Key Points
The real-life incidence rates of bleeding in older patients with VTE are high and are associated with a substantial disease burden.
Active cancer, low physical activity, and a high risk of falls are associated with an increased bleeding risk.
Abstract
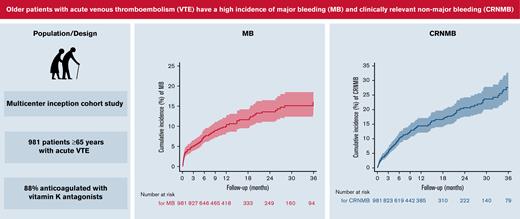
Older patients anticoagulated for venous thromboembolism (VTE) have an increased risk of bleeding compared with younger patients. Little is known about the clinical impact of anticoagulation-related bleeding in this growing patient group. To prospectively assess the incidence, clinical impact, and predictors of bleeding in older patients anticoagulated for VTE, we analyzed 981 patients aged ≥65 years with acute VTE in a prospective multicenter cohort. Eight-eight percent were anticoagulated with vitamin K antagonists. Outcomes were the occurrence of major bleeding (MB) or clinically relevant nonmajor bleeding (CRNMB) event during the initial anticoagulation period up to 36 months. We described the incidence and clinical impact of bleeding and examined the association between risk factors and time to a first bleeding using competing risk regression; 100 MB and 125 CRNMB events occurred during follow-up. The incidence of MB and CRNMB was 8.5 (95% confidence interval [CI], 7.0-10.4) and 13.4 events (95% CI, 11.4-15.7) per 100 patient-years, respectively. In patients with MB, 79% required hospitalization, 18% required surgical intervention, and 19% required permanent discontinuation of anticoagulation; 15% of MB were intracranial and 6% were fatal. After adjustment, active cancer (subhazard ratio [SHR], 1.81; 95% CI, 1.12-2.93) and low physical activity (SHR, 1.88; 95% CI, 1.19-2.98) were associated with MB and high risk of falls with CRNMB (SHR, 2.04; 95% CI, 1.39-3.00). Older patients anticoagulated for VTE had a high incidence of MB and CRNMB, and these bleeding episodes caused a great burden of disease. Physicians should carefully weigh the risks/benefits of extended anticoagulation in the older population with VTE.
Introduction
Anticoagulation effectively reduces the risk of recurrent venous thromboembolism (VTE) by 80% to 90%1 but carries a risk of major bleeding (MB) of 1% to 3% per year.2,3 The most serious manifestations of anticoagulation-related bleeding include fatal and intracranial bleeding, which accounts for 13.4% and 8.7% of MBs, respectively.3 Anticoagulation-related MB also is associated with substantial health care costs4 and a reduction of quality of life.5,6 Prior studies have shown that the incidence of anticoagulation-related bleeding risk is not linear, with a higher risk of MB in the early phase than during subsequent anticoagulation periods.3,7
Anticoagulated older patients have a 2-fold greater risk of MB and intracranial and fatal bleeding than younger patients,8-11 possibly due to comorbid diseases,12 comedications (eg, platelet inhibitors),13,14 drug interactions,15,16 and age-related conditions, such as cerebral amyloid microangiopathy and leukoaraiosis.17,18 Although >50% of patients with VTE are aged ≥65 years, the older population is underrepresented in clinical VTE trials,19,20 and little is known about the effect of predisposing factors on the risk and the clinical impact of bleeding in older patients with VTE. Earlier studies about anticoagulation-related bleeding in older patients with VTE were limited by the use of administrative data,11,21,22 retrospective design,21,22 focus on subgroups (ie, very old or frail patients),9,21,23-27 or patients with atrial fibrillation28,29 or who had a short follow-up period.9,27 Moreover, evidence from patients anticoagulated for atrial fibrillation may not be extrapolable to patients with VTE who may have a higher risk of bleeding.29 To fill this gap of knowledge, we described the incidence and clinical impact of bleeding in a prospective cohort of older patients anticoagulated for acute VTE and explored the association among predisposing factors, anticoagulation quality, and the occurrence of bleeding.
Methods
Cohort sample
The study was conducted between September 2009 and December 2013 as part of the SWIss venous Thromboembolism COhort study 65+ (SWITCO65+), a prospective multicenter inception cohort study of consecutive in- and outpatients aged ≥65 years with acute symptomatic objectively confirmed deep vein thrombosis and/or pulmonary embolism. The patients were enrolled at all 5 Swiss university and 4 high-volume nonuniversity hospitals and followed up to assess long-term clinical outcomes. We excluded patients with a thrombosis at a different site than the lower limb (eg, mesenteric vein thrombosis) and patients with a catheter-related thrombosis because the natural history of these conditions might be different.30 Other exclusion criteria were an insufficient ability to speak German or French, an inability to provide informed consent (eg, due to severe dementia), follow-up not possible (eg, terminally ill patients), and a prior enrollment in the cohort. For the sake of this analysis, we included only patients who received at least 1 dose of an anticoagulant. Because the study was of purely observational nature, management decisions with respect to anticoagulation and complications were left to the discretion of the treating physicians. Anticoagulant treatment in the outpatient setting was managed by primary care physicians. A full description of study methods, including follow-up procedures, has been reported previously.31 The central ethics committee in Bern and the ethics committee of northeastern Switzerland approved the study, and all patients provided written informed consent.
