Key Points
HRG attenuates catheter-induced clotting in human and rabbit plasma.
HRG depletion with an ASO enhances catheter thrombosis in rabbits, whereas FXII depletion attenuates it.
Abstract
Factor XII (FXII) knockdown attenuates catheter thrombosis in rabbits. Because histidine-rich glycoprotein (HRG) modulates FXIIa activity, we hypothesized that HRG depletion would promote catheter thrombosis. To test this, rabbits were given either antisense oligonucleotides (ASOs) against HRG or FXII, a control ASO, or saline. The activated partial thromboplastin time (aPTT), prothrombin time (PT), and catheter-induced thrombin generation were determined in blood collected before and after treatment. Compared with the controls, the HRG- and FXII-directed ASOs reduced hepatic messenger RNA and plasma levels of HRG and FXII, respectively, by >90%. Although HRG knockdown shortened the aPTT by 2.5 fold, FXII knockdown prolonged it by fourfold; neither of the ASOs affected the PT. Catheter segments shortened the lag time and increased peak thrombin in the plasma from control rabbits; effects were significantly enhanced and attenuated in the plasma from rabbits given the HRG- and FXII-directed ASOs, respectively. Catheters were then inserted into the right external jugular vein of the rabbits, and the time for catheter occlusion was determined. The catheter occlusion times with the control ASO or saline were 62 ± 8 minutes and 60 ± 11 minutes, respectively. The occlusion time was significantly reduced to 34 ± 9 minutes, with HRG knockdown and significantly prolonged to 128 ± 19 minutes with FXII knockdown. HRG levels are decreased with sepsis or cancer, and such patients are prone to catheter thrombosis. Because HRG modulates catheter thrombosis, our findings suggest that HRG supplementation may prevent this problem.
Introduction
Central venous catheters (CVCs) are often used for venous access and to deliver chemotherapy, antibiotics, blood products, and parenteral nutrition.1-3 CVCs often cause thrombosis, which can lead to infection, device failure, upper extremity deep vein thrombosis, and risk of pulmonary embolism, which can be fatal.1-3 The reported incidence of symptomatic catheter–related deep vein thrombosis ranges from 1% to 5% and is higher in patients admitted to the intensive care unit or in those with cancer.4-6 CVC thrombosis is associated with significant morbidity because it can delay treatments, necessitate CVC removal, and lead to postthrombotic syndrome.4,5,7 Consequently, there is a need for effective treatments to prevent CVC thrombosis.
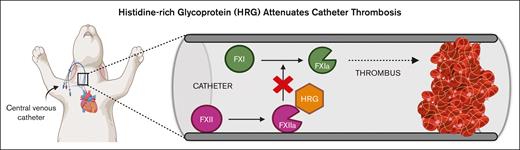
Catheters trigger clotting via the intrinsic pathway of coagulation.2,8,9 Factor XII (FXII) binds to negatively charged surfaces, including catheters, after which it undergoes autoactivation.10-13 The resultant FXIIa propagates coagulation by activating FXI, thereby inducing thrombin generation and fibrin formation.2,10,11 The importance of FXII in this process is highlighted by the observations that (1) catheter-induced clotting in vitro is attenuated in plasma depleted of FXII or FXI but not FVII9; (2) the addition of corn trypsin inhibitor (CTI), a potent and specific inhibitor of FXIIa, attenuates catheter clotting in vitro14; (3) in a rabbit catheter thrombosis model, the time to occlusion is longer with catheters coated with CTI than that with uncoated catheters14; and (4) knockdown of FXII or FXI with target-specific antisense oligonucleotides (ASOs) prolongs the time to catheter occlusion in rabbits, whereas the knockdown of FVII does not.9 Therefore catheter-induced clotting occurs via the intrinsic pathway of coagulation.
Histidine-rich glycoprotein (HRG) is a 75 kDa glycoprotein that is synthesized in the liver and circulates in plasma at a concentration of ranging between 1 and 3 μM.15,16 HRG plays a key role in various biological processes, including immunity, angiogenesis, and coagulation.17-19 We have previously shown that HRG inhibits the contact system by binding FXIIa with high affinity, thereby attenuating FXII autoactivation and subsequent FXIIa-mediated FXI activation.20 Furthermore, FeCl3- and polyphosphate-induced thromboses are enhanced in HRG-deficient mice compared with those in wild-type mice.21,22 These results suggest that HRG serves as a molecular brake for the contact system.
Because catheter thrombosis is triggered by FXII activation and HRG modulates FXIIa activity,2,20,23 we hypothesized that HRG depletion would promote catheter thrombosis in vitro and in rabbits. To test this hypothesis, we knocked down the levels of HRG in rabbits with a specific HRG-directed ASO and assessed the impact on ex vivo and in vivo catheter-induced clotting. A FXII-directed ASO was used as a positive control, whereas a control ASO and saline were used as negative controls.
Methods
Materials
A detailed description of materials is provided in the supplemental File.
Rabbit acquisition and housing
Male New Zealand white rabbits (2.5-3.0 kg), purchased from Charles River Canada (Sherbrooke, QC, Canada), were housed in individual cages in rooms maintained at a constant 12-hour light-dark cycle with controlled temperature and humidity and were given free access to food and water. Studies were approved by the Animal Research Ethics Board at McMaster University, and procedures complied with Canadian Council on Animal Care guidelines.
