Key Points
EASIX assessed at 1 year after allo-SCT identifies patients at high risk for late NRM.
These findings suggest an important role of the endothelium for late NRM in long-term survivors after allo-SCT.
Abstract
Patients with hematological malignancies who survive the first year after allogeneic stem cell transplantation (allo-SCT) without relapse have a substantial risk of nonrelapse mortality (NRM) and missing predictive markers. The Endothelial Activation and Stress Index (EASIX) predicts endothelial complications and NRM early after allo-SCT. We hypothesized that EASIX assessed 1 year after allo-SCT in survivors who were disease free may predict late NRM. Survivors who were relapse-free at 1 year after allo-SCT were retrospectively studied in 2 independent cohorts (training cohort, n = 610; merged validation cohort, n = 852). EASIX determined 1 year after allo-SCT correlated with the overall survival (OS), NRM, and relapse. Serum endothelial and inflammatory markers were measured in the training cohort and correlated with EASIX-1year, which predicted OS and NRM but not relapse risk in both the training and validation cohorts in univariable and multivariable Cox regression analyses. Brier score and c-index analyses validated the univariable EASIX effects. There was no significant interaction between EASIX-1year and incidence of chronic graft-versus-host disease (GVHD) on OS. EASIX-1year predicted the outcome irrespective of preexisting comorbidities. Principal causes of NRM in both training and validation cohorts were infections with and without GVHD as well as cardiovascular complications. EASIX-1year correlated with sCD141 and interleukin-18 but not with C-reactive protein, suppressor of tumorigenicity-2, angiopoietin-2, CXCL9, or CXCL8. To our knowledge, EASIX-1year is the first validated predictor of late overall and NRM. Patients who are high risk as defined by EASIX-1year might be considered for intensified surveillance and prophylactic measures.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-SCT) is a well-established and effective treatment option for a variety of malignant and nonmalignant hematological diseases. The success of allo-SCT is impeded by relapse of the underlying disease and by nonrelapse mortality (NRM). NRM is often associated with immunological complications, most importantly graft-versus-host disease (GVHD). Relapse and GVHD significantly contribute to early mortality after allo-SCT. Overall survival (OS) for patients alive after the first 2 or 5 years after allo-SCT has increasingly improved and currently can be expected to be from 70% to 85% at 10 years after transplantation.1-4
Nonetheless, the risk of late mortality continues to be higher in these patients than in the age-matched general population.2,3 Although the relative mortality risk declines over time, it does not align with the expected general population rates.4,5 Several large cohort studies have investigated the reasons for continuing excess mortality of survivors of allo-SCT. Consistently, late mortality was caused by secondary malignancies, relapse of the hematological disease, infections, and pulmonary, renal, and cardiovascular disorders, often in the context of chronic GVHD (cGVHD).2,4,6,7 This ongoing increased mortality risk for survivors of allo-SCT necessitates a life-long risk-adjusted monitoring.
Therefore, reliable and easy-to-implement methods for estimating the individual risk of late mortality are urgently needed. This would enable clinicians to tailor follow-up and toxicity assessments as well as interventions for relapse prevention individually for each patient.
In recent years, evidence has accumulated that endothelial dysfunction contributes to severe complications after allo-SCT, such as refractory GVHD, transplantation-associated thrombotic microangiopathy (TMA), and sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD).8-11 Based on the hypothesis that TMA is the end stage of endothelial dysfunction, diagnostic criteria of TMA were merged with the Endothelial Activation and Stress Index (EASIX). This simple formula of routine laboratory parameters, measured at the onset of acute GVHD, enabled the prediction of GVHD outcome.12 Moreover, EASIX assessed before conditioning (“EASIX-pre”) predicted OS and NRM after allo-SCT.13 Besides allo-SCT, EASIX predicts mortality in lower-risk myelodysplastic syndrome (MDS),14 multiple myeloma,15 and COVID-19.16 Given the overrepresentation of cardiovascular events in long-term survivors of allotransplantation,17 we hypothesized that EASIX assessed 1 year after allo-SCT in patients who were disease free (ie, EASIX-1year) may also predict late mortality. To test this hypothesis, we analyzed the association of EASIX-1year with OS and NRM.
