCL6 mutation status could not be assessed (n = 9), (6) lost to follow-up within 2 years (n = 15), or (7) one of the individual IPI components was missing (n = 4). Patients who died within 2 years without signs of progression (n = 12) were excluded from the development of the prediction model and TTP survival analysis but included for PFS and OS.
In total, 245 patients with MYC-WT DLBCL, 24 with MYC-SH DLBCL, and 54 with DH/TH were included in this study (Table 1). For 3 patients with MYC and BCL2 rearrangements, BCL6 rearrangement status could not be assessed. The 2-year TTP of patients with MYC-WT DLBCL was 85.7% (95% confidence interval [CI], 81.4-90.2), compared with 66.7% (95% CI, 50.2-88.5) for patients with MYC-SH DLBCL and 57.4% (95% CI, 45.6-72.2) for patients with DH/TH. Both MYC-SH and DH/TH subgroups had a more pronounced male predominance compared with patients with MYC-WT DLBCL. Patients with MYC-SH DLBCL had similar baseline characteristics as those with MYC-WT DLBCL, whereas patients with DH/TH more often had higher frequencies of advanced-stage disease, elevated lactate dehydrogenase levels, and extranodal involvement leading to higher IPI scores compared with patients with MYC-SH DLBCL and MYC-WT DLBCL. In the MYC-WT DLBCL cohort, 1 patient was treated with the Burkitt protocol and the rest were treated with R-CHOP regimens. Sixty-seven percent of patients with MYC-SH DLBCL vs 76% of patients with DH/TH received other induction therapies than R-CHOP. Patient characteristics for individual trials are presented in supplemental Table 1.
Patient characteristics
| . | Total (n = 323) . | MYC-WT DLBCL (n = 245) . | MYC-SH DLBCL (n = 24) . | DH/TH (n = 54) . |
|---|---|---|---|---|
| Gender | ||||
| Male | 185 (57) | 131 (53) | 15 (63) | 39 (72) |
| Female | 138 (43) | 114 (47) | 9 (38) | 15 (28) |
| Age (interquartile range), y | 63 (53-71) | 64 (54-71) | 57 (45-66) | 63 (55-71) |
| Ann Arbor stage | ||||
| I | 23 (7) | 20 (8) | 2 (8) | 1 (2) |
| II | 54 (17) | 44 (18) | 5 (21) | 5 (9) |
| III | 73 (23) | 62 (25) | 3 (13) | 8 (15) |
| IV | 173 (54) | 119 (49) | 14 (58) | 40 (74) |
| Lactate dehydrogenase | ||||
| Normal | 124 (38) | 103 (42) | 9 (38) | 12 (22) |
| >Normal | 199 (62) | 142 (58) | 15 (63) | 42 (78) |
| Extranodal involvement | ||||
| 0-1 | 204 (63) | 166 (68) | 16 (67) | 22 (41) |
| >1 | 119 (37) | 79 (32) | 8 (33) | 32 (59) |
| World Health Organization performance status | ||||
| 0 | 186 (58) | 142 (58) | 15 (63) | 29 (54) |
| 1 | 100 (31) | 74 (30) | 8 (33) | 18 (33) |
| 2 | 33 (10) | 26 (11) | 1 (4) | 6 (11) |
| 3 | 4 (1) | 3 (1) | 1 (2) | |
| IPI | ||||
| Low | 83 (26) | 68 (28) | 8 (33) | 7 (13) |
| Low-intermediate | 65 (20) | 51 (21) | 4 (17) | 10 (19) |
| High-intermediate | 104 (32) | 75 (31) | 8 (33) | 21 (39) |
| High | 71 (22) | 51 (21) | 4 (17) | 16 (30) |
| Treatment | ||||
| 6 × R-CHOP | 99 (31) | 94 (38) | 1 (4) | 4 (7) |
| 8 × R-CHOP | 29 (9) | 24 (10) | 2 (8) | 3 (6) |
| 6 × R-CHOP + 2 × R | 71 (22) | 67 (27) | 2 (8) | 2 (4) |
| 6 × RR-CHOP | 44 (14) | 41 (17) | 2 (8) | 1 (2) |
| 8 × RR-CHOP | 22 (7) | 18 (7) | 1 (4) | 3 (6) |