Synthesis of ASOs
HRG, FXII, and control ASOs conjugated to N-acetyl galactosamine were synthesized by Ionis Pharmaceuticals (Carlsbad, CA), and the ASO sequences are listed in supplemental Table 1. N-acetyl galactosamine conjugation was undertaken to enhance the hepatic uptake of the ASOs.24
ASO dosing
A preliminary study was performed to identify the doses of the HRG- and FXII-directed ASOs that produced maximum knockdown. As controls, rabbits were given either a control ASO, consisting of a scrambled sequence of oligonucleotides or an equivalent volume of saline. The ASOs were given by subcutaneous injection twice weekly for 4 weeks at a dose of 8 or 16 mg/kg. Rabbits were monitored daily for evidence of toxicity, and body weight was determined twice weekly. Both ASO doses produced maximum knockdown; thus, the lower 8 mg/kg dose of the HRG, FXII, and control ASO was selected for this study (supplemental Figures 1 and 2).
Preparation of platelet-poor plasma
Blood samples (5 mL) were collected from a marginal ear vein into 3.8% sodium citrate (9:1, vol/vol) at baseline and 4 weeks while rabbits were under anesthesia with a mixture of 1 L/min of oxygen, and between 1% and 4% isoflurane was delivered via face masks. Samples were immediately mixed and kept on ice before centrifugation at 2000g for 20 minutes at 23°C. Plasma was subjected to a second centrifugation step under the same conditions and was then frozen in aliquots at −80°C. To create pools of control rabbit or human platelet-poor plasma, blood from 4 healthy rabbits or 10 healthy human volunteers was collected into 3.8% sodium citrate (9:1, vol/vol) and processed as described, and the plasma was then pooled and frozen in aliquots.
Hepatic HRG and FXII mRNA expression
To assess messenger RNA (mRNA) expression, 1 cm3 sections of liver collected from each rabbit post mortem was submerged in RNALater solution (Life Technologies, Burlington, ON), stored overnight at 4°C, and then frozen at −80°C. After homogenization of thawed samples, the mRNA was isolated using the PureLink Pro 96 Total RNA Purification Kit (Life Technologies), and mRNA levels were quantified using OneStepPlus real-time polymerase chain reaction (Applied Biosystems, Foster City, CA) and normalized against a rabbit 18S ribosomal RNA primer probe set.9
Global tests of coagulation
Clotting times of rabbit plasma were determined by incubating 40 μL of plasma with 50 μL of 20 mM HEPES buffer at pH 7.4 for 10 minutes at 37°C. After addition of 26 mM CaCl2, absorbance was monitored at 405 nm using a THERMOmax plate reader (Molecular Devices, Sunnyvale, CA), and clotting times were recorded as the time to the half-maximum absorbance, as determined by instrument software (SoftMax Pro, version 5.4). The dilute activated partial thromboplastin time (aPTT) and prothrombin time (PT) were determined in rabbit plasma collected before and after ASO administration. Briefly, 40 μL of plasma were incubated with 40 μL of a 1:10 dilution of aPTT-SP reagent or a 1:100 dilution of RecombiPlasTin (Instrumentation Laboratory Co., Bedford, MA) and 10 μL of 20 mM HEPES buffer at pH 7.4 for 10 minutes at 37°C. After addition of 10 μL of 260 mM CaCl2 (final concentration of 26 mM), absorbance was monitored at 405 nm using a THERMOmax plate reader (Molecular Devices, Sunnyvale, CA), and clotting times were determined as described earlier.
HRG and FXII antigen levels
Plasma was subjected to electrophoresis on sodium dodecyl sulfate 4% or 15% polyacrylamide gradient gels (Bio-Rad Laboratories, Hercules, CA) under nonreducing conditions, and separated proteins were then transferred to Immuno-Blot polyvinylidene fluoride membranes (Bio-Rad), as previously described.9,22 HRG and FXII were detected using a 1:1000 dilution of peroxidase-labeled sheep antihuman HRG antibody20 or a 1:2000 dilution of peroxidase-labeled goat antihuman FXII antibody (Affinity Biologicals, Ancaster, ON), respectively. Blots were imaged using the ChemiDoc (Bio-Rad, Hercules, CA), and protein levels were quantified via densitometry using ImageLab version 3.0 software (Bio-Rad).
In vitro catheter-induced clotting and thrombin generation
Plate-based assays were used to examine the effects of HRG or FXII depletion in rabbits upon ex vivo catheter-induced clotting and thrombin generation. Catheter-induced clotting assays were conducted as previously described with some modifications.23 Briefly, 6F catheters were pressed flat and cut into 1.7 cm segments to fit the circumference of wells of a 96-well, clear, flat-bottom plate. To wells with or without catheter segments, 75 μL of 20 mM HEPES at pH 7.4 and 100 μL of rabbit plasma were added. After incubation for 20 minutes at 37°C, 25 μL of 160 mM CaCl2 was added (final concentration, 20 mM CaCl2); absorbance was monitored at 405 nm using a plate reader, and clotting times were recorded as the time to 50% absorbance, as determined by the instrument software.
Catheter-induced thrombin generation was quantified as previously described.25 Briefly, to wells of a 96-well Costar, black, flat-bottom plate with or without catheter segments, 100 μL of rabbit plasma was added. After incubation for 15 minutes at 37°C, 100 μL of a solution containing 1 mM Z-GGR-AMC (Bachem, Bubendorf, Switzerland) and 15 mM CaCl2 in 20 mM HEPES at pH 7.4 was added, and substrate hydrolysis was monitored for 90 minutes at excitation and emission wavelengths of 360 nm and 460 nm, respectively, using a SpectraMax M3 plate reader (Molecular Devices). Thrombin generation profiles were analyzed using Technothrombin TGA software (Technoclone, Vienna, Austria).