Methods
Study population
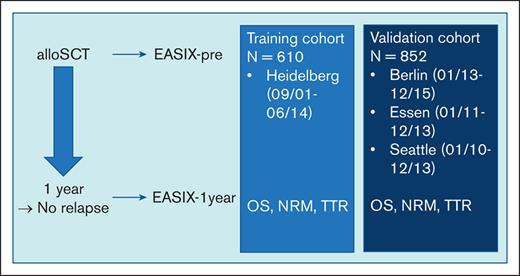
A total of 1565 consecutive adult patients who survived relapse-free the first year after allo-SCT were retrospectively recruited in 4 independent institutions. The training cohort included 610 patients who had received allografts at the University Hospital Heidelberg between September 2001 and June 2014. The validation cohort consisted of patients who had undergone allo-SCT at the Charité, Campus Benjamin Franklin, Berlin (Germany) between January 2013 and December 2015 (cohort I, n = 199); the University Hospital Essen (Germany) between January 2011 and December 2013 (cohort II, n = 233); and the Fred Hutchinson Cancer Research Center, Seattle, WA, between January 2010 and December 2013 (cohort III, n = 420). Acute and cGVHD was diagnosed and graded according to accepted clinical standards. Written informed consent according to the Declaration of Helsinki was obtained from all patients who were eligible, and the study was approved by the responsible institutional review boards.
Prognostic scores
EASIX was calculated as previously described (lactate dehydrogenase [LDH] [U/L] × creatinine [mg/dL]/thrombocytes [109 cells per L]).12-14 LDH, creatinine, and platelet counts were measured in clinically routine blood samples and values were retrieved from electronic files, either at the 1-year time point (±28 days) after allo-SCT (EASIX-1year) or day 0 until day −28 before conditioning therapy (EASIX-pre). The hematopoietic cell transplantation (HCT)–comorbidity index (CI) score,18 with its focus on patient-related factors, and the European Society for Blood and Marrow Transplantation (EBMT) score, considering patient and donor data,19 were available in the training cohorts (both) and for cohort 3 (Seattle, HCT-CI).
Cytokine enzyme-linked immunosorbent assays
Patients of the training cohort were recruited to a prospective observational study, collecting blood samples before conditioning therapy and in weekly/second weekly intervals for 1 year thereafter (ethic vote: S120-2002). Serum after 1 year was available for 551 patients of the training cohort (median time of sampling, day +346; range, 317-459). Serum levels of interleukin-18 (IL-18), sCD141 (soluble thrombomodulin), suppressor of tumorigenicity-2 (ST2), angiopoietin-2, CXCL8 (interleukin-8), and CXCL9 (monokine induced by interferon-gamma) were measured using the respective commercial enzyme-linked immunosorbent assay kits (DuoSet, R&D Systems, Wiesbaden, Germany) according to the manufacturer instructions.
Statistical analyses
Time-to-event end points considered were OS, time to relapse (TTR), and NRM, measured 1 year after allo-SCT. Survival and incidence curves for OS and TTR/NRM were computed based on Kaplan-Meier and Nelson-Aalen estimators, respectively. The effects of different variables on OS and TTR/NRM were assessed using univariable and multivariable Cox proportional hazards models. Because NRM and TTR are competing events, for these end points, cause-specific hazard ratios (HRs) were estimated.
To check for generalizability and prediction accuracy of the multivariable model, prediction error estimates were calculated for all event times using a time-dependent adaption of the Brier score and the concordance index (c-index).20 In order to determine the added value of including EASIX in the prediction model, c-index and Brier score have been computed for multivariable models with and without EASIX. We used log2 transformed index values for modeling as follows: log2 (EASIX) = log2 (LDH) + log2 (creatinine) – log2 (thrombocytes). Besides log2 (EASIX), the following variables were included in multivariable models: age >50 years, recipient sex, HLA mismatch (<10/10), reduced-intensity conditioning, antithymocyte globulin, methotrexate, myeloid vs lymphoid disease, and history of acute GVHD. Additional models were trained to check a possible interaction between EASIX and cGVHD and assess EASIX effects on survival in the context of comorbidities.
Pearson correlation between log2-transformed EASIX and log2-transformed serum markers were estimated to measure strength and direction of linear relationship. Jonckheere-Terpstra test was applied to check whether distribution of continuous serum markers differs between EASIX quartiles.