Baseline data collection
For all enrolled patients, trained study nurses prospectively collected baseline patient characteristics, including demographic information, location of VTE, and known risk factors for bleeding, including arterial hypertension, cardiac disease, cerebrovascular disease, diabetes mellitus, chronic renal disease, chronic liver disease, active malignancy, history of MB, recent surgery, low physical activity level, high risk of falls, anemia, thrombocytopenia, and concomitant antiplatelet or nonsteroidal antiinflammatory drug therapy.29,32-39 We further ascertained VTE-related treatments (parenteral and oral anticoagulants, thrombolysis, and inferior vena cava filter insertion). All collected data were recorded on standardized forms.
Study outcomes and follow-up
The outcomes were the occurrence of an MB or clinically relevant nonmajor bleeding (CRNMB) event during the initial anticoagulation period up to 36 months. MB was defined as fatal bleeding, bleeding at a critical site (intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardical, intramuscular with compartment syndrome), bleeding contributing to a fall in hemoglobin of ≥20 g/L, or bleeding leading to transfusion of ≥2 units of whole blood or red cells.40 CRNMB was defined as bleeding that did not meet the criteria for MB but required physician consultation or evaluation at an emergency department.32
Follow-up included 1 telephone interview and 2 surveillance face-to-face evaluations during the first year of study participation and then semiannual contacts, alternating between face-to-face evaluations (clinic or home visits) and telephone calls as well as periodic reviews of the patient’s hospital chart. If bleeding or death occurred, the information was complemented by reviewing hospital discharge letters, medical charts, and autopsy reports and interviewing patients’ primary care physicians and/or family members to obtain information about the date and location of bleeding as well as the main therapeutic processes of care performed (transfusions, administration of fresh frozen plasma or prothrombin complex concentrates, need for surgical hemostasis, and insertion of a vena cava inferior filter), including whether anticoagulation treatment was definitely discontinued (ie, >14 days) after the bleed. For patients in whom bleeding occurred in the outpatient setting, the site of management (hospital admission vs outpatient) was also recorded.
Based on all available information, a committee of three blinded clinical experts adjudicated all bleeding events and determined the cause of death. Death was considered bleeding-related if it followed an intracranial hemorrhage or a bleeding episode leading to hemodynamic deterioration. Final classifications were made based on the full consensus of this committee. In patients receiving vitamin K antagonists (VKAs), study nurses also recorded international normalized ratio (INR) values throughout the initial anticoagulation period.
Statistical analyses
We calculated the overall incidence rates of MB and CRNMB (events per 100 patient-years of observation) as well as the 36-month cumulative incidences of MB and CRNMB during initial anticoagulation using Kaplan-Meier analysis. The time at risk of bleeding was defined as the interval between initiation of anticoagulation and a first MB or CRNMB, cessation of anticoagulation, loss to follow-up, or death from a nonbleeding-related cause. In patients with bleeding, we described the bleeding location, main management decisions (processes of care, anticoagulation stop), and outcomes (hospitalization, death).
We explored the association between previously described bleeding risk factors29,32-39 and the time to a first MB and first CRNMB using 2 separate competing-risk regression models according to Fine and Gray,41 accounting for nonhemorrhagic death in the MB model, overall death in the CRNMB model, and the definite discontinuation of anticoagulation (ie, >14 days) in both models as competing events. The strength of the association between predictors and the occurrence of bleeding was expressed as a subhazard ratio (SHR) with corresponding 95% confidence interval (CI). Missing values were assumed to be normal. In patients treated with VKAs, we used analysis of variance to compare the mean percentage of time spent in a given INR range (<2.0, 2.0-3.0, >3.0),42 excluding the first 7 days of treatment. A 2-sided P value <.05 was considered statistically significant. All analyses were performed using Stata 16 (Stat Corporation, College Station, TX).
Results
Study sample
Of the 1003 patients enrolled in the cohort, we excluded 12 who withdrew consent/refused the use of their data, 9 who did not receive any anticoagulation therapy, and 1 who bled before starting anticoagulation, leaving a final study sample of 981 patients. The median age was 75 years (interquartile range [IQR], 69-81 years), and 53% were men. The median duration of initial anticoagulation was 9 months (IQR, 5.2-24.4) and varied by type of VTE (7.0 months [IQR, 3.4-22.1] for provoked, 12.7 [IQR, 6.1-29.7] for unprovoked, and 6.1 [IQR, 3.0-16.3] for active cancer-related VTE). A total of 861 patients (88%) were treated with VKAs, and 102 patients (10.4%) died.