| Burkitt protocol | 4 (1) | 1 (1) | 2 (8) | 1 (2) |
| 6 × R2-CHOP + 2 × R | 54 (17) | 14 (59) | 40 (74) |
| . | Total (n = 323) . | MYC-WT DLBCL (n = 245) . | MYC-SH DLBCL (n = 24) . | DH/TH (n = 54) . |
|---|---|---|---|---|
| Gender | ||||
| Male | 185 (57) | 131 (53) | 15 (63) | 39 (72) |
| Female | 138 (43) | 114 (47) | 9 (38) | 15 (28) |
| Age (interquartile range), y | 63 (53-71) | 64 (54-71) | 57 (45-66) | 63 (55-71) |
| Ann Arbor stage | ||||
| I | 23 (7) | 20 (8) | 2 (8) | 1 (2) |
| II | 54 (17) | 44 (18) | 5 (21) | 5 (9) |
| III | 73 (23) | 62 (25) | 3 (13) | 8 (15) |
| IV | 173 (54) | 119 (49) | 14 (58) | 40 (74) |
| Lactate dehydrogenase | ||||
| Normal | 124 (38) | 103 (42) | 9 (38) | 12 (22) |
| >Normal | 199 (62) | 142 (58) | 15 (63) | 42 (78) |
| Extranodal involvement | ||||
| 0-1 | 204 (63) | 166 (68) | 16 (67) | 22 (41) |
| >1 | 119 (37) | 79 (32) | 8 (33) | 32 (59) |
| World Health Organization performance status | ||||
| 0 | 186 (58) | 142 (58) | 15 (63) | 29 (54) |
| 1 | 100 (31) | 74 (30) | 8 (33) | 18 (33) |
| 2 | 33 (10) | 26 (11) | 1 (4) | 6 (11) |
| 3 | 4 (1) | 3 (1) | 1 (2) | |
| IPI | ||||
| Low | 83 (26) | 68 (28) | 8 (33) | 7 (13) |
| Low-intermediate | 65 (20) | 51 (21) | 4 (17) | 10 (19) |
| High-intermediate | 104 (32) | 75 (31) | 8 (33) | 21 (39) |
| High | 71 (22) | 51 (21) | 4 (17) | 16 (30) |
| Treatment | ||||
| 6 × R-CHOP | 99 (31) | 94 (38) | 1 (4) | 4 (7) |
| 8 × R-CHOP | 29 (9) | 24 (10) | 2 (8) | 3 (6) |
| 6 × R-CHOP + 2 × R | 71 (22) | 67 (27) | 2 (8) | 2 (4) |
| 6 × RR-CHOP | 44 (14) | 41 (17) | 2 (8) | 1 (2) |
| 8 × RR-CHOP | 22 (7) | 18 (7) | 1 (4) | 3 (6) |
| Burkitt protocol | 4 (1) | 1 (1) | 2 (8) | 1 (2) |
| 6 × R2-CHOP + 2 × R | 54 (17) | 14 (59) | 40 (74) |
All data are presented as number of patients (%), unless indicated otherwise.
Differences in radiomics features between MYC subgroups
Patients with MYC-SH DLBCL showed significantly lower intensity values (SUVmean and SUVmax; P < .04) (Table 2; supplemental Tables 2 and 3) than those with MYC-WT DLBCL and DH/TH and more homogeneous intensity between lesions, as shown by the lower maximum difference in SUVpeak between 2 lesion (DSUVpeakpatient; P = .10) values. MTV and differences in MTV between lesions of patients with MYC-SH DLBCL were comparable to those with MYC-WT DLBCL. Patients with DH/TH had comparable uptake intensity and differences in intensity between lesions compared with patients with MYC-WT DLBCL. However, MTV was significantly higher (P < .001) (supplemental Tables 2 and 3), the spread of the disease was significantly larger (all: P < .04), differences in volume between lesions were significantly larger (all: P < .001), and the sum of differences between all lesions was significantly higher in patients with DH/TH compared with patients with MYC-WT DLBCL. MTV was highly correlated with TLG (r = 0.98-0.99) in all MYC subgroups, but not highly correlated with other radiomic features (r < 0.7 for all features). SUVpeak correlated highly with SUVmean, SUVmax, DSUVpeakbulk, and DSUVpeakpatient for all MYC subgroups (r > 0.7 for all features).