Results
Patient characteristics
Detailed patient characteristics by cohort are given in Table 1 and supplemental Table 1. Median age was >50 years in all cohorts. Recipient and donor sex, the frequency of HLA mismatch, and ongoing or cleared cGVHD were similar, and most patients suffered from acute leukemia or MDS. With respect to stem cell source, cohort 3 (Seattle) differed because it was the only 1 with cord blood transplantations. In addition, there were significant deviations among individual cohorts in terms of underlying disease, GVHD prophylaxis, antithymocyte globulin use, and conditioning intensity. Note that only patients who had a disease-free survival for 1 year were eligible for this analysis.
Patient characteristics
| . | Training cohort . | Validation cohort . | P∗ . |
|---|---|---|---|
| n = 610 . | n = 852 . | ||
| Date of allo-SCT | 09/2001-06/2014 | 01/2010-12/2013 | |
| Median age at allo-SCT (y, range) | 53 (18-75) | 52 (17-78) | .090 |
| Recipient sex | .830 | ||
| Female | 236 (38.7%) | 335 (39%) | |
| Male | 374 (61.3%) | 517 (61%) | |
| Donor sex | <.001 | ||
| Female | 203 (33.3%) | 312 (37%) | |
| Male | 407 (66.7%) | 494 (58%) | |
| Missing | 0 (0.0%) | 46 (5%) | |
| Donor relation | <.001 | ||
| MRD | 193 (31.6%) | 211 (25%) | |
| MUD | 304 (49.8%) | 473 (56%) | |
| MMUD | 100 (16.4%) | 106 (12%) | |
| MMRD | 7 (1.1%) | 8 (1%) | |
| Haplo | 6 (1.0%) | 13 (2%) | |
| UCB | 0 (0.0%) | 41 (5%) | |
| HLA mismatch | .590 | ||
| No | 497 (81.5%) | 684 (80%) | |
| Yes | 113 (18.5%) | 168 (20%) | |
| Disease | <.001 | ||
| AML | 184 (30.2%) | 375 (44%) | |
| MPN | 47 (7.7%) | 131 (15%) | |
| Lymphoma | 176 (28.9%) | 99 (12%) | |
| MM | 70 (11.5%) | 31 (4%) | |
| MDS | 66 (10.8%) | 93 (11%) | |
| ALL | 67 (11.0%) | 93 (11%) | |
| Other | 0 (0.0%) | 30 (4%) | |
| ATG | <.001 | ||
| No | 221 (36.2%) | 504 (59%) | |
| Yes | 389 (63.8%) | 347 (41%) | |
| NA | 0 (0.0%) | 1 (0%) | |
| GVHD prophylaxis | <.001 | ||
| MMF | 397 (65.1%) | 362 (43%) | |
| MTX | 213 (34.9%) | 490 (57%) | |
| Conditioning | <.001 | ||
| MAC, apl | 107 (17.5%) | 474 (56%) | |
| RIC | 503 (82.5%) | 378 (44%) | |
| History of aGVHD | <.001 | ||
| None | 379 (62%) | 255 (27%) | |
| Grades 1-2 | 206 (34%) | 634 (66%) | |
| Grades 3-4 | 25 (4%) | 65 (7%) | |
| cGVHD (positive/available [%]) | 323/554 (58%) | 379/653 (58%) | .926 |
| . | Training cohort . | Validation cohort . | P∗ . |
|---|---|---|---|
| n = 610 . | n = 852 . | ||
| Date of allo-SCT | 09/2001-06/2014 | 01/2010-12/2013 | |
| Median age at allo-SCT (y, range) | 53 (18-75) | 52 (17-78) | .090 |
| Recipient sex | .830 | ||
| Female | 236 (38.7%) | 335 (39%) | |
| Male | 374 (61.3%) | 517 (61%) | |
| Donor sex | <.001 | ||
| Female | 203 (33.3%) | 312 (37%) | |