Experiments with or without catheter segments were repeated in human control plasma or HRG- or FXII-depleted human plasma, and the time to clot formation and thrombin generation were determined. The effect of addition of human HRG up to 5 μM on catheter-induced clotting and thrombin generation was also assessed in control and HRG-depleted human plasma.
Catheter thrombosis in rabbits
Using a web-based system (www.randomization.com) balanced using randomly permuted blocks, a total of 30 rabbits were randomly assigned to receive twice weekly subcutaneous injections of 8 mg/kg of the HRG, FXII, or control ASO or an equivalent volume of saline for 4 weeks. The ASOs or saline were prepared and aliquoted by an investigator who was aware of the treatment allocations (R.H.); treatments were administered, and catheter occlusion studies were performed by investigators blinded to treatment allocation (R.A.M., P.L., and J.Z.).
To determine the effect of HRG or FXII knockdown on catheter thrombosis in vivo, a catheter thrombosis model with some modifications was used (supplemental Figure 3).9 Briefly, rabbits were anesthetized with a combination of oxygen (1 L/min) and isoflurane (1%-4%) and maintained on anesthesia via a 2-mm endotracheal tube. Catheters (7F) from Access Technologies (Skokie, IL) cut into preweighed, 7 cm segments were inserted into the right external jugular vein toward the heart and secured with a ligature. A pressure transducer (Baxter Healthcare, Deerfield, IL) was attached to the catheter. Aliquots of blood (3 mL) were drawn from the catheter into 5 mL plastic syringes every 5 minutes for up to 4 hours, maintained in the syringe for 2 minutes, and then slowly reinjected, after which the catheter was flushed with 2 mL of 0.9% sterile saline. The time to catheter occlusion was defined as that when blood could no longer be withdrawn from the catheter or when the pressure reached 90 mmHg. When catheter occlusion occurred or at the 4-hour point, rabbits were given IV injections of 1000 U/kg unfractionated heparin and 360 mg/kg sodium pentobarbital via a marginal ear vein. The catheter was then excised, weighed to determine the intralumenal thrombus weight, and photographed.
Statistical analysis
All results are presented as mean ± standard deviation (SD). Unless otherwise stated, experiments were performed at least 3 times. Significance of differences between groups was determined using one or two-way analysis of variance (ANOVA) followed by the Holm-Sidak comparison test. SigmaPlot version 11 (Systat Software, San Jose, CA) and GraphPad Prism 9 (GraphPad Software, San Diago, CA) were used to create graphs, and IBM SPSS Statistics software (Armonk, NY) was used to perform the statistical analyses. For all analyses, P < .05 was considered statistically significant.
Results
HRG and FXII ASOs reduce hepatic mRNA and protein expression
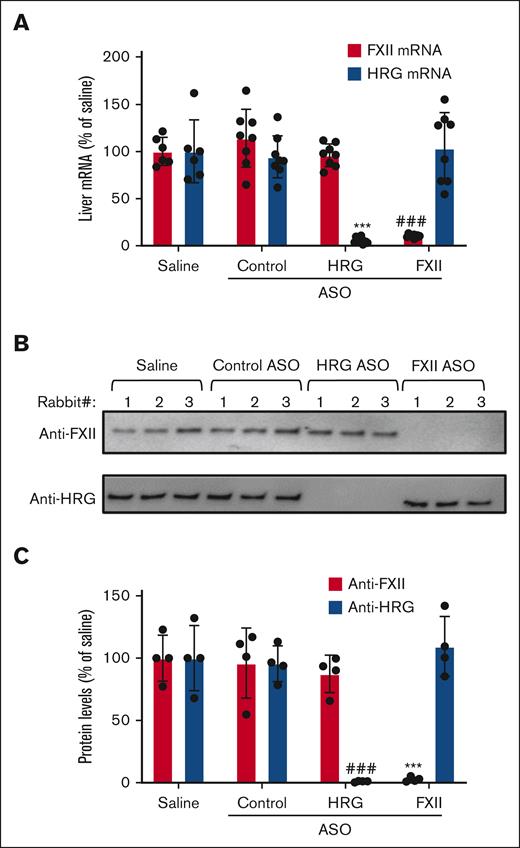
After a 4-week course of ASO treatment, hepatic mRNA and protein expression were quantified (supplemental Figure 3). Compared with baseline, the HRG or FXII-directed ASOs significantly reduced hepatic mRNA expression of HRG and FXII by 95% and 85%, respectively (Figure 1). Compared with the saline control values, the HRG-directed and FXII-directed ASO significantly (P < .0001) reduced the mean plasma HRG level from 142 ± 29 μg/mL to 4 ± 1 μg/mL and the mean plasma FXII level from 24 ± 3 μg/mL to 2 ± 0.2 μg/mL (supplemental Figure 4). In contrast, neither the control ASO nor saline had any effect on hepatic mRNA or plasma levels of HRG or FXII.