Descriptive statistics of radiomics features stratified for MYC-R status
| . | MYC-WT DLBCL (n = 245) . | MYC-SH DLBCL (n = 24) . | DH/TH (n = 54) . |
|---|---|---|---|
| SUVpeak | 17.0 (11.8-22.4) | 12.0 (8.7-16.7) | 17.4 (12.9-23.4) |
| MTV | 256.6 (54.9-777.0) | 292.5 (15.9-1 098.7) | 709.5 (304.6-1 280.1) |
| No. of lesions | 7 (3-16) | 5 (1-15) | 10 (3-24) |
| Spreadpatient | 3 122.0 (156.7-25 383.9) | 2 099.7 (0-18 197.4) | 11 828.1 (1 034.3-77 878.9) |
| Dmaxbulk | 28.2 (8.8-43.6) | 28.6 (0-50.2) | 35.3 (23.0-51.5) |
| Volumemaxpatient | 114.5 (11.0-497.0) | 118.2 (0-700.7) | 444.7 (88.3-752.8) |
| DSUVpeakpatient | 10.5 (2.2-16.7) | 5.0 (0-10.3) | 10.8 (5.6-19.2) |
| . | MYC-WT DLBCL (n = 245) . | MYC-SH DLBCL (n = 24) . | DH/TH (n = 54) . |
|---|---|---|---|
| SUVpeak | 17.0 (11.8-22.4) | 12.0 (8.7-16.7) | 17.4 (12.9-23.4) |
| MTV | 256.6 (54.9-777.0) | 292.5 (15.9-1 098.7) | 709.5 (304.6-1 280.1) |
| No. of lesions | 7 (3-16) | 5 (1-15) | 10 (3-24) |
| Spreadpatient | 3 122.0 (156.7-25 383.9) | 2 099.7 (0-18 197.4) | 11 828.1 (1 034.3-77 878.9) |
| Dmaxbulk | 28.2 (8.8-43.6) | 28.6 (0-50.2) | 35.3 (23.0-51.5) |
| Volumemaxpatient | 114.5 (11.0-497.0) | 118.2 (0-700.7) | 444.7 (88.3-752.8) |
| DSUVpeakpatient | 10.5 (2.2-16.7) | 5.0 (0-10.3) | 10.8 (5.6-19.2) |
All values are denoted as median (interquartile range). Corresponding P values between subgroups are presented in supplemental Table 3.
Prediction model
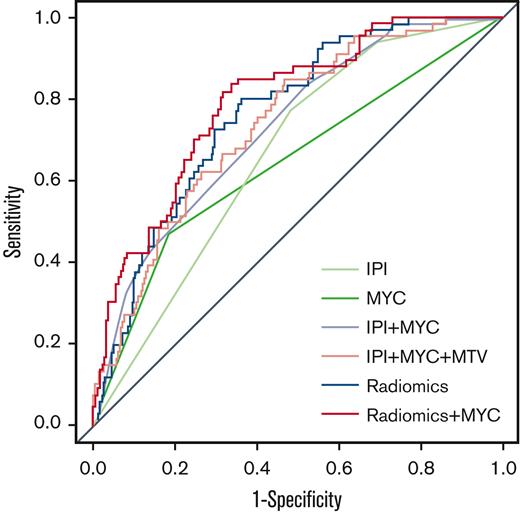
The logistic regression model with IPI using 2-year TTP as outcome yielded a CV-AUC of 0.65 ± 0.07 (95% CI, 0.52-0.83) (Table 3; Figure 1). The model with IPI applied to patients with DH/TH yielded a CV-AUC of 0.56 ± 0.15 (95% CI, 0.28-0.85). The logistic regression model with MYC-R status resulted in a CV-AUC of 0.58 ± 0.08 (95% CI, 0.43-0.72). The model that combined IPI with MYC-R status yielded a CV-AUC of 0.68 ± 0.08 (95% CI, 0.55-0.87). Adding the natural logarithm of MTV to IPI and MYC-R improved the CV-AUC to 0.74 ± 0.08 (95% CI, 0.59-0.87).