| Male | 407 (66.7%) | 494 (58%) | |
| Missing | 0 (0.0%) | 46 (5%) | |
| Donor relation | <.001 | ||
| MRD | 193 (31.6%) | 211 (25%) | |
| MUD | 304 (49.8%) | 473 (56%) | |
| MMUD | 100 (16.4%) | 106 (12%) | |
| MMRD | 7 (1.1%) | 8 (1%) | |
| Haplo | 6 (1.0%) | 13 (2%) | |
| UCB | 0 (0.0%) | 41 (5%) | |
| HLA mismatch | .590 | ||
| No | 497 (81.5%) | 684 (80%) | |
| Yes | 113 (18.5%) | 168 (20%) | |
| Disease | <.001 | ||
| AML | 184 (30.2%) | 375 (44%) | |
| MPN | 47 (7.7%) | 131 (15%) | |
| Lymphoma | 176 (28.9%) | 99 (12%) | |
| MM | 70 (11.5%) | 31 (4%) | |
| MDS | 66 (10.8%) | 93 (11%) | |
| ALL | 67 (11.0%) | 93 (11%) | |
| Other | 0 (0.0%) | 30 (4%) | |
| ATG | <.001 | ||
| No | 221 (36.2%) | 504 (59%) | |
| Yes | 389 (63.8%) | 347 (41%) | |
| NA | 0 (0.0%) | 1 (0%) | |
| GVHD prophylaxis | <.001 | ||
| MMF | 397 (65.1%) | 362 (43%) | |
| MTX | 213 (34.9%) | 490 (57%) | |
| Conditioning | <.001 | ||
| MAC, apl | 107 (17.5%) | 474 (56%) | |
| RIC | 503 (82.5%) | 378 (44%) | |
| History of aGVHD | <.001 | ||
| None | 379 (62%) | 255 (27%) | |
| Grades 1-2 | 206 (34%) | 634 (66%) | |
| Grades 3-4 | 25 (4%) | 65 (7%) | |
| cGVHD (positive/available [%]) | 323/554 (58%) | 379/653 (58%) | .926 |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATG, antithymocyte globulin; apl, aplasia conditioning; MAC, myeloablative conditioning; MM, multiple myeloma; MMF, mycofenolat mofetil; MMRD, mismatched related donor; MMUD, mismatched unrelated donor; MPN, myeloproliferative neoplasm; MRD, matched related donor; MUD, matched unrelated donor; MTX, methotrexate; NA, not available; RIC, reduced-intensity conditioning; UCB, umbilical cord blood.
Kruskal-Wallis test; all other statistics were calculated with Mann Whitney U test.
EASIX-1year and mortality
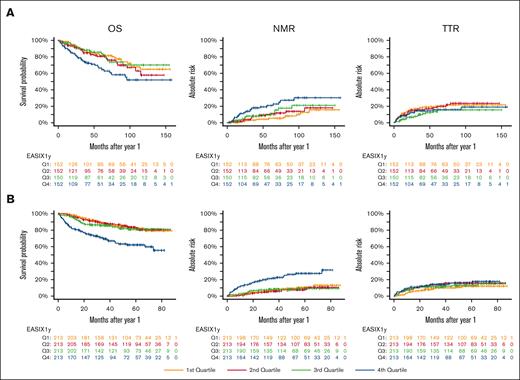
In the training cohort, EASIX-1year significantly correlated with OS and NRM in both univariable and multivariable Cox regression analyses (Table 2). This effect was similar in the pooled validation cohort (Table 3). EASIX-1year did not correlate with relapse risk in the training cohort (Table 2), whereas it associated with a small but significant hazard of relapse in the validation cohort (HR, 1.22; 95% confidence interval, 1.02-1.46; P = .031; Table 3). We visualized the influence of EASIX-1year on outcome in the training and validation cohorts, as given in Figure 1A-B. The effects of EASIX-1year quartiles on outcome for the 3 individual validation cohorts are shown in supplemental Figure 1. Similar to the results reported for EASIX-pre, the 3 individual variables composing EASIX-1year (LDH, creatinine, and platelets) showed independent, significant effects in a 3-variable Cox regression analysis, with NRM as the end point (supplemental Table 2).