Effect of ASO treatment on hepatic mRNA and plasma levels of HRG and FXII. (A) Liver mRNA levels were quantified in rabbits that were given a 4-week course of treatment with the control, HRG, or FXII ASOs and compared with the levels in rabbits given saline. FXII (red bars) and HRG (blue bars) hepatic mRNA levels are plotted as percentages relative to those in the saline-treated rabbits. Bars represent mean ± SD from 6 to 8 rabbits per treatment group. ∗∗∗P < .001 HRG mRNA levels compared with those in saline controls. ###P < .001 FXII mRNA levels compared with those in saline controls (one-way ANOVA and Holm-Sidak method). (B) Plasma levels of HRG and FXII after ASO treatments were quantified via immunoblot analysis using peroxidase-labeled sheep antihuman HRG antibody and peroxidase-labeled goat antihuman FXII antibody, respectively. Representative image from 4 blots. (C) Bands from panel B were quantified via densitometry. ∗∗∗P < .001 comparison of FXII levels in saline controls. ###P < .001 comparison of HRG levels in saline controls. Bars represent mean ± SD from 6 to 8 rabbits per treatment group (one-way ANOVA and Holm-Sidak method).
Effect of ASO treatment on hepatic mRNA and plasma levels of HRG and FXII. (A) Liver mRNA levels were quantified in rabbits that were given a 4-week course of treatment with the control, HRG, or FXII ASOs and compared with the levels in rabbits given saline. FXII (red bars) and HRG (blue bars) hepatic mRNA levels are plotted as percentages relative to those in the saline-treated rabbits. Bars represent mean ± SD from 6 to 8 rabbits per treatment group. ∗∗∗P < .001 HRG mRNA levels compared with those in saline controls. ###P < .001 FXII mRNA levels compared with those in saline controls (one-way ANOVA and Holm-Sidak method). (B) Plasma levels of HRG and FXII after ASO treatments were quantified via immunoblot analysis using peroxidase-labeled sheep antihuman HRG antibody and peroxidase-labeled goat antihuman FXII antibody, respectively. Representative image from 4 blots. (C) Bands from panel B were quantified via densitometry. ∗∗∗P < .001 comparison of FXII levels in saline controls. ###P < .001 comparison of HRG levels in saline controls. Bars represent mean ± SD from 6 to 8 rabbits per treatment group (one-way ANOVA and Holm-Sidak method).
HRG knockdown shortens the aPTT, whereas FXII knockdown prolongs it
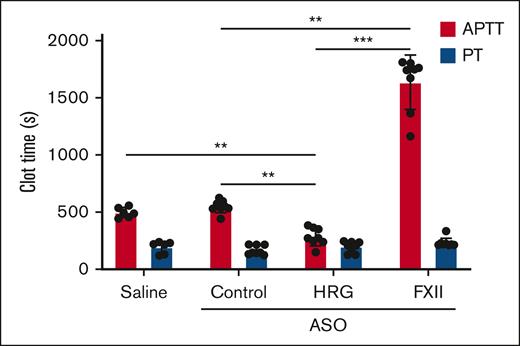
Compared with baseline, the HRG-directed ASO significantly shortened the aPTT (P < .01) twofold, whereas treatment with the FXII-directed ASO significantly prolonged it threefold (P < .01; Figure 2). The threefold prolongation of the aPTT with FXII knockdown is consistent with the observation that the aPTT is approximately threefold longer in mice that are deficient in FXII than in wild-type mice (68 seconds and 24 seconds, respectively).26 The fact that the aPTT is prolonged but measurable with FXII depletion may reflect the contribution of residual FXII, prekallikrein or FXI autoactivation, or kallikrein-induced FIX activation27 contributing to contact-mediated clotting. Neither the control ASO nor saline had any effect on the aPTT. The PT was unaffected by treatment with the HRG or FXII-directed ASOs, thereby localizing their effect to the intrinsic pathway of coagulation (Figure 2).
Effect of treatment with saline, or control, HRG, or FXII ASOs on the aPTT and PT. The dilute aPTT (red bars) and PT (blue bars) were determined in the plasma collected from rabbits after a 4-week course of treatment with saline (n = 6) or control, or HRG or FXII ASO (N = 8 per group). Bars represent mean ± SD from 3 determinations for each rabbit. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001 comparison of aPTT results as indicated by the lines (one-way ANOVA and Holm-Sidak method).
Effect of treatment with saline, or control, HRG, or FXII ASOs on the aPTT and PT. The dilute aPTT (red bars) and PT (blue bars) were determined in the plasma collected from rabbits after a 4-week course of treatment with saline (n = 6) or control, or HRG or FXII ASO (N = 8 per group). Bars represent mean ± SD from 3 determinations for each rabbit. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001 comparison of aPTT results as indicated by the lines (one-way ANOVA and Holm-Sidak method).
HRG-directed ASO enhances catheter-induced clotting and thrombin generation, whereas the FXII-directed ASO attenuates them
To confirm that catheters promote FXII activation, FXII was incubated in the absence or presence of catheter segments, and FXIIa generation was quantified via a chromogenic assay. Although <5 nM FXIIa was generated in the absence of catheter segments, there was a significant (P < .001) increase in FXIIa generation to 61 nM in their presence (supplemental Figure 5). HRG attenuated FXII autoactivation with a 50% inhibitory concentration of 344 ± 12 nM and abrogated activation at 2 μM. These data confirm that HRG directly attenuates FXII autoactivation.20 The role of FXII in catheter-induced clotting was further evaluated using CTI, a potent and specific inhibitor of FXIIa. CTI attenuated plasma clotting in both the absence and presence of catheter segments (supplemental Figure 6).