Model performances of all models
| . | CV-AUC ± standard deviation (95% CI) . | AIC . |
|---|---|---|
| IPI | 0.65 ± 0.07 (0.50-0.78) | 191.1 |
| MYC | 0.58 ± 0.08 (0.43-0.72) | 197.3 |
| IPI + MYC | 0.68 ± 0.08 (0.52-0.83) | 186.7 |
| IPI + MYC + MTV | 0.74 ± 0.07 (0.59-0.87) | 180.1 |
| Radiomics | 0.77 ± 0.07 (0.62-0.89) | 175.0 |
| Radiomics + MYC | 0.77 ± 0.07 (0.63-0.90) | 173.1 |
| . | CV-AUC ± standard deviation (95% CI) . | AIC . |
|---|---|---|
| IPI | 0.65 ± 0.07 (0.50-0.78) | 191.1 |
| MYC | 0.58 ± 0.08 (0.43-0.72) | 197.3 |
| IPI + MYC | 0.68 ± 0.08 (0.52-0.83) | 186.7 |
| IPI + MYC + MTV | 0.74 ± 0.07 (0.59-0.87) | 180.1 |
| Radiomics | 0.77 ± 0.07 (0.62-0.89) | 175.0 |
| Radiomics + MYC | 0.77 ± 0.07 (0.63-0.90) | 173.1 |
Receiver operating characteristic curves for 2-year TTP for IPI, MYC, IPI + MYC, IPI + MYC + MTV, radiomics, and radiomics + MYC prediction models.
Receiver operating characteristic curves for 2-year TTP for IPI, MYC, IPI + MYC, IPI + MYC + MTV, radiomics, and radiomics + MYC prediction models.
The highest model performance for the radiomics model after backward feature selection was observed for the natural logarithm of MTV combined with the maximum distance between the largest lesion and any other lesion (Dmaxbulk), the maximum difference in SUVpeak between 2 lesions (DSUVpeakpatient), and the sum of distances between all lesions (Spreadpatient), yielding an improved CV-AUC of 0.77 ± 0.07 (95% CI, 0.62-0.89) (Figure 2). The same radiomics features were retained after backward feature selection when adding MYC-R status to the model (natural logarithm of MTV, Dmaxbulk, DSUVpeakpatient, and Spreadpatient), which together with MYC-R status yielded comparable model performance (CV-AUC of 0.77 ± 0.07; 95% CI, 0.63-0.90; and lowest AIC). IPI was not retained in the combined model after backward feature selection, therefore, the combined model included the same features as the radiomics + MYC model thereby yielding the same CV-AUC and AIC. After the backward feature selection, the COO was not retained in the IPI + MYC and combined model. MTV was the most important radiomics feature in the radiomics model and the radiomics + MYC model, followed by Dmaxbulk (supplemental Table 4).
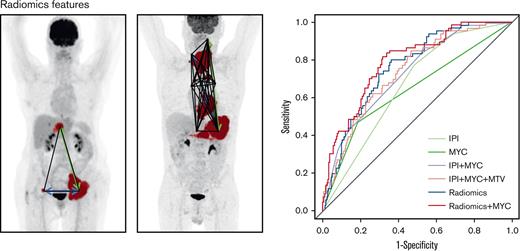
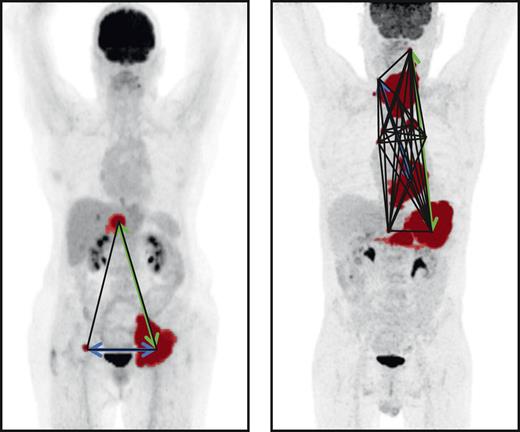
Maximum intensity projections of 2 patients. MTV is indicated in red, Dmaxbulk with a green arrow, DSUVpeakpatient with a blue arrow, and Spreadpatient with black lines.
