TO THE EDITOR:
Diffuse large B-cell lymphoma (DLBCL) is a disease prevalent among older adults, with a median age at diagnosis of 66 years.1 The treatment of these patients is often complicated because of frailty and the presence of underlying comorbidities. Many older patients are ineligible for aggressive salvage therapy, including high-dose chemotherapy or autologous stem cell transplantation (auto-HCT) because of increased toxicity.2-4 Interestingly, older adults receiving chimeric-antigen receptor T-cell (CAR-T) therapy for relapsed/refractory (r/r) DLBCL appear to have outcomes comparable with those of younger patients.5-9 Older adults experienced superior quality-of-life improvements in the CAR-T arm vs the standard of care arm in the phase 3 ZUMA-7 trial.7 However, the optimal approach in older patients with r/r DLBCL responding to salvage chemotherapy is unclear, particularly when both CAR-T therapy and auto-HCT are potential options.
In this study, we used data publicly available from the Center for International Blood and Marrow Transplant Research registry to compare outcomes of older adults receiving auto-HCT vs CAR-T therapy for r/r DLBCL in partial response (PR) after salvage chemotherapy.10 Shadman et al10 previously reported that patients receiving auto-HCT had superior overall survival (OS) compared with those receiving CAR-T therapy; however, outcomes in older patients were not reported. Considering the increased risk of toxicity from auto-HCT in older adults, our aim was to compare outcomes specifically in the older patients in this cohort, using propensity-scoring to match patients aged ≥65 years in both the auto-HCT and CAR-T therapy groups.
All patients in this cohort had r/r DLBCL and had achieved a PR, per international working group criteria, as the best response to salvage chemotherapy before receiving auto-HCT (2013-2019) or CAR-T therapy (2018-2019).11 The primary focus of this analysis was the subgroup of patients aged ≥65 years. Definitions of end points are identical to those previously described and can be found in the supplemental Appendix, with an expanded description of statistical methods.10 Additional details on the data source are also available from the original publication, which was approved by the institutional review boards of the Medical College of Wisconsin and the National Marrow Donor Program.10 In addition, this study was submitted for local approval by the scientific review committee at Moffit Cancer Center (Tampa, FL) and was deemed exempt from institutional review board approval. The study was conducted in accordance with the Declaration of Helsinki.
Of 409 patients with available data, 125 (30.6%) were older adults (aged ≥65 years), of whom 72 (58%) received auto-HCT, and 53 (42%) received CAR-T therapy. Baseline characteristics of these 125 patients are described in Table 1. A higher proportion of patients in the CAR-T group had received >2 prior lines of therapy (70% vs 33%; P < .001) than that in the auto-HCT arm and had node size of >5 cm (45% vs 18%; P = .017). Median follow-up in the auto-HCT group was 24.9 months (range, 3-72 months) and in the CAR-T group was 12.1 months (range, 3-25 months).
Baseline characteristics of patients aged ≥65 years per the treatment group
| Characteristic . | Auto-HCT n = 72∗ . | CAR-T n = 53∗ . | P value† . |
|---|---|---|---|
| Sex | .024 | ||
| Male | 52 (72%) | 27 (51%) | |
| Female | 20 (28%) | 26 (49%) | |
| Age at infusion, y | 68.4 (66.6, 73.2) | 70.0 (67.8, 74.3) | .096 |
| Karnofsky performance score | .305 | ||
| 90-100 | 29 (40%) | 14 (26%) | |
| <90 | 42 (58%) | 33 (62%) | |
| Missing | 1 (1.4%) | 6 (11%) | |
| Race | .025 | ||
| White | 46 (64%) | 45 (85%) | |
| Black | 11 (15%) | 3 (5.7%) | |
| Asian | 8 (11%) | 2 (3.8%) | |
| Others | 4 (5.6%) | 0 (0%) | |
| Missing | 3 (4.2%) | 3 (5.7%) | |
| Disease stage | .876 | ||
| Early (stage I/II) | 14 (19%) | 9 (17%) | |
| Advanced (stage III/IV) | 45 (62%) | 35 (66%) | |
| Missing | 13 (18%) | 9 (17%) | |
| Extranodal involvement at diagnosis | .697 | ||
| No | 30 (42%) | 20 (38%) | |
| Yes | 31 (43%) | 26 (49%) | |
| Missing | 11 (15%) | 7 (13%) | |