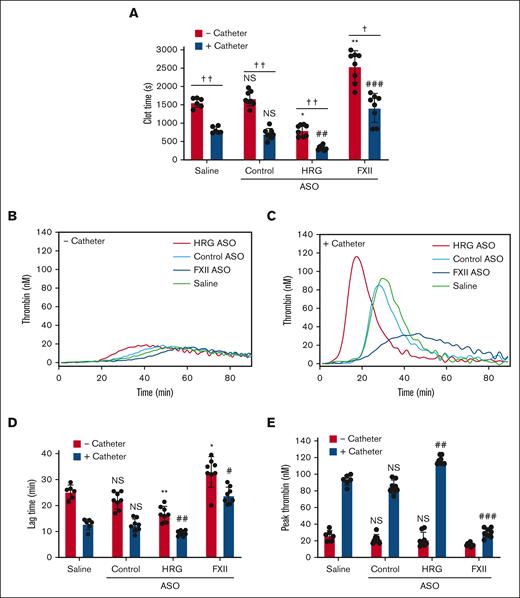
Catheter-induced clotting and thrombin generation were used to evaluate the effects of HRG and FXII knockdown. Catheter segments significantly (P < .01) shortened the recalcification time in plasma from saline-treated rabbits by 2.2-fold, from 1614 ± 133 seconds to 732 ± 122 seconds, confirming that catheter segments are prothrombotic (Figure 3A). Compared with results in plasma from rabbits given the control ASO, catheter segments significantly (P < .01) shortened the recalcification time by 2.1-fold, from 805 ± 117 seconds to 370 ± 96 seconds in plasma from rabbits given the HRG-directed ASO, and significantly (P < .001) prolonged the recalcification time from 805 ± 117 seconds to 1665 ± 457 seconds in plasma from rabbits given the FXII-directed ASO (Figure 3A). Addition of human HRG to control rabbit plasma or to plasma from rabbits given the HRG-directed ASO attenuated the prothrombotic effect of catheters in a concentration-dependent manner (supplemental Figure 7).
Effect of treatment with saline, or control, HRG, or FXII ASOs on ex vivo catheter-induced thrombin generation. Rabbit plasma was collected after a 4-week course of treatment with saline (n = 6 per group), or HRG or FXII ASOs (n = 8). (A) Plasma was incubated in the absence or presence of catheter segments for 15 minutes at 37°C before clotting was initiated by the addition of 20 mM CaCl2. Absorbance was monitored, and plasma recalcification clot time was determined as the half-time of maximal absorbance. Thrombin generation was initiated by addition of 20 mM CaCl2 and was quantified by monitoring the hydrolysis of 1 mM Z-Gly-Gly-Arg-7-amino-4-methyl coumarin (Z-GGR-AMC) thrombin substrate. (B-C) Representative thrombin generation profiles in the absence (B) or presence (C) of catheter segments. (D) Mean lag time and (E) peak thrombin in the absence (red bars) or presence (blue bars) of catheter segments are shown. Bars represent mean ± SD from 3 determinations per rabbit, each performed in duplicate. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001 comparison of saline-treated plasma in the absence of catheter segments; #P < .05 and ##P < .01 comparison of saline-treated plasma in the absence of catheter segments; †P < .05 and ††P < .01 as indicated by the lines (one-way ANOVA and Holm-Sidak method).
Effect of treatment with saline, or control, HRG, or FXII ASOs on ex vivo catheter-induced thrombin generation. Rabbit plasma was collected after a 4-week course of treatment with saline (n = 6 per group), or HRG or FXII ASOs (n = 8). (A) Plasma was incubated in the absence or presence of catheter segments for 15 minutes at 37°C before clotting was initiated by the addition of 20 mM CaCl2. Absorbance was monitored, and plasma recalcification clot time was determined as the half-time of maximal absorbance. Thrombin generation was initiated by addition of 20 mM CaCl2 and was quantified by monitoring the hydrolysis of 1 mM Z-Gly-Gly-Arg-7-amino-4-methyl coumarin (Z-GGR-AMC) thrombin substrate. (B-C) Representative thrombin generation profiles in the absence (B) or presence (C) of catheter segments. (D) Mean lag time and (E) peak thrombin in the absence (red bars) or presence (blue bars) of catheter segments are shown. Bars represent mean ± SD from 3 determinations per rabbit, each performed in duplicate. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001 comparison of saline-treated plasma in the absence of catheter segments; #P < .05 and ##P < .01 comparison of saline-treated plasma in the absence of catheter segments; †P < .05 and ††P < .01 as indicated by the lines (one-way ANOVA and Holm-Sidak method).
Catheter-induced thrombin generation was significantly (P < .01) enhanced in the plasma from rabbits given the HRG-directed ASO compared with that in the plasma from rabbits treated with the control ASO, as evidenced by a shorter lag time (8 ± 0.5 minutes vs 15 ± 3 minutes; P < .01) and a higher peak thrombin (118 ± 4 nM vs 86 ± 7 nM; P < .01; Figure 3D-E). In contrast, catheter-induced thrombin generation was significantly attenuated in the plasma from rabbits given the FXII ASO compared with that in the plasma from those given the control ASO, as evidenced by a longer lag time (from 15 ± 3 minutes to 23 ± 2 minutes; P < .05) and a reduced peak thrombin (from 86 ± 7.2 nM to 32 ± 6 nM; P < .001). These results suggest that HRG knockdown is associated with a prothrombotic phenotype that enhances catheter-induced clotting and thrombin generation, whereas FXII knockdown is associated with an antithrombotic phenotype that attenuates catheter-induced clotting and thrombin generation.