Maximum intensity projections of 2 patients. MTV is indicated in red, Dmaxbulk with a green arrow, DSUVpeakpatient with a blue arrow, and Spreadpatient with black lines.
Diagnostic performance
Sensitivity (31.8%) and PPV (29.6%) were the lowest for the IPI model. The NPV was comparable for all models and always >82% (Table 4). The PPV increased by 10% when combining radiomics features and MYC-R status compared with the IPI + MYC model (40.4% vs 50.0%) and increased by 20% in comparison with the IPI model (29.6% vs 50.0%). PPV and NPV were highest in the radiomics + MYC model. However, the model that only included radiomics features had comparable diagnostic performance to the radiomics + MYC model. Nineteen patients with DH/TH were classified as low risk by our radiomics + MYC model (supplemental Table 5), of which 4 patients showed progression within 2 years. Thirty-eight patients with DH/TH were classified as low risk by the IPI model, of which 15 patients showed progression within 2 years.
Diagnostic measures of prediction models
| . | Sensitivity . | Specificity . | PPV . | NPV . |
|---|---|---|---|---|
| IPI | 31.8 (20.9-44.4) | 80.5 (75.2-85.2) | 29.6 (21.4-39.3) | 82.1 (79.4-84.6) |
| MYC | 34.9 (23.5-47.6) | 87.9 (83.3-91.7) | 42.6 (31.8-54.2) | 84.0 (81.4-86.3) |
| IPI + MYC | 60.6 (47.8-72.4) | 77.0 (71.4-82.0) | 40.4 (33.5-47.7) | 88.4 (84.9-91.2) |
| IPI + MYC + MTV | 40.9 (29.0-53.7) | 84.8 (79.8-89.0) | 40.9 (31.5-51.0) | 84.8 (82.0-87.3) |
| Radiomics | 48.5 (36.0-61.1) | 86.8 (82.0-90.7) | 48.5 (38.7-58.4) | 86.8 (83.8-89.3) |
| Radiomics + MYC | 50.0 (37.4-62.6) | 87.2 (82.4-91.0) | 50.0 (40.1-59.9) | 87.2 (84.2-89.7) |
| . | Sensitivity . | Specificity . | PPV . | NPV . |
|---|---|---|---|---|
| IPI | 31.8 (20.9-44.4) | 80.5 (75.2-85.2) | 29.6 (21.4-39.3) | 82.1 (79.4-84.6) |
| MYC | 34.9 (23.5-47.6) | 87.9 (83.3-91.7) | 42.6 (31.8-54.2) | 84.0 (81.4-86.3) |
| IPI + MYC | 60.6 (47.8-72.4) | 77.0 (71.4-82.0) | 40.4 (33.5-47.7) | 88.4 (84.9-91.2) |
| IPI + MYC + MTV | 40.9 (29.0-53.7) | 84.8 (79.8-89.0) | 40.9 (31.5-51.0) | 84.8 (82.0-87.3) |
| Radiomics | 48.5 (36.0-61.1) | 86.8 (82.0-90.7) | 48.5 (38.7-58.4) | 86.8 (83.8-89.3) |
| Radiomics + MYC | 50.0 (37.4-62.6) | 87.2 (82.4-91.0) | 50.0 (40.1-59.9) | 87.2 (84.2-89.7) |
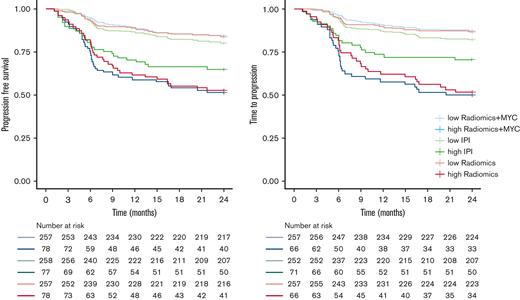
High-risk IPI patients had a 2-year TTP of 70.4% (95% CI, 60.6-81.9) (Table 5; Figure 3), a 2-year PFS of 64.9% (95% CI, 55.1-76.5) (Figure 3), and a 2-year OS of 68.8% (95% CI, 59.2-80.0) (supplemental Figure 1), compared with a much lower 2-year TTP of 50.0% (95% CI, 39.3-63.6) for the high-risk patients identified with the radiomics + MYC model. High-risk patients according to the radiomics + MYC model had a 2-year PFS of 50.6% (95% CI, 40.7-63.0) and a 2-year OS of 57.4% (95% CI, 45.6-72.2). Two-year TTP for high-risk patients identified with the radiomics model was 51.5% (95% CI, 40.8-65.1). Survival rates for other prediction models using 2-year PFS and 2-year OS as outcome parameters are presented in supplemental Table 6.