Cox regression analysis of the training cohort
| Univariable Cox . | OS . | NRM . | TTR . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| EASIX-1y (twofold increase) | 1.50 | 1.26-1.74 | <.001 | 1.74 | 1.45-2.08 | < .001 | 0.95 | 0.76-1.17 | .618 |
| Multivariable Cox | n = 550, events = 125 | n = 550, events = 91 | n = 550, events = 66 | ||||||
| EASIX-1y (twofold increase) | 1.45 | 1.22-1.72 | < .001 | 1.68 | 1.34-2.10 | < .001 | 0.94 | 0.75-1.19 | .610 |
| Age above 50 y (yes vs no) | 1.68 | 1.12-2.51 | .011 | 2.02 | 1.16-3.54 | .014 | 1.30 | 0.82-2.07 | .266 |
| Recipient sex (male vs female) | 0.98 | 0.68-1.42 | .908 | 0.99 | 0.59-1.65 | .959 | 1.11 | 0.72-1.73 | .630 |
| HLA-mismatch (yes vs no) | 1.19 | 0.75-1.90 | .455 | 1.61 | 0.87-2.99 | .131 | 1.14 | 0.66-1.99 | .634 |
| RIC (yes vs no) | 1.20 | 0.68-2.12 | .520 | 1.31 | 0.60-2.88 | .495 | 0.80 | 0.43-1.49 | .479 |
| ATG (yes vs no) | 0.73 | 0.49-1.09 | .127 | 0.65 | 0.38-1.16 | .144 | 0.66 | 0.42-1.04 | .072 |
| MTX (yes vs no) | 0.80 | 0.53-1.19 | .269 | 0.62 | 0.35-1.09 | .096 | 0.86 | 0.53-1.38 | .533 |
| Diagnosis (myeloid vs lymphoid) | 0.96 | 0.66-1.39 | .819 | 1.24 | 0.75-2.07 | .403 | 0.57 | 0.36-0.89 | .013 |
| History of acute GVHD | 1.77 | 1.23-2.53 | .002 | 1.65 | 1.00-2.71 | .051 | 1.42 | 0.93-2.16 | .103 |
| Univariable Cox . | OS . | NRM . | TTR . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| EASIX-1y (twofold increase) | 1.50 | 1.26-1.74 | <.001 | 1.74 | 1.45-2.08 | < .001 | 0.95 | 0.76-1.17 | .618 |
| Multivariable Cox | n = 550, events = 125 | n = 550, events = 91 | n = 550, events = 66 | ||||||
| EASIX-1y (twofold increase) | 1.45 | 1.22-1.72 | < .001 | 1.68 | 1.34-2.10 | < .001 | 0.94 | 0.75-1.19 | .610 |
| Age above 50 y (yes vs no) | 1.68 | 1.12-2.51 | .011 | 2.02 | 1.16-3.54 | .014 | 1.30 | 0.82-2.07 | .266 |
| Recipient sex (male vs female) | 0.98 | 0.68-1.42 | .908 | 0.99 | 0.59-1.65 | .959 | 1.11 | 0.72-1.73 | .630 |
| HLA-mismatch (yes vs no) | 1.19 | 0.75-1.90 | .455 | 1.61 | 0.87-2.99 | .131 | 1.14 | 0.66-1.99 | .634 |
| RIC (yes vs no) | 1.20 | 0.68-2.12 | .520 | 1.31 | 0.60-2.88 | .495 | 0.80 | 0.43-1.49 | .479 |
| ATG (yes vs no) | 0.73 | 0.49-1.09 | .127 | 0.65 | 0.38-1.16 | .144 | 0.66 | 0.42-1.04 | .072 |
| MTX (yes vs no) | 0.80 | 0.53-1.19 | .269 | 0.62 | 0.35-1.09 | .096 | 0.86 | 0.53-1.38 | .533 |
| Diagnosis (myeloid vs lymphoid) | 0.96 | 0.66-1.39 | .819 | 1.24 | 0.75-2.07 | .403 | 0.57 | 0.36-0.89 | .013 |
| History of acute GVHD | 1.77 | 1.23-2.53 | .002 | 1.65 | 1.00-2.71 | .051 | 1.42 | 0.93-2.16 | .103 |
ATG, antithymocyte globulin; MTX, methotrexate; RIC, reduced-intensity conditioning; 95% CI, 95% confidence interval.