Overall, 100 patients (10%) experienced an MB event and 125 (13%) experienced a CRNMB event during anticoagulation. Patients with MB and CRNMB were older than those without bleeding, with a median age of 77, 78, and 74 years, respectively (Table 1). Patients who bled were more likely to have pulmonary embolism, cardiac disease, low physical activity, high risk of falls, and treatment with antiplatelet drugs/nonsteroidal antiinflammatory drugs than those without bleeding. More than half of the patients with MB were anemic at presentation, compared with 39% and 37% of patients who had CRNMB and no bleeding, respectively.
Baseline patient characteristics and treatments
| Characteristic/treatment . | All patients (N = 981) . | No bleeding (N = 756) . | Major bleeding (N = 100) . | Clinically relevant nonmajor bleeding (N = 125) . |
|---|---|---|---|---|
| n (%) or median (interquartile range)∗ . | ||||
| Age, y | 75 (69; 81) | 74 (69; 80) | 77 (70; 81) | 78 (71; 84) |
| Male sex | 524 (53) | 405 (54) | 51 (51) | 68 (54) |
| Localization of index VTE | ||||
| PE ± DVT | 682 (70) | 508 (67) | 77 (77) | 97 (78) |
| DVT only | 299 (30) | 248 (33) | 23 (23) | 28 (22) |
| Type of index VTE | ||||
| Provoked | 212 (22) | 160 (21) | 20 (20) | 32 (26) |
| Unprovoked† | 594 (61) | 466 (62) | 54 (54) | 74 (59) |
| Active cancer-related‡ | 175 (18) | 130 (17) | 26 (26) | 19 (15) |
| Arterial hypertension | 631 (64) | 483 (64) | 65 (65) | 83 (66) |
| Cardiac disease§ | 240 (24) | 167 (22) | 36 (36) | 37 (30) |
| Cerebrovascular disease‖ | 91 (9) | 65 (9) | 10 (10) | 16 (13) |
| Diabetes mellitus | 152 (15) | 114 (15) | 13 (13) | 25 (20) |
| Chronic renal disease¶ | 184 (19) | 133 (18) | 26 (26) | 25 (20) |
| Chronic liver disease# | 14 (1) | 10 (1) | 3 (3) | 1 (1) |
| History of major bleeding∗∗ | 95 (10) | 67 (9) | 11 (11) | 17 (14) |
| Recent surgery†† | 148 (15) | 113 (15) | 20 (20) | 15 (12) |
| Low physical activity‡‡ | 360 (37) | 251 (33) | 53 (53) | 56 (45) |
| High risk of fallsa | 450 (46) | 316 (42) | 54 (54) | 80 (64) |
| Anemiab | 382 (39) | 280 (37) | 53 (53) | 49 (39) |
| Thrombocytopeniac | 139 (14) | 102 (13) | 13 (13) | 24 (19) |
| Antiplatelet/NSAID therapyd | 377 (38) | 266 (35) | 49 (49) | 62 (50) |
| AC prior to index VTE | 51 (5) | 36 (5) | 7 (7) | 8 (6) |
| Initial parenteral AC | ||||
| LMWH | 465 (47) | 364 (48) | 40 (40) | 61 (49) |
| Unfractionated heparin | 332 (34) | 247 (33) | 38 (38) | 47 (38) |
| Fondaparinux | 158 (16) | 126 (17) | 16 (16) | 16 (13) |
| Danaparoid | 1 (0) | 0 (0) | 1 (1) | 0 (0) |
| No parenteral AC | 25 (3) | 19 (3) | 5 (5) | 1 (1) |
| Initial VKA therapy | 861 (88) | 668 (88) | 81 (81) | 112 (90) |
| Thrombolysise | 30 (3) | 23 (3) | 4 (4) | 3 (2) |
| Inferior vena cava filter | 10 (1) | 8 (1) | 0 (0) | 2 (2) |
| Characteristic/treatment . | All patients (N = 981) . | No bleeding (N = 756) . | Major bleeding (N = 100) . | Clinically relevant nonmajor bleeding (N = 125) . |
|---|---|---|---|---|
| n (%) or median (interquartile range)∗ . | ||||
| Age, y | 75 (69; 81) | 74 (69; 80) | 77 (70; 81) | 78 (71; 84) |
| Male sex | 524 (53) | 405 (54) | 51 (51) | 68 (54) |
| Localization of index VTE | ||||
| PE ± DVT | 682 (70) | 508 (67) | 77 (77) | 97 (78) |
| DVT only | 299 (30) | 248 (33) | 23 (23) | 28 (22) |
| Type of index VTE | ||||
| Provoked | 212 (22) | 160 (21) | 20 (20) | 32 (26) |
| Unprovoked† | 594 (61) | 466 (62) | 54 (54) | 74 (59) |
| Active cancer-related‡ | 175 (18) | 130 (17) | 26 (26) | 19 (15) |
| Arterial hypertension | 631 (64) | 483 (64) | 65 (65) | 83 (66) |
| Cardiac disease§ | 240 (24) | 167 (22) | 36 (36) | 37 (30) |
| Cerebrovascular disease‖ | 91 (9) | 65 (9) | 10 (10) | 16 (13) |
| Diabetes mellitus | 152 (15) | 114 (15) | 13 (13) | 25 (20) |