Survival rates of high-risk and low-risk patients according to prediction models
| . | TTP (95% CI) . |
|---|---|
| IPI | |
| Low | 95.2 (90.7-99.9) |
| Low-intermediate | 83.1 (74.4-92.7) |
| High-intermediate | 71.2 (63.0-80.4) |
| High∗ | 70.4 (60.6-81.9) |
| MYC | |
| MYC-WT | 85.7 (81.4-90.2) |
| MYC-SH | 66.7 (50.2-88.5) |
| DH/TH | 57.4 (45.6-72.2) |
| IPI +MYC† | |
| Low | 88.4 (84.3-92.7) |
| High | 59.6 (50.7-70.1) |
| IPI+MYC+MTV | |
| Low | 84.8 (80.5-89.3) |
| High | 59.1 (48.3-72.2) |
| Radiomics | |
| Low | 86.8 (82.7-91.0) |
| High | 51.5 (40.8-65.1) |
| Radiomics +MYC | |
| Low | 87.2 (83.2-91.3) |
| High | 50.0 (39.3-63.6) |
| . | TTP (95% CI) . |
|---|---|
| IPI | |
| Low | 95.2 (90.7-99.9) |
| Low-intermediate | 83.1 (74.4-92.7) |
| High-intermediate | 71.2 (63.0-80.4) |
| High∗ | 70.4 (60.6-81.9) |
| MYC | |
| MYC-WT | 85.7 (81.4-90.2) |
| MYC-SH | 66.7 (50.2-88.5) |
| DH/TH | 57.4 (45.6-72.2) |
| IPI +MYC† | |
| Low | 88.4 (84.3-92.7) |
| High | 59.6 (50.7-70.1) |
| IPI+MYC+MTV | |
| Low | 84.8 (80.5-89.3) |
| High | 59.1 (48.3-72.2) |
| Radiomics | |
| Low | 86.8 (82.7-91.0) |
| High | 51.5 (40.8-65.1) |
| Radiomics +MYC | |
| Low | 87.2 (83.2-91.3) |
| High | 50.0 (39.3-63.6) |
n = 72 patients as high risk.
n= 99 patients as high risk, all other models included n = 66 patients as high risk.
Kaplan-Meier survival curves of high- and low-risk groups for 2-year TTP and 2-year PFS.
Kaplan-Meier survival curves of high- and low-risk groups for 2-year TTP and 2-year PFS.
Discussion
Our study shows that baseline PET radiomics features can identify high-risk patients with aggressive B-cell lymphoma, and that a prediction model based only on radiomics features can select high-risk patients more accurately than a model that combines IPI and MYC-R status. Moreover, adding dissemination and intensity features to MTV improves the predictive value and diagnostic accuracy of our prediction model. Better selection of high-risk patients is clinically relevant because it offers these patients a timely switch to innovative new treatment options, as well as including these patients in clinical trials offering them chimeric antigen receptor T-cell or bispecific monoclonal therapy.
Our results show that MTV values of patients with MYC-SH DLBCL are comparable to those with MYC-WT DLBCL. In addition, SUV metrics are significantly lower too. Patients with DH/TH had higher MTVs, higher SUVs, and larger dissemination at baseline than patients with MYC-SH DLBCL and MYC-WT DLBCL. To the best of our knowledge, no other studies compared either MTV, SUV, or dissemination features stratified for MYC and BCL2 and/or BCL6 rearrangement status in aggressive B-cell lymphoma. The higher intensity values and MTV of patients with DH/TH could be explained by the different pathological behavior (higher cell metabolism). We previously showed that dissemination expressed as distance does not correlate with MTV.16 This current study shows that SUV metrics and dissemination in volume and intensity, respectively, do not correlate with MTV and are independent predictors of the outcome.