| Largest node before treatment | .017 | ||
| <3 cm | 15 (21%) | 11 (21%) | |
| 3-5 cm | 19 (26%) | 8 (15%) | |
| >5 cm | 13 (18%) | 24 (45%) | |
| Missing | 25 (35%) | 10 (19%) | |
| Prior lines of therapy | <.001 | ||
| 0-2 | 45 (62%) | 14 (26%) | |
| >2 | 24 (33%) | 37 (70%) | |
| Missing | 3 (4.2%) | 2 (3.8%) | |
| Refractory to first-line therapy | .402 | ||
| No | 30 (42%) | 24 (45%) | |
| Yes | 39 (54%) | 21 (40%) | |
| Missing | 3 (4.2%) | 8 (15%) |
| Characteristic . | Auto-HCT n = 72∗ . | CAR-T n = 53∗ . | P value† . |
|---|---|---|---|
| Sex | .024 | ||
| Male | 52 (72%) | 27 (51%) | |
| Female | 20 (28%) | 26 (49%) | |
| Age at infusion, y | 68.4 (66.6, 73.2) | 70.0 (67.8, 74.3) | .096 |
| Karnofsky performance score | .305 | ||
| 90-100 | 29 (40%) | 14 (26%) | |
| <90 | 42 (58%) | 33 (62%) | |
| Missing | 1 (1.4%) | 6 (11%) | |
| Race | .025 | ||
| White | 46 (64%) | 45 (85%) | |
| Black | 11 (15%) | 3 (5.7%) | |
| Asian | 8 (11%) | 2 (3.8%) | |
| Others | 4 (5.6%) | 0 (0%) | |
| Missing | 3 (4.2%) | 3 (5.7%) | |
| Disease stage | .876 | ||
| Early (stage I/II) | 14 (19%) | 9 (17%) | |
| Advanced (stage III/IV) | 45 (62%) | 35 (66%) | |
| Missing | 13 (18%) | 9 (17%) | |
| Extranodal involvement at diagnosis | .697 | ||
| No | 30 (42%) | 20 (38%) | |
| Yes | 31 (43%) | 26 (49%) | |
| Missing | 11 (15%) | 7 (13%) | |
| Largest node before treatment | .017 | ||
| <3 cm | 15 (21%) | 11 (21%) | |
| 3-5 cm | 19 (26%) | 8 (15%) | |
| >5 cm | 13 (18%) | 24 (45%) | |
| Missing | 25 (35%) | 10 (19%) | |
| Prior lines of therapy | <.001 | ||
| 0-2 | 45 (62%) | 14 (26%) | |
| >2 | 24 (33%) | 37 (70%) | |
| Missing | 3 (4.2%) | 2 (3.8%) | |
| Refractory to first-line therapy | .402 | ||
| No | 30 (42%) | 24 (45%) | |
| Yes | 39 (54%) | 21 (40%) | |
| Missing | 3 (4.2%) | 8 (15%) |
n (%); median (interquatile range).
Pearson χ2 test; Wilcoxon rank sum test; and Fisher exact test.
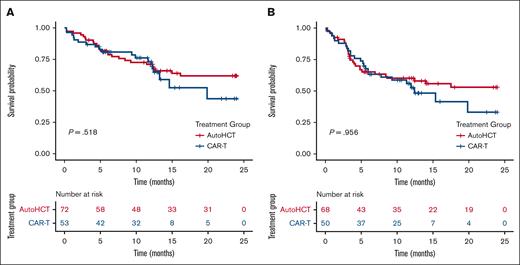
In an unadjusted Kaplan-Meier analysis with log-rank test, there was no difference in progression-free survival (PFS) between the auto-HCT and CAR-T groups (1-year PFS, 52% vs 51%; P = .96; Figure 1). Similarly, there was no difference in OS between older adults receiving auto-HCT and those receiving CAR-T therapy (1-year OS, 71% vs 73%; P = .52; Figure 1). Similar findings were noted when restricting the analysis to high-risk subgroups (supplemental Table 1). Finally, for older adults, the 1-year nonrelapse mortality (NRM) was 11% in the auto-HCT group vs 6% in the CAR-T group (P = .5; supplemental Table 1).
Overall and progression free survival in older patients receiving either auto-HCT or CAR-T therapy. OS (A) and PFS (B) in older patients aged ≥65 years with r/r DLBCL receiving auto-HCT or CAR-T therapy in PR (follow-up restricted to 24 months).
Overall and progression free survival in older patients receiving either auto-HCT or CAR-T therapy. OS (A) and PFS (B) in older patients aged ≥65 years with r/r DLBCL receiving auto-HCT or CAR-T therapy in PR (follow-up restricted to 24 months).
Upon multivariable analysis, CAR-T therapy was not associated with inferior PFS (hazard ratio [HR], 1.27; P = .5) or OS (HR, 1.71; P = .2), when compared with auto-HCT (supplemental Tables 2 and 3). In contrast, younger patients (aged <65 years) in the CAR-T group had inferior PFS (HR, 1.79; P = .02) and OS (HR, 2.3; P = .004; supplemental Tables 4-6; supplemental Figure 1).
We used propensity-matched analysis to compare outcomes among a matched-group of older patients in the auto-HCT group (n = 25) and those in the CAR-T group (n = 33). We found no statistically significant differences in 1-year OS (68% vs 72%; P = .539), 1-year PFS (56% vs 59%; P = .804), or 1-year NRM (12% vs 3.2%; P = .2) between the 2 groups.
In a sensitivity analysis, we performed these analyses using an age cut-off of ≥70 years to define an older adult subgroup (n = 53). Although there were a fewer number of events, the results were similar. Importantly, among patients aged ≥70 years, the 1-year NRM was 19% in the auto-HCT group (n = 27), compared with only 4% in the CAR-T group (n = 26), although this difference did not achieve statistical significance (P = .2).
