In this issue of Blood Advances, Malik et al have shown that plasma concentrations of histidine-rich glycoprotein (HRG) modulate factor XIIa (FXIIa) activity and, in turn, catheter thrombosis.1 This observation suggests that high concentrations of HRG may reduce contact activation and, thus, thrombosis. These studies may be the first step toward characterizing and translating a target that reduces thrombosis risk without affecting hemostasis.
HRG is produced in the liver at 75 kDa and circulates in plasma at a concentration between 1 and 3 μM. It is rich in histidine and proline and has a unique consensus sequence GHHPH that promotes its interaction with negatively charged ligands, such as sulfated glycosaminoglycans and many divalent cations, such as nickel and zinc.2 Upon protonation, it acquires a large positive charge. It is also pH sensitive, binding heparin, heparan sulfate, or dermatan sulfate. It has been shown to participate in fibrinolysis, complement activation, angiogenesis, immunity, and coagulation. It was first described to interact with the lysine binding sites on plasminogen, thereby interfering with plasminogen activation.3 Likewise, it was found to bind fibrinogen with high affinity to prolong the thrombin time.4 The concept developed that this protein is a negative regulator of plasma proteolytic processes, but its major function has only been recently clarified. It is a member of the cystatin superfamily of proteins with domain 1 and domain 2 most homologous to the heavy chain of kininogen and human cystatin S, respectively, 2 proteins that are cysteine protease inhibitors.5
The first investigation showing that HRG inhibited contact activation was performed by Vestergaard AB et al.6 These investigators showed that ellagic acid– or sulfatide-induced generation of plasma chromogenic substrate hydrolysis of HD-Pro-Phe-Arg-para nitroaniline (the FXIIa and plasma kallikrein substrate) was inhibited by HRG in a concentration-dependent manner. Degradation of HRG by plasmin reduced its inhibitory activity. The laboratory of Jahnen-Dechent recognized that Hrg–/– mice (HRG-null mice) have heightened coagulation and fibrinolysis.7 These animals demonstrated shortened prothrombin times, higher antithrombin activity, shorter tail tip bleeding times, and spontaneous fibrinolytic activity.
The focus on contact activation arose with the observation performed at the Weitz Laboratory that HRG binds FXIIa to inhibit contact activation-initiated blood coagulation.8 HRG was noted to prolong the activated partial thromboplastin time in a concentration-dependent manner. It did not influence the prothrombin time. It inhibited the autoactivation of FXII on various surfaces without inhibiting the active site of FXIIa on various chromogenic substrates. However, it inhibited FXIIa activation of FXI and prekallikrein. HRG bound FXIIa with physiologic concentrations of Zn2+ but did not bind FXII. HRG also neutralized polyanions and DNA, thereby reducing their procoagulant activity. HRG was found to bind polyphosphate (polyP) and neutralize its effect in vitro and in vivo. In HRG-null mice, polyP-induced coagulation shortens the activated partial thromboplastin time, increases thrombin generation and thrombin-antithrombin complexes, and promotes more fibrin deposition in the lungs.9 These studies indicated that contact activation coagulation was enhanced in HRG-null mice because it was blocked by FXII depletion with an antisense oligonucleotide.
HRG-null mice are constitutively prothrombotic.10 In additional work from the Weitz Laboratory, thrombin generation induced by DNA or RNA was found to be enhanced in the HRG-null mice. Additionally, carotid artery thrombosis initiated by ferric chloride was accelerated in the HRG-null mice. Although DNA depletion or FVII knockdown had no effect on ferric chloride arterial thrombosis in HRG-null mice, depletion of RNA or FXII knockdown attenuated the shortened time to thrombosis in these animals. These investigations provided clear information that HRG is a strong regulator of FXIIa initiation of thrombin generation.
This background set the stage for the investigations of this article.1 In these studies, Malik et al questioned whether HRG depletion would promote catheter thrombosis. It is generally agreed that FXII is the major promoter of catheter thrombosis because its knockdown attenuates it. In rabbits, HRG depletion by an antisense oligonucleotide shortened the activated partial thromboplastin time without effect on the prothrombin time. In vitro, catheter segments shortened the thrombin generation lag time and increased peak thrombin generation in rabbit plasma. These effects were enhanced in HRG-depleted plasma and attenuated in FXII-depleted plasma. After inserting catheters in the right external jugular vein of rabbits, the catheter occlusion time was significantly reduced in the HRG-depleted animals and significantly prolonged with FXII knockdown.
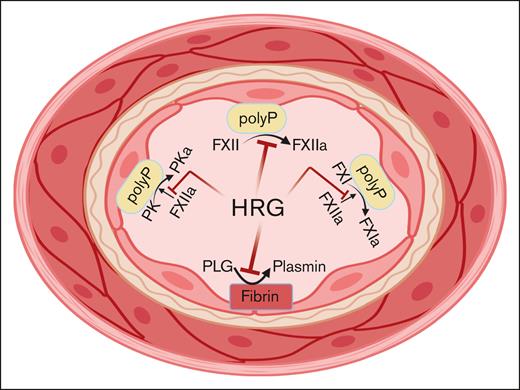
These investigations suggest that HRG repletion at times of consumption, for example, in some patients with sepsis or cancer, may attenuate the risk for catheter thrombosis, a common problem that increases morbidity for these patients. This notion needs to be proven experimentally. There are few clinical data to support this interpretation. One case report described a case of hereditary HRG deficiency associated with familial thrombophilia. However, another report described the opposite, that is, the association of familial elevation of HRG in a family with thrombophilia. The experimental data do not support the association of familial elevation of HRG with thrombophilia. Although the plasma concentration of HRG appears to most influence FXII activation and activation of the intrinsic coagulation system, it is unknown what its effects will be on plasminogen activation (see figure). Because it attenuates FXII activation and FXIIa activation of factor XI and prekallikrein simultaneously, it may markedly reduce plasminogen activation thus producing a prothrombotic situation that counterbalances its anticoagulant state.
HRG blocks FXII autoactivation to FXIIa, FXI activation to FXIa by FXIIa, and prekallikrein activation to plasma kallikrein by FXIIa on polyPs. All of these functions reduce thrombin formation. Alternatively, HRG, by binding to lysines on plasminogen, reduce plasmin formation such that if it were the only activity, it would contribute to thrombosis risk.
HRG blocks FXII autoactivation to FXIIa, FXI activation to FXIa by FXIIa, and prekallikrein activation to plasma kallikrein by FXIIa on polyPs. All of these functions reduce thrombin formation. Alternatively, HRG, by binding to lysines on plasminogen, reduce plasmin formation such that if it were the only activity, it would contribute to thrombosis risk.
In summary, these investigations show that pathways other than anticoagulation targeting thrombin, FXa, and FXIa need to be recognized and examined as a possible therapy to reduce thrombosis risk without increasing the risk of bleeding. This characterization of what HRG does in the blood coagulation system is an important first step in that direction.
Conflict-of-interest disclosure: A.H.S. declares no competing financial interests.