Several studies have shown that radiomics features extracted from baseline 18F-FDG PET scans are predictive of outcome in DLBCL16,17,20,34 and the independent predictive value of both MTV and dissemination is expressed as distance.16,28,35 In this study, we showed that both features were retained in the prediction model when adding MYC-R status and adding new dissemination features. Cottereau et al36 showed that double expressor and patients with MYC-positive DLBCL using complementary DNA–mediated annealing, selection, ligation, and extension technology had an increased risk of relapse or progression, regardless of their MTV. Complementary DNA–mediated annealing, selection, ligation, and extension provides an expression profile, therefore, this signature does not capture MYC DH/TH translocation status. To the best of our knowledge, no studies incorporated both molecular genotypes and radiomics features extracted from 18F-FDG PET/CT scans.
Our model that only included radiomics features showed almost identical model performance compared with the model that included radiomics features and MYC-R related to the CV-AUC, standard deviation, 95% CI, and the AIC index. Moreover, the PPV and progression rates of the high-risk group identified with only radiomics features were very comparable to the PPV and progression and PFS rates of the model that combined radiomics features and MYC-R status. However, it should be noted that the model that included radiomics features and MYC-R status showed a steeper initial decline in the high-risk group using 2-year TTP and is superior in identifying primary refractory patients. For OS, 2-year survival rates dropped by an additional 5% when MYC-R status was added. Both models show the high predictive value of baseline radiomics features in patients with aggressive B-cell lymphoma. Furthermore, the survival rate of high-risk patients identified by the radiomics + MYC model dropped by 20% using 2-year TTP, by 14.3% using 2-year PFS, and by 11.3% using 2-year OS as an outcome parameter compared with high-risk IPI patients. Furthermore, our model that included both MYC-R status and radiomics features correctly identified 15 patients with DH/TH (25% of the population) as low risk. Moreover, compared with the IPI and MYC-R status, another advantage of our radiomics + MYC prediction model is the fact that it allows individual risk prediction per patient. Individual patients with poorer outcomes in need of treatment escalation can be identified and the optimal cutoff of the model can be selected based on the clinical context.
This study showed that when adding radiomics features extracted from baseline 18F-FDG PET/CT scans to MYC-R status, the selection of high-risk patients became more accurate with a higher PPV and CV-AUC. These data are important to place in the context of modifications to frontline therapy. Several approaches are being explored to improve outcomes in high-risk subgroups. However, so far, no alternative frontline therapy has improved survival rates.24,25,37,38 Widespread adoption of alternative treatments for biologically high-risk patients can increase treatment toxicity and health care expenditure. Therefore, a high PPV or upfront diagnosis is important to avoid unnecessary intensive therapy in a subset of patients with relatively favorable outcomes.39,40
MYC-R status is not always available before treatment and patients frequently receive 1 cycle of R-CHOP before they shift to intensified chemotherapy. The radiomics features that were extracted in this study can be calculated easily from the baseline PET without treatment delay allowing rapid stratification of patients starting with frontline therapy. Multiple vendors of PET/CT systems have implemented algorithms to calculate MTV in their clinical software. If the workflow is optimized, MTV can be calculated in 3 to 6 minutes, with complex cases taking up to 10 to 20 minutes.41 Dissemination features are currently only extracted in research settings. However, these features are also relatively simple to calculate and relatively insensitive to differences in acquisition, reconstruction, and delineation methods.42,43 Therefore, the implementation of the calculation of these features should be feasible in a reproducible manner in most clinical PET centers. We expect and hope that vendors implement the calculation of radiomics features in their software in the foreseeable future once more evidence of their clinical value becomes apparent. In the meantime, our image analysis tool, ACCURATE, is provided as an open tool to facilitate research.