| Chronic renal disease¶ | 184 (19) | 133 (18) | 26 (26) | 25 (20) |
| Chronic liver disease# | 14 (1) | 10 (1) | 3 (3) | 1 (1) |
| History of major bleeding∗∗ | 95 (10) | 67 (9) | 11 (11) | 17 (14) |
| Recent surgery†† | 148 (15) | 113 (15) | 20 (20) | 15 (12) |
| Low physical activity‡‡ | 360 (37) | 251 (33) | 53 (53) | 56 (45) |
| High risk of fallsa | 450 (46) | 316 (42) | 54 (54) | 80 (64) |
| Anemiab | 382 (39) | 280 (37) | 53 (53) | 49 (39) |
| Thrombocytopeniac | 139 (14) | 102 (13) | 13 (13) | 24 (19) |
| Antiplatelet/NSAID therapyd | 377 (38) | 266 (35) | 49 (49) | 62 (50) |
| AC prior to index VTE | 51 (5) | 36 (5) | 7 (7) | 8 (6) |
| Initial parenteral AC | ||||
| LMWH | 465 (47) | 364 (48) | 40 (40) | 61 (49) |
| Unfractionated heparin | 332 (34) | 247 (33) | 38 (38) | 47 (38) |
| Fondaparinux | 158 (16) | 126 (17) | 16 (16) | 16 (13) |
| Danaparoid | 1 (0) | 0 (0) | 1 (1) | 0 (0) |
| No parenteral AC | 25 (3) | 19 (3) | 5 (5) | 1 (1) |
| Initial VKA therapy | 861 (88) | 668 (88) | 81 (81) | 112 (90) |
| Thrombolysise | 30 (3) | 23 (3) | 4 (4) | 3 (2) |
| Inferior vena cava filter | 10 (1) | 8 (1) | 0 (0) | 2 (2) |
AC, anticoagulant; DVT, deep vein thrombosis; LMWH, low-molecular-weight heparin; NSAID, nonsteroidal antiinflammatory drug; PE, pulmonary embolism.
Patients with both major and clinically relevant nonmajor bleeding are shown in the major bleeding category.
Values were missing for history of major bleeding (0.1%), low physical activity (0.3%), high risk of falls 0.2%), anemia (6.3%), and thrombocytopenia (6.3%).
Absence of major surgery, estrogen therapy, immobilization, or active cancer during the last 3 months before the index VTE.
VTE in a patient with solid or hematologic cancer requiring chemotherapy, radiotherapy, surgery, or palliative care during last 3 months.
Systolic or diastolic heart failure, left or right heart failure, forward or backward heart failure, left ventricular ejection fraction <40%, acute heart failure during the last 3 months, a myocardial infarction with or without ST elevation during the last 3 months, or history of coronary heart disease.
Ischemic or hemorrhagic stroke with hemiparesis, hemiplegia, or paraplegia.
Diabetic or hypertensive nephropathy, chronic glomerulonephritis or interstitial nephritis, myeloma-related nephropathy, or cystic kidney disease.
Liver cirrhosis, chronic hepatitis, chronic liver failure, or hemochromatosis.
Any bleeding that led to a hospital stay or transfusions.
Surgery requiring general or spinal anesthesia during the last 3 months.
Mostly lying/sitting activity or avoidance to climb stairs/carry light weight.
Self-reported fall during the previous year or any problem with gait, balance, or mobility.
Serum hemoglobin <13 g/dL in men or <12 g/dL in women.
Platelet count <150 g/L.
Aspirin, clopidogrel, prasugrel, aspirin/dipyridamole, or nonsteroidal antiinflammatory drugs.
Catheter-directed or systemic thrombolysis.
Incidence and clinical impact of bleeding
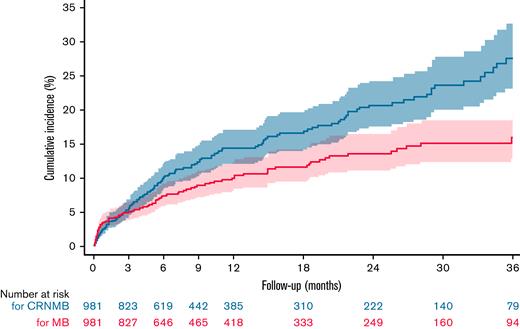
The overall incidence rates of MB and CRNMB were 8.5 (95% CI, 7.0-10.4) and 13.4 (95% CI, 11.4-15.7) events per 100 patient-years, respectively. The incidence rate of intracranial bleeding was 1.3 (95% CI, 0.8-2.1), and the rate of fatal bleeding was 0.6 (95% CI, 0.3-1.2) events per 100 patient-years. Bleeding rates were highest during early anticoagulation and decreased over time (Table 2). The 36-month cumulative incidence of MB and CRNMB was 16.0% (95% CI, 12.8% to 19.9%) and 27.6% (95% CI, 23.1% to 32.8%), respectively (Figure 1). Almost half of MB (47%) and a third of CRNMB (34%) episodes occurred during the initial 3 months, and 20% of bleeds were fall-related.