Time to catheter occlusion is shortened in rabbits given the HRG-directed ASO and prolonged in those given the FXII-directed ASO
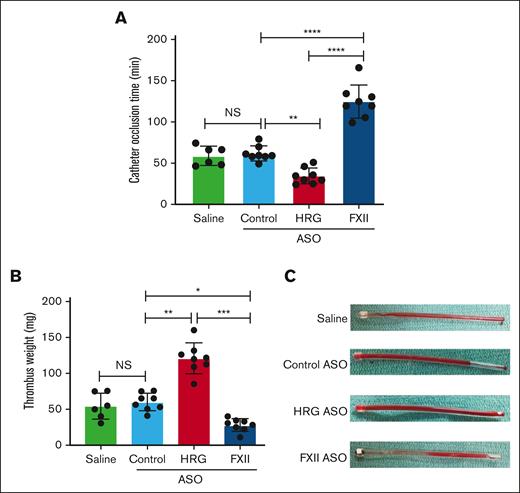
To confirm the findings in vivo, the time to occlusion of catheters implanted in the jugular vein of rabbits was quantified (supplemental Figure 3), as described previously by Yau et al.9,23 The times to catheter occlusion were 59.7 ± 10.6 minutes and 61.8 ± 8.4 minutes in rabbits given the control ASO or saline, respectively (Figure 4A). In contrast, the time to catheter occlusion was significantly (P < .01) reduced to 35 ± 9 minutes in rabbits given the HRG-directed ASO and was significantly (P < .0001) prolonged to 125 ± 19 minutes in those given the FXII-directed ASO (Figure 4A). In keeping with these findings, compared with the thrombus weights in rabbits given the control ASO or saline (53.4 ± 16.3 mg and 43.2 ± 10.4 mg, respectively) the thrombus weight was significantly (P < .01) greater in rabbits given the HRG-directed ASO (129 ± 34 mg) and significantly (P < .05) lower in those given the FXII-directed ASO (20 ± 13 mg; Figure 4B-C). To evaluate the extent of activation of coagulation, the plasma levels of TAT and FXIIa-C1 inhibitor complex were also quantified. Compared with the plasma levels after catheter implantation in control rabbits, the plasma levels of FXIIa-C1 inhibitor complex were significantly higher (P < .01) in rabbits that were given the HRG-directed ASO and significantly lower (P < .05) in rabbits that received the FXII-directed ASO (supplemental Figure 8A). Compared with control rabbits, the mean levels of thrombin-antithrombin (TAT) complex were higher after catheter implantation in rabbits given the HRG-directed ASO and lower in rabbits given the FXII-directed ASO, but the differences were not statistically significantly (supplemental Figure 8B). Together, these results suggest that HRG knockdown enhances the prothrombotic activity of catheters in rabbits, whereas FXII knockdown attenuates their prothrombotic activity.
Effect of treatment with saline, or control, HRG, or FXII ASOs on time to catheter occlusion and thrombus weight. After a 4-week course of treatment with saline (n = 6 per group), or control, HRG, or FXII ASOs (n = 8 per group), catheters were implanted in the jugular vein and the time to catheter occlusion (A) and the weight of the explanted catheter thrombi (B) were determined. Mean ± SD, with the symbols representing the results in individual rabbits. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001 comparisons between catheter occlusion times or thrombus weights as indicated by the lines; (one-way ANOVA and Holm-Sidak method). (C) Representative images of explanted catheters with intralumenal thrombi. NS, not significant.
Effect of treatment with saline, or control, HRG, or FXII ASOs on time to catheter occlusion and thrombus weight. After a 4-week course of treatment with saline (n = 6 per group), or control, HRG, or FXII ASOs (n = 8 per group), catheters were implanted in the jugular vein and the time to catheter occlusion (A) and the weight of the explanted catheter thrombi (B) were determined. Mean ± SD, with the symbols representing the results in individual rabbits. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001 comparisons between catheter occlusion times or thrombus weights as indicated by the lines; (one-way ANOVA and Holm-Sidak method). (C) Representative images of explanted catheters with intralumenal thrombi. NS, not significant.
Depletion of HRG or FXII in human plasma has the same effect as it does in rabbit plasma
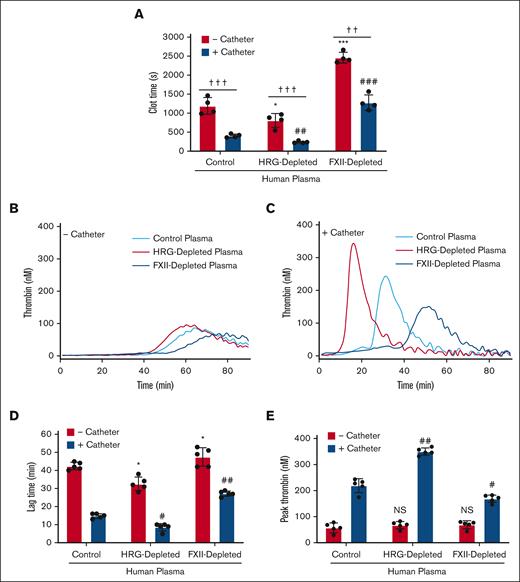
To determine whether the findings in rabbits apply to those in humans, catheter-induced clotting and thrombin generation were examined in human plasma. Catheter segments accelerated the clotting time in control plasma threefold compared with that determined in their absence (354 ± 51 seconds and 1088 ± 329 seconds, respectively; P < .001; Figure 5A). The catheter-induced clotting time was significantly shorter in HRG-depleted plasma than that in control plasma (202 ± 24 seconds and 354 ± 51 seconds, respectively; P < .01), whereas the clotting time in FXII-depleted plasma was significantly longer (1225 ± 243 seconds; P < .001).