To the best of our knowledge, our study is the first to incorporate both quantitative PET metrics and genetic markers with relatively large subsets of patients available with MYC-SH and DH/TH. The uniform analysis of baseline 18F-FDG PET/CT scans and uniform FISH analysis in this study resulted in high-quality data. Nevertheless, several limitations of the current study should be noted. First, due to the retrospective nature of this study, treatment subgroups were heterogeneous. Patients with MYC-WT DLBCL were almost exclusively treated with R-CHOP regimens; whereas, one-third of the MYC-SH patients and 25% of patients with DH/TH received R-CHOP–based treatment. However, as we sampled patients for each fold based on MYC-R, only 5% of the patients in each fold were not treated according to current standards. Consequently, the treatment effect in our study was likely limited; yet an effect cannot be precluded. Moreover, not all patients in the prospective clinical trials had a baseline PET/CT scan available and/or sufficient biopsy material to assess MYC, BCL2, and BCL6 rearrangement status. As a result, not all enrolled patients could be included in our analysis, possibly resulting in patient selection bias. Finally, our results regarding differences in radiomics features between patients with MYC-SH DLBCL and DH/TH could be suffering from relatively small sample sizes and should be validated in a larger cohort.
We chose to increase the internal validity of our model instead of leaving out 1 of the 3 trials or selecting a holdout set a priori. Even though the sample size of this study is large for a PET study, from a statistical perspective it was rather small. Small internal or external data sets suffer from large uncertainties when predicting outcomes, therefore, appropriate internal validation approaches using the full training data set are preferred over a small external data set or a holdout set, which is essentially the same as onefold in the cross-validation as the patient characteristics of the train and test set are identical if you leave out 1 part of the data.33,44-46
In summary, robust and easy-to-use biomarkers for the early identification of poor responders in this patient group are essential. We showed that radiomics features extracted from baseline 18F-FDG PET/CT scans accurately predict outcomes in aggressive B-cell lymphoma and an integrative approach with both molecular data and quantitative PET metrics could improve the prediction of prognosis and guide the choice of therapies.
Acknowledgments
This work was financially supported by the Dutch Cancer Society (VU 2018–11648). The PETAL trial was supported by grants from the Deutsche Krebshilfe (107592 and 110515).
Authorship
Contribution: J.J.E., G.J.C.Z., Y.W.S.J., O.S.H., H.C.W.d.V., R.B., and J.M.Z. contributed to the concept and design of this study; P.J.L., M.E.D.C., U.D., and A.H. were responsible for acquiring the data; J.J.E., S.E.W., S.P., C.H., and G.J.C.Z. performed positron emission tomography/computed tomography analyses; D.d.J., B.Y., M.M., J.R., and W.K. performed fluorescence in situ hybridization analyses; and all authors contributed to the interpretation of the data and critically reviewed and approved the manuscript.
Conflict-of-interest disclosure: M.E.D.C. received financial support for clinical trials from Celgene, Bristol-Myers Squibb, and Gilead. J.M.Z. achieved financial support for clinical trials from Roche, Gilead, and Takeda. The remaining authors declare no competing financial interests.
A complete list of the members of the PETRA consortium appears in “Appendix.”
Correspondence: Jakoba Johanna Eertink, Department of Hematology, Amsterdam University Medical Center (UMC), Vrije Universiteit Amsterdam, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; e-mail: j.eertink@amsterdamumc.nl.
Appendix
The members of the PETRA consortium are Josée Zijlstra, Riekie de Vet, Otto Hoekstra, Ronald Boellaard, Corinne Eertink, Coreline Burggraaff, Sanne Wiegers, Simone Pieplenbosch, Maria Ferrandez Ferrandez, Sandeep Golla, Ben Zwezerijnen, Annelies Bes, Martijn Heijmans, Yvonne Jauw, Elly Lugtenburg, Martine Chamuleau, Sally Barrington, George Mikhaeel, Ulrich Dührsen, Andreas Hüttmann, Lars Kurch, Christine Hanoun, Emanuele Zucca, Luca Ceriani, Robert Carr, Tamás Györke, Sándor Czibor, Stefano Fanti, Lale Kostakoglu, Annika Loft, Martin Hutchings, and Sze Ting Lee.
References
Author notes
Deidentified individual participant data can be requested through the PETRA consortium (https://petralymphoma.org). Contact and more information can be obtained either via the contact form or the email address of the consortium (petra@amsterdamumc.nl).
The full-text version of this article contains a data supplement.