Bleeding incidence rates during anticoagulant therapy
| . | No. of patients . | Bleeds per 100 patient-years (95% CI) . |
|---|---|---|
| Major bleeding, mo | ||
| 0-3 | 981 | 21.4 (16.1-28.4) |
| 0-1 | 981 | 44.9 (32.2-62.5) |
| 1-3 | 910 | 8.9 (5.2-15.3) |
| 3-36 | 827 | 5.4 (4.1-7.1) |
| 3-12 | 827 | 7.5 (5.3-10.6) |
| 12-36 | 418 | 3.7 (2.4-5.8) |
| Clinically relevant nonmajor bleeding, mo | ||
| 0-3 | 981 | 22.3 (16.9-29.4) |
| 0-1 | 981 | 33.2 (22.6-48.7) |
| 1-3 | 913 | 16.5 (11.0-24.5) |
| 3-36 | 823 | 11.0 (9.0-13.5) |
| 3-12 | 823 | 14.8 (11.5-19.0) |
| 12-36 | 385 | 7.8 (5.6-10.8) |
| . | No. of patients . | Bleeds per 100 patient-years (95% CI) . |
|---|---|---|
| Major bleeding, mo | ||
| 0-3 | 981 | 21.4 (16.1-28.4) |
| 0-1 | 981 | 44.9 (32.2-62.5) |
| 1-3 | 910 | 8.9 (5.2-15.3) |
| 3-36 | 827 | 5.4 (4.1-7.1) |
| 3-12 | 827 | 7.5 (5.3-10.6) |
| 12-36 | 418 | 3.7 (2.4-5.8) |
| Clinically relevant nonmajor bleeding, mo | ||
| 0-3 | 981 | 22.3 (16.9-29.4) |
| 0-1 | 981 | 33.2 (22.6-48.7) |
| 1-3 | 913 | 16.5 (11.0-24.5) |
| 3-36 | 823 | 11.0 (9.0-13.5) |
| 3-12 | 823 | 14.8 (11.5-19.0) |
| 12-36 | 385 | 7.8 (5.6-10.8) |
Kaplan-Meier estimates for CRNMB and MB during anticoagulant therapy. The 36-month cumulative incidence of CRNMB was 27.6% (95% CI, 23.1% to 32.8%). The 36-month cumulative incidence of MB was 16.0% (95% CI, 12.8% to 19.9%). The dashed lines indicate the upper and lower boundary of the 95% CI.
Kaplan-Meier estimates for CRNMB and MB during anticoagulant therapy. The 36-month cumulative incidence of CRNMB was 27.6% (95% CI, 23.1% to 32.8%). The 36-month cumulative incidence of MB was 16.0% (95% CI, 12.8% to 19.9%). The dashed lines indicate the upper and lower boundary of the 95% CI.
MB was most often gastrointestinal (34%), whereas most patients with CRNMB had (sub)cutaneous bleeds (41%) (Table 3). Fifteen percent of MB events were intracranial. Approximately half of the patients with MB received ≥2 units of blood, and 12% were treated with fresh frozen plasma or prothrombin complex concentrates (Table 3). Eighteen percent of patients with MB (18%) and CRNMB (11%) underwent surgical hemostasis. Anticoagulants were definitely discontinued in 19% of patients with MB and in 6% of those with CRNMB. When bleeding occurred in the outpatient setting, 79% of patients with MB and 15% of those with CRNMB were hospitalized (Table 3). Overall, 6% of MB episodes were fatal (4 intracranial, 1 retroperitoneal, and 1 intramuscular bleeds).