Catheter-induced clotting and thrombin generation in control, HRG-depleted, or FXII-depleted human plasma. (A) Plasma was incubated for 15 minutes at 37°C in the absence or presence of catheter segments before initiating clotting by addition of 20 mM CaCl2. Absorbance was monitored, and plasma recalcification times were determined as the time to half maximal absorbance. Bars represent the mean ± SD of 4 determinations. ∗P < .05 and ∗∗∗P < .001 comparison of the plasma recalcification time in the absence of catheter segments (red bars); ##P < .01 and ###P < .001 comparisons of the plasma recalcification time in the presence of catheter segments (blue bars); ††P < .01 and †††P < .001 as indicated by the lines (one-way ANOVA and Holm-Sidak method). (B-E) Plasma was incubated in the absence or presence of catheter segments for 15 minutes at 37°C, and thrombin generation was initiated by the addition of 20 mM CaCl2 and was quantified by monitoring the hydrolysis of 1 mM Z-GGR-AMC. Representative thrombin generation profiles in the absence (B) or presence (C) of catheter segments. Mean lag time (D) and peak thrombin (E) in the absence (red bars) or presence (blue bars) of catheter segments are shown. Bars represent mean ± SD from 5 determinations each performed in duplicate. ∗P < .05 comparison of the control plasma in the absence of catheter segments. #P < .05 and ##P < .01 comparisons of the control plasma in the absence of catheter segments; P > .05 (one-way ANOVA and Holm-Sidak method).
Catheter-induced clotting and thrombin generation in control, HRG-depleted, or FXII-depleted human plasma. (A) Plasma was incubated for 15 minutes at 37°C in the absence or presence of catheter segments before initiating clotting by addition of 20 mM CaCl2. Absorbance was monitored, and plasma recalcification times were determined as the time to half maximal absorbance. Bars represent the mean ± SD of 4 determinations. ∗P < .05 and ∗∗∗P < .001 comparison of the plasma recalcification time in the absence of catheter segments (red bars); ##P < .01 and ###P < .001 comparisons of the plasma recalcification time in the presence of catheter segments (blue bars); ††P < .01 and †††P < .001 as indicated by the lines (one-way ANOVA and Holm-Sidak method). (B-E) Plasma was incubated in the absence or presence of catheter segments for 15 minutes at 37°C, and thrombin generation was initiated by the addition of 20 mM CaCl2 and was quantified by monitoring the hydrolysis of 1 mM Z-GGR-AMC. Representative thrombin generation profiles in the absence (B) or presence (C) of catheter segments. Mean lag time (D) and peak thrombin (E) in the absence (red bars) or presence (blue bars) of catheter segments are shown. Bars represent mean ± SD from 5 determinations each performed in duplicate. ∗P < .05 comparison of the control plasma in the absence of catheter segments. #P < .05 and ##P < .01 comparisons of the control plasma in the absence of catheter segments; P > .05 (one-way ANOVA and Holm-Sidak method).
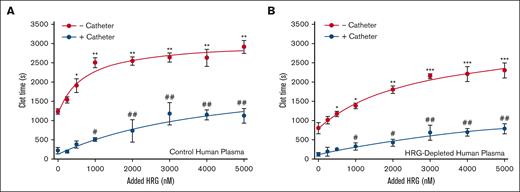
Comparing the results in the control rabbit plasma, catheter-induced thrombin generation was enhanced in HRG-depleted human plasma as evidenced by a significantly shorter lag time (from 15 ± 2 minutes to 9 ± 3 minutes; P < .05) and a higher peak thrombin (from 216 ± 22 nM to 350 ± 11 nM; P < .01; Figure 5B-E). In contrast, catheter-induced thrombin generation was attenuated in FXII-depleted human plasma compared with that in control human plasma, as evidenced by a prolonged lag time (from 15 ± 2 minutes to 29 ± 6 minutes; P < .01) and had a significantly reduced thrombin peak (from 216 ± 22 nM to 168 ± 11 nM; P < .05). Addition of human HRG to control or HRG-depleted human plasma attenuated the prothrombotic effect of catheters in a concentration-dependent manner (Figure 6). Therefore, the findings in human plasma mirror those in rabbit plasma.
Effect of HRG on catheter-induced clotting in control or HRG-depleted human plasma. Control human plasma (A), or HRG-depleted human plasma (B) was incubated with human HRG up to 5 μM in the absence (red line) or presence (blue line) of catheter segments for 15 minutes at 37°C before initiating clotting via the addition of 20 mM CaCl2. Absorbance was monitored and clot time was determined as the half-time of maximal absorbance. Mean ± SD of 4 determinations. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001 comparisons of the clot time in the absence of catheter segments and without HRG; #P < .05 and ##P < .01 compared with clot time in the presence of catheter segments and without HRG (one-way ANOVA and Holm-Sidak method).
Effect of HRG on catheter-induced clotting in control or HRG-depleted human plasma. Control human plasma (A), or HRG-depleted human plasma (B) was incubated with human HRG up to 5 μM in the absence (red line) or presence (blue line) of catheter segments for 15 minutes at 37°C before initiating clotting via the addition of 20 mM CaCl2. Absorbance was monitored and clot time was determined as the half-time of maximal absorbance. Mean ± SD of 4 determinations. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001 comparisons of the clot time in the absence of catheter segments and without HRG; #P < .05 and ##P < .01 compared with clot time in the presence of catheter segments and without HRG (one-way ANOVA and Holm-Sidak method).