Our data highlight the safety and efficacy of CAR-T therapy in older adults with chemosensitive r/r DLBCL. We found that in older adults with r/r DLBCL achieving a PR to salvage chemotherapy, outcomes were almost identical using either auto-HCT or CAR-T therapy, which is in stark contrast to the findings of Shadman et al.10 Despite the fact that patients in the CAR-T cohort were enriched for negative prognostic factors, such as bulky disease, and were more heavily pretreated, they had comparable outcomes with those of the older patients who were fit enough to tolerate auto-HCT, who, in this real-world setting, are likely to be a highly selected population. Importantly, older adults in the auto-HCT group had numerically higher 1-year NRM rates compared with those in the CAR-T group, although this did not reach statistical significance. These findings have potential implications for clinical practice and suggest that CAR-T therapy could be preferable for the fit older adults with relapse beyond 1 year who could be eligible for either approach.7
Our results are in line with findings from post hoc analyses from clinical trials, including ZUMA-7, and data from the Center for International Blood and Marrow Transplant Research, which highlight the safety and efficacy of CAR-T therapy in older patients.6,7 Although outcomes with auto-HCT have improved over time, 1-year NRM rates among older adults still range between 6% and 8%, and older adults experience significantly higher rates of organ toxicity than younger patients.2,3 In fact, in the original study by Shadman et al, age ≥60 years was independently associated with increased risk for NRM (in the overall cohort).10 In this analysis, the 1-year NRM in the auto-HCT group was 11% for patients aged ≥65 years, and 19% for patients aged ≥70 years. Younger patients (aged <65 years) in the auto-HCT group had a numerically lower 1-year NRM (5.3%). In contrast, the 1-year NRM in the CAR-T group was 6% in patients aged ≥65 years and 4% in those aged ≥70 years. Although these differences did not approach statistical significance likely because of the small number of events, they are likely to be clinically relevant.
Our study has several limitations. Firstly, this was a retrospective analysis with all the inherent limitations of such a study. As noted in the original article, interpretations of what constitutes a PR might also have varied among institutions and diagnostic modality. Secondly, follow-ups and number of events were more limited in the CAR-T group than in the auto-HCT group, which could have contributed to the lack of statistical significance in some analyses. Thirdly, our study was limited by the unavailability of objective measures of frailty.12
Nonetheless, our findings are provocative, showing that in older patients with r/r DLBCL achieving a response to salvage chemotherapy, CAR-T therapy resulted in outcomes comparable with those of auto-HCT, with a trend toward lower rates of NRM, despite being used in higher-risk disease and in later lines of therapy. CAR-T therapy should, therefore, be considered early in the disease course of older patients.
Acknowledgment: The authors thank the Center for International Blood and Marrow Transplantation Research for making this data set publicly available for further analysis.
Contribution: O.S.A. and C.L.F. conceived and designed the study, and prepared the first draft of the manuscript; B.C. and X.W. helped with data analysis; and all authors interpreted the data and helped revise the manuscript.
Conflict-of-interest disclosure: P.T. reports consultancy fees from TG Therapeutics, ADC Therapeutics, Genentech, GenMab, and Lilly USA. F.L.L. reports a scientific advisory role for and/or consulting fees from A2, Allogene, Amgen, bluebird bio, Bristol Myers Squibb (BMS)/Celgene, Calibr, Caribou, Cellular Biomedicine Group, Cowen, Daiichi Sankyo, EcoR1, Emerging Therapy Solutions, GammaDelta Therapeutics, Gerson Lehrman Group, Iovance, Kite Pharma, Janssen, Legend Biotech, Novartis, Sana, Takeda, Wugen, and Umoja; contracts for service for Kite Pharma (Institutional), Allogene (Institutional), CERo Therapeutics (Institutional), Novartis (Institutional), bluebird bio (Institutional), BMS (Institutional), National Cancer Institute, and Leukemia and Lymphoma Society; and patents, royalties, and other intellectual property for several patents held by the institution in his name (unlicensed) in the field of cellular immunotherapy; and education or editorial activity for Aptitude Health, ASH, BioPharma Communications CARE Education, Clinical Care Options Oncology, Imedex, and the Society for Immunotherapy of Cancer. C.L.F. reports honoraria/consulting for BMS, Seattle Genetics, Celgene, AbbVie, Sanofi, Incyte, Amgen, ONK therapeutics, and Janssen, and research funding from BMS, Janssen, and Roche/Genentech. The remaining authors declare no competing financial interests.
Correspondence: Ciara L. Freeman, Department of Blood and Marrow Transplantation and Cellular Immunotherapy, H. Lee Moffitt Cancer Center, 12902 USF Magnolia Dr, Tampa, FL 33612; e-mail: ciara.freeman@moffitt.org.
References
Author notes
The data reported in this article have already been published by Shadman et al10 and has been made publicly available by the Center for International Blood and Marrow Transplant Research at https://cibmtr.org/CIBMTR/Resources/Publicly-Available-Datasets1#2022.
The full-text version of this article contains a data supplement.