Clinical impact of bleeding
| Clinical impact . | Major bleeding, n/N (%) . | Clinically relevant nonmajor bleeding . |
|---|---|---|
| Location∗ | ||
| Intracranial | 15/100 (15) | N/A |
| Intraspinal | 1/100 (1) | N/A |
| Intraocular | 2/100 (2) | N/A |
| Retroperitoneal | 4/100 (4) | N/A |
| Intraarticular | 4/100 (4) | N/A |
| Intramuscular with compartment syndrome | 1/100 (1) | N/A |
| Intramuscular | 16/100 (16) | 5/125 (4) |
| Gastrointestinal† | 34/100 (34) | 21/125 (17) |
| Cutaneous/subcutaneous | 15/100 (15) | 51/125 (41) |
| Hemoptysis | 1/100 (1) | 3/125 (2) |
| Urogenital | 11/100 (11) | 22/125 (18) |
| Epistaxis | 2/100 (2) | 21/125 (17) |
| Unknown | 1/100 (1) | 3/125 (2) |
| Management | ||
| Transfusion of ≥2 units of packed red blood cells | 47/100 (47) | N/A |
| Administration of fresh frozen plasma or prothrombin complex concentrates | 12/100 (12) | 2/125 (2) |
| Vena cava filter insertion | 7/100 (7) | 0/125 (0) |
| Surgical hemostasis | 18/100 (18) | 14/125 (11) |
| Definite discontinuation of anticoagulation‡ | 19/100 (19) | 8/125 (6) |
| Outcomes | ||
| Need for hospital admission§ | 26/33 (79) | 14/91 (15) |
| Bleeding-related death‖ | 6/100 (6) | N/A |
| Clinical impact . | Major bleeding, n/N (%) . | Clinically relevant nonmajor bleeding . |
|---|---|---|
| Location∗ | ||
| Intracranial | 15/100 (15) | N/A |
| Intraspinal | 1/100 (1) | N/A |
| Intraocular | 2/100 (2) | N/A |
| Retroperitoneal | 4/100 (4) | N/A |
| Intraarticular | 4/100 (4) | N/A |
| Intramuscular with compartment syndrome | 1/100 (1) | N/A |
| Intramuscular | 16/100 (16) | 5/125 (4) |
| Gastrointestinal† | 34/100 (34) | 21/125 (17) |
| Cutaneous/subcutaneous | 15/100 (15) | 51/125 (41) |
| Hemoptysis | 1/100 (1) | 3/125 (2) |
| Urogenital | 11/100 (11) | 22/125 (18) |
| Epistaxis | 2/100 (2) | 21/125 (17) |
| Unknown | 1/100 (1) | 3/125 (2) |
| Management | ||
| Transfusion of ≥2 units of packed red blood cells | 47/100 (47) | N/A |
| Administration of fresh frozen plasma or prothrombin complex concentrates | 12/100 (12) | 2/125 (2) |
| Vena cava filter insertion | 7/100 (7) | 0/125 (0) |
| Surgical hemostasis | 18/100 (18) | 14/125 (11) |
| Definite discontinuation of anticoagulation‡ | 19/100 (19) | 8/125 (6) |
| Outcomes | ||
| Need for hospital admission§ | 26/33 (79) | 14/91 (15) |
| Bleeding-related death‖ | 6/100 (6) | N/A |
N/A, not applicable.
Sum of percentages exceeds 100% because several bleeding locations were possible.
Hematemesis, melena, or hematochezia.
Anticoagulation was stopped at the time of bleeding and not resumed within 14 days.
Subgroup of 124 patients in whom bleeding occurred in the outpatient setting.
Overall, 4 intracranial, 1 retroperitoneal, and 1 intramuscular bleed were fatal.
Predictors of bleeding and anticoagulation quality
After adjustment, active cancer (SHR, 1.81; 95% CI, 1.12-2.93) and low physical activity (SHR, 1.88; 95% CI, 1.19-2.98) were significantly associated with MB (Table 4). A high risk of falls doubled the risk of CRNMB (SHR, 2.04; 95% CI, 1.39-3.00).
Multivariate analysis for risk factors of first bleeding
| Predictors . | Major bleeding . | Clinically relevant nonmajor bleeding . | ||
|---|---|---|---|---|
| Adjusted SHR∗ (95% CI) . | P . | Adjusted SHR∗ (95% CI) . | P . | |
| Patient age, per year | 0.99 (0.96-1.02) | .639 | 1.02 (0.99-1.04) | .155 |
| Male sex | 0.92 (0.59-1.44) | .710 | 1.17 (0.82-1.67) | .391 |
| Arterial hypertension | 0.78 (0.49-1.24) | .291 | 0.96 (0.67-1.37) | .824 |
| Cardiac disease | 1.51 (0.96-2.38) | .075 | 1.14 (0.76-1.70) | .532 |
| Cerebrovascular disease | 0.82 (0.40-1.65) | .575 | 0.91 (0.52-1.62) | .760 |
| Diabetes mellitus | 0.65 (0.34-1.25) | .199 | 1.10 (0.70-1.72) | .681 |
| Chronic renal disease | 1.30 (0.79-2.13) | .299 | 0.99 (0.65-1.51) | .968 |
| Chronic liver disease | 1.65 (0.46-5.87) | .439 | 0.92 (0.18-4.84) | .926 |
| Active cancer | 1.81 (1.12-2.93) | .016 | 1.51 (0.99-2.31) | .056 |