Discussion
Catheter thrombosis is a common complication in patients with CVCs, but its pathogenesis is poorly understood.1,8,28 The results of this study identify HRG as a modulator of catheter thrombosis because the prothrombotic effect of catheters is enhanced when rabbit or human plasma is depleted of HRG and attenuated when HRG is added. Consistent with these in vitro findings, the time to catheter occlusion in rabbits is shorter when the HRG levels are reduced with an HRG-directed ASO compared with the times to catheter occlusion in rabbits given saline or a control ASO. Therefore, HRG modulates catheter thrombosis in vitro and in vivo.
Protein adsorption onto the surface of medical devices occurs rapidly after implantation.29-32 FXII, prekallikrein, and high molecular weight kininogen can bind directly to the surface, and by forming complexes of components of the contact system, high molecular weight kininogen can promote contact factor deposition on the surface of medical devices.30,33 Catheters induce clotting by promoting the autoactivation of bound FXII.9,14 The resultant FXIIa then initiates coagulation on the catheter surface by activating bound FXI and promoting thrombin generation.30,33 Consistent with our previous findings, the central role of FXII in this process is highlighted by our current observation that catheter thrombosis in vitro or in vivo is attenuated by FXII depletion.9,14,23 Therefore, this study provides additional evidence that catheters trigger clotting by activating FXII.
In purified or plasma systems, we have previously shown that HRG binds to FXIIa and attenuates FXII autoactivation and FXIIa-mediated activation of FXI.20 In this study, we extend these findings by showing that (1) catheter-induced FXII autoactivation and thrombin generation is enhanced in HRG-depleted plasma and attenuated in FXII-depleted plasma, and (2) compared with the levels in the control rabbits, catheter implantation is associated with significantly higher and lower plasma levels of FXIIa-C1 inhibitor complexes with HRG and FXII depletion, respectively. The interaction of HRG with FXIIa is primarily mediated by its central histidine-rich region (HRR) because peptide or recombinant fragment analogs of the HRR recapitulate the effects of intact HRG.34 HRG binds to an exosite on FXIIa that is distinct from its active site because HRG has no effect on the chromogenic activity of FXIIa, nor does it influence the rate of FXIIa inhibition by C1 inhibitor.20 The results of this study suggest that the HRG binding exosite on FXIIa remains accessible when FXII undergoes autoactivation on the catheter surface, thereby explaining the capacity of HRG to modulate catheter thrombosis.
Similar to the findings in rabbit plasma, catheter clotting is enhanced when human plasma is depleted of HRG, and is attenuated when FXII is depleted. Therefore, the results in rabbits are likely to mirror those in humans. HRG levels are reduced in patients with severe sepsis or in those with advanced cancer.35-37 The low levels of HRG in such patients may contribute to a higher risk of catheter thrombosis compared with that in patients without these disorders.35,36 The fact that addition of HRG attenuates the prothrombotic effect of catheters in HRG-depleted rabbit or human plasma raises the possibility that systemic administration of HRG or peptides or recombinant analogs of the HRR will prevent catheter thrombosis in patients with reduced HRG levels.
In summary, we have shown that HRG modulates catheter thrombosis. These findings support the concept that HRG serves as a molecular brake for the contact system by attenuating the procoagulant activity of FXIIa regardless of whether FXII activation occurs locally on the surface of catheters, is triggered systemically with polyphosphate,22 or is induced by vascular injury with ferric chloride.21 The fact that therapeutic levels of FXa-directed oral anticoagulants are unable to prevent catheter-induced clotting in vitro suggests that alternative approaches are needed.38 Therefore, supplementation with HRG or HRG analogs34 has the potential to attenuate catheter thrombosis without perturbing hemostasis in individuals with hereditary or acquired HRG deficiency.
Acknowledgments
The authors thank Iqbal Jaffer of the Hamilton Health Sciences Heart Investigation Unit for generously providing the percutaneous coronary intervention catheters used for the in vitro studies. J.I.W. holds the Tier I Canada Research Chair in Thrombosis, and the Heart and Stroke Foundation and the J. Fraser Mustard Chair in Cardiovascular Research at McMaster University. This work was supported, in part, by grants-in-aid from the Canadian Institutes of Health Research (FDN-159928) and the Heart and Stroke Foundation of Canada (G-16-00013163).
Authorship
Contribution: R.A.M. designed and performed experiments, analyzed data, and wrote the paper; P.L. designed experiments and performed the catheter placement surgery; J.Z. designed and performed experiments; R.H. performed treatment randomization; L.H. and A.S.R. provided vital reagents and reviewed the manuscript; and J.C.F. and J.I.W. designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: L.H. and A.S.R. are employees of Ionis Pharmaceuticals. J.I.W. has served as a consultant for and has received honoraria from Ionis Pharmaceuticals, Anthos, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, Janssen, Merck, Pfizer, and Servier. The remaining authors declare no competing financial interests.
Correspondence: Jeffrey I. Weitz, Thrombosis and Atherosclerosis Research Institute, 237 Barton St E, Hamilton, ON L8L 2X2, Canada; e-mail: weitzj@taari.ca.
References
Author notes
Data are available on request from the corresponding author, Jeffrey I. Weitz (weitzj@taari.ca).
The full-text version of this article contains a data supplement.