| History of major bleeding | 0.87 (0.45-1.68) | .671 | 1.15 (0.69-1.93) | .588 |
| Recent surgery | 1.41 (0.82-2.45) | .215 | 0.94 (0.53-1.67) | .824 |
| Low physical activity | 1.88 (1.19-2.98) | .007 | 1.00 (0.70-1.44) | .990 |
| High risk of falls | 1.12 (0.71-1.76) | .629 | 2.04 (1.39-3.00) | <.001 |
| Anemia | 1.54 (0.99-2.41) | .056 | 1.23 (0.86-1.75) | .254 |
| Thrombocytopenia | 0.90 (0.49-1.65) | .740 | 1.38 (0.89-2.14) | .156 |
| Concomitant antiplatelet/NSAID therapy | 1.35 (0.85-2.16) | .203 | 1.43 (0.99-2.05) | .055 |
| Predictors . | Major bleeding . | Clinically relevant nonmajor bleeding . | ||
|---|---|---|---|---|
| Adjusted SHR∗ (95% CI) . | P . | Adjusted SHR∗ (95% CI) . | P . | |
| Patient age, per year | 0.99 (0.96-1.02) | .639 | 1.02 (0.99-1.04) | .155 |
| Male sex | 0.92 (0.59-1.44) | .710 | 1.17 (0.82-1.67) | .391 |
| Arterial hypertension | 0.78 (0.49-1.24) | .291 | 0.96 (0.67-1.37) | .824 |
| Cardiac disease | 1.51 (0.96-2.38) | .075 | 1.14 (0.76-1.70) | .532 |
| Cerebrovascular disease | 0.82 (0.40-1.65) | .575 | 0.91 (0.52-1.62) | .760 |
| Diabetes mellitus | 0.65 (0.34-1.25) | .199 | 1.10 (0.70-1.72) | .681 |
| Chronic renal disease | 1.30 (0.79-2.13) | .299 | 0.99 (0.65-1.51) | .968 |
| Chronic liver disease | 1.65 (0.46-5.87) | .439 | 0.92 (0.18-4.84) | .926 |
| Active cancer | 1.81 (1.12-2.93) | .016 | 1.51 (0.99-2.31) | .056 |
| History of major bleeding | 0.87 (0.45-1.68) | .671 | 1.15 (0.69-1.93) | .588 |
| Recent surgery | 1.41 (0.82-2.45) | .215 | 0.94 (0.53-1.67) | .824 |
| Low physical activity | 1.88 (1.19-2.98) | .007 | 1.00 (0.70-1.44) | .990 |
| High risk of falls | 1.12 (0.71-1.76) | .629 | 2.04 (1.39-3.00) | <.001 |
| Anemia | 1.54 (0.99-2.41) | .056 | 1.23 (0.86-1.75) | .254 |
| Thrombocytopenia | 0.90 (0.49-1.65) | .740 | 1.38 (0.89-2.14) | .156 |
| Concomitant antiplatelet/NSAID therapy | 1.35 (0.85-2.16) | .203 | 1.43 (0.99-2.05) | .055 |
Adjustments were done for all other variables and competing risk of death.
In the 837 patients who received VKAs and for whom INR values were available, the mean percentage of time in the therapeutic INR range (2.0-3.0) was 62% for the overall cohort. Patients without bleeding spent slightly more time in the therapeutic INR range (63%) than patients with MB (55%) and CRNMB (60%) (Table 5). Patients with CRNMB spent slightly more time in a supratherapeutic INR (>3.0) range (18%) than those with MB (16%) and without bleeding (14%).
Anticoagulation quality and bleeding events
| Anticoagulation quality . | No bleeding . | Major bleeding . | Clinically relevant nonmajor bleeding . | P . |
|---|---|---|---|---|
| Mean percent (SD) . | ||||
| Time in a given INR range | ||||
| <2.0 | 23 (22) | 29 (22) | 21 (19) | .052 |
| 2.0-3.0 | 63 (23) | 55 (23) | 60 (21) | .017 |
| >3.0 | 14 (17) | 16 (17) | 18 (16) | .038 |
| Anticoagulation quality . | No bleeding . | Major bleeding . | Clinically relevant nonmajor bleeding . | P . |
|---|---|---|---|---|
| Mean percent (SD) . | ||||
| Time in a given INR range | ||||
| <2.0 | 23 (22) | 29 (22) | 21 (19) | .052 |
| 2.0-3.0 | 63 (23) | 55 (23) | 60 (21) | .017 |
| >3.0 | 14 (17) | 16 (17) | 18 (16) | .038 |
SD, standard deviation.
We could obtain an INR value in 146 of 225 patients (65%) at the time of bleeding. Overall, a substantial proportion of patients with MB (44%) and CRNMB (43%) was overanticoagulated (INR >3.0) at the time of bleeding.
Discussion
In this prospective multicenter cohort study of older patients with acute VTE, we found a high cumulative incidence of both MB and CRNMB. The bleeding incidence rate was highest in the first 30 days after the index VTE and declined over time. In patients with MB, most required hospital admission and a substantial proportion had intracranial or fatal bleeding. Thus, bleeding complications represent a substantial clinical and economic burden of disease in older patients with VTE.
We found a high 36-month cumulative incidence of MB of 16%, with almost half of MB occurring in the initial phase of anticoagulation. Our results are consistent with the findings from 2 community-based North American studies of patients with VTE aged ≥65 years showing a 3-year cumulative MB incidence of 10.6% and 16.9%, respectively.11,22 In other studies of older patients with VTE, the incidence rates of MB were substantially lower than in ours (0.8-2.4 vs 8.5 events per 100 patient-years).23,24,26 Potential reasons include the enrollment of lower-risk patients,24,26 the use of a more restrictive definition of MB,23 and the management of anticoagulation by specialized clinics.26 In our study, anticoagulation was managed by primary care physicians, and the anticoagulation quality was slightly lower than in older patients managed by specialized anticoagulation and thrombosis clinics (time spent in the INR therapeutic range of 62% vs 60% to 74%).26,28 Finally, the median initial anticoagulation duration for provoked VTE was 7 months, indicating that many patients may have received a longer anticoagulant treatment than recommended by guidelines (3 months) and thus may have been unnecessarily exposed to bleeding complications.43,44
The MB rates were much higher in our cohort of older patients than in a meta-analysis of clinical trials of patients who received VKAs for VTE,3 which showed an incidence rate of MB of 2.1 events per 100 patient-years during the first 3 months and a rate of 2.7 events per 100 patient-years thereafter. This finding is hardly a surprise because older patients who have an increased bleeding risk are often excluded from randomized anticoagulation trials.19,20
Our results suggest that MB often has major clinical consequences in older patients with VTE. Not only were 4 of 5 outpatients with MB hospitalized but also a substantial proportion of patients suffered intracranial (15%) or fatal bleeding (6%), needed surgical hemostasis (18%), or had anticoagulation permanently stopped (19%). In a meta-analysis of clinical trials and cohort studies of younger patients anticoagulated for VTE, intracranial bleeding accounted for 8.7% of MB, and the case-fatality rate of MB was 13.4%.3 Although our results are consistent with findings that advanced age is associated with a 2-fold higher risk of intracranial bleeding,8,45 the low case-fatality of MB (6%) is more difficult to interpret. Overall, reported case-fatality of MB varies widely in older persons with VTE, ranging from 2% to 55%,7,9,23,26,28 and may be attributable to differing patient selection and the lack of a standardized definition of bleeding-related death. It is possible that our definition of death after an intracranial bleed or bleeding with hemodynamic deterioration was more restrictive than in other studies. Of note, MB was followed by permanent discontinuation of anticoagulation in 19% of cases, exposing patients to the risk of recurrent VTE.
Two factors, active cancer and a low physical activity, almost doubled the risk of MB. Active cancer is a known predictor of recurrence and bleeding in older and younger patients with VTE and atrial fibrillation.11,29,46 Physical activity is associated with less fall-related bleeds, may have a stabilizing effect on the response to VKAs, or may reflect a lower comorbid burden.33 Although the anticoagulation quality was similar between patients who bled and those who did not, 44% of patients were overanticoagulated at the time of MB.
Although CRNMB lacks the devastating clinical effects of MB, it is associated with a decreased quality of life,5,6 hospital admissions,47 interventions,48 and costs of care.4 In our study, approximately twice as many patients experienced CRNMB than MB, with 15% of outpatients requiring hospital admission and 11% of patients requiring surgical hemostasis. A high risk of falls was associated with a 2-fold increased risk of CRNMB.
To date, no trial has specifically compared anticoagulation strategies in older patients with VTE. In a cost-effectiveness analysis, 3 months of anticoagulation with VKAs were more effective and less costly than prolonged anticoagulation in patients aged ≥80 years with unprovoked VTE.49
The strengths of our study include its prospective design, a long-term follow-up of up to 36 months, the inclusion of CRNMB, and a near complete outcomes assessment by independent, blinded adjudicators using explicit definitions. Our study also has limitations. First, our study excluded severely demented and terminally ill patients and may not represent the sickest of the sick. Second, most patients were anticoagulated using VKAs in our study whereas direct oral anticoagulants (DOACs) have become the standard of care for most patients with acute VTE.43,44 In meta-analyses of clinical trials, DOACs reduced the risk of MB by up to 60% compared with VKAs in older persons with VTE, at least during the first 3 months of treatment.20,50,51 MB may also have a lower case-fatality in patients treated with DOACs.52 Thus, our results based on data from the pre-DOAC era may not extrapolable to older patients with VTE who are treated with DOACs.
In conclusion, in older patients with VTE, most of whom received anticoagulant treatment with VKAs, the incidence of clinically relevant bleedings was substantially higher in our study than reported in clinical trials of younger patients. Bleeding complications occurred early in the course of anticoagulant treatment and carried major clinical consequences. The presence of active cancer, a low physical activity level, and an increased risk of falls were independently associated with bleeding. Given the great clinical and economic burden related to bleeding complications, the risks vs benefits of extended anticoagulation must be carefully weighed in older patients with acute VTE. Because DOACs are increasingly used for treatment of VTE and are associated with a lower bleeding risk, the incidence and impact of bleeding in older person with VTE should be reexamined using prospective data from the DOAC era.
Acknowledgment
This study was supported by the Swiss National Science Foundation (grant no. 33CSO-122659/139470).
Authorship
Contribution: All authors have read and approved the submission of this manuscript; E.F., M.M., and D.A. were responsible for study concept and design; O.S. and A.L. carried out the statistical analyses; E.F. and D.A. wrote the manuscript; M.M. and N.R. revised the manuscript; and M.M., N.R., and D.A. collected data and/or obtained funding from the Swiss National Science Foundation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elisa Ferrazzini, Department of General Internal Medicine, Inselspital, Bern University Hospital, Freiburgstr 18, 3010 Bern, Switzerland; e-mail: elisa.ferrazzini@insel.ch.
References
Author notes
Requests for data sharing should be e-mailed to elisa.ferrazzini@insel.ch.