Cox regression analysis of the validation cohort
| Univariable Cox . | OS . | NRM . | TTR . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| EASIX-1y (twofold increase) | 1.61 | 1.42-1.82 | < .001 | 1.76 | 1.52-2.03 | < .001 | 1.22 | 1.03-1.46 | .023 |
| Multivariable Cox | n = 851 events = 162 | n = 851, events = 101 | n = 851, events = 110 | ||||||
| EASIX-1y (twofold increase) | 1.60 | 1.41-1.83 | <.001 | 1.77 | 1.50-2.05 | <.001 | 1.22 | 1.02-1.46 | .031 |
| Age above 50 y (yes vs no) | 1.26 | 0.91-1.76 | .168 | 1.56 | 1.02-2.40 | .041 | 1.20 | 0.80-1.82 | .378 |
| Recipient sex (male vs female) | 1.15 | 0.83-1.61 | .403 | 1.17 | 0.76-1.78 | .475 | 1.09 | 0.73-1.62 | .670 |
| HLA-mismatch (yes vs no) | 0.94 | 0.63-1.39 | .756 | 0.91 | 0.55-1.49 | .702 | 0.95 | 0.58-1.58 | .858 |
| RIC (yes vs no) | 0.98 | 0.66-1.47 | .934 | 1.23 | 0.75-2.03 | .416 | 1.02 | 0.62-1.68 | .943 |
| ATG (yes vs no) | 1.26 | 0.91-1.77 | .169 | 1.15 | 0.74-1.78 | .530 | 1.64 | 1.11-2.44 | .014 |
| MTX (yes vs no) | 1.24 | 0.83-1.86 | .290 | 1.13 | 0.68-1.88 | .664 | 0.86 | 0.52-1.41 | .542 |
| Diagnosis (myeloid vs lymphoid) | 1.14 | 0.80-1.65 | .464 | 1.10 | 0.69-1.76 | .675 | 0.80 | 0.53-1.22 | .304 |
| History of acute GVHD | 0.88 | 0.61-1.28 | .507 | 1.75 | 1.00-3.04 | .049 | 0.42 | 0.28-0.63 | < .001 |
| Univariable Cox . | OS . | NRM . | TTR . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| EASIX-1y (twofold increase) | 1.61 | 1.42-1.82 | < .001 | 1.76 | 1.52-2.03 | < .001 | 1.22 | 1.03-1.46 | .023 |
| Multivariable Cox | n = 851 events = 162 | n = 851, events = 101 | n = 851, events = 110 | ||||||
| EASIX-1y (twofold increase) | 1.60 | 1.41-1.83 | <.001 | 1.77 | 1.50-2.05 | <.001 | 1.22 | 1.02-1.46 | .031 |
| Age above 50 y (yes vs no) | 1.26 | 0.91-1.76 | .168 | 1.56 | 1.02-2.40 | .041 | 1.20 | 0.80-1.82 | .378 |
| Recipient sex (male vs female) | 1.15 | 0.83-1.61 | .403 | 1.17 | 0.76-1.78 | .475 | 1.09 | 0.73-1.62 | .670 |
| HLA-mismatch (yes vs no) | 0.94 | 0.63-1.39 | .756 | 0.91 | 0.55-1.49 | .702 | 0.95 | 0.58-1.58 | .858 |
| RIC (yes vs no) | 0.98 | 0.66-1.47 | .934 | 1.23 | 0.75-2.03 | .416 | 1.02 | 0.62-1.68 | .943 |
| ATG (yes vs no) | 1.26 | 0.91-1.77 | .169 | 1.15 | 0.74-1.78 | .530 | 1.64 | 1.11-2.44 | .014 |
| MTX (yes vs no) | 1.24 | 0.83-1.86 | .290 | 1.13 | 0.68-1.88 | .664 | 0.86 | 0.52-1.41 | .542 |
| Diagnosis (myeloid vs lymphoid) | 1.14 | 0.80-1.65 | .464 | 1.10 | 0.69-1.76 | .675 | 0.80 | 0.53-1.22 | .304 |
| History of acute GVHD | 0.88 | 0.61-1.28 | .507 | 1.75 | 1.00-3.04 | .049 | 0.42 | 0.28-0.63 | < .001 |
EASIX-1year predicts NRM in patients surviving without disease progression for 1 year after allo-SCT. Kaplan-Maier curves for OS and cumulative incidences of NRM and TTR, according to EASIX quartiles calculated 1 year after allo-SCT in patients without relapse. (A) Training cohort. (B) Validation cohort. Q, quartile.
EASIX-1year predicts NRM in patients surviving without disease progression for 1 year after allo-SCT. Kaplan-Maier curves for OS and cumulative incidences of NRM and TTR, according to EASIX quartiles calculated 1 year after allo-SCT in patients without relapse. (A) Training cohort. (B) Validation cohort. Q, quartile.
Validation of the univariable model
Brier score and c-index analyses for the validation cohort, with offset of the training cohort, revealed a predictive impact of EASIX-1year on both the OS and NRM, validating the univariable model (supplemental Figure 2). This allowed us to incorporate EASIX-1year into our online EASIX calculator.
Causes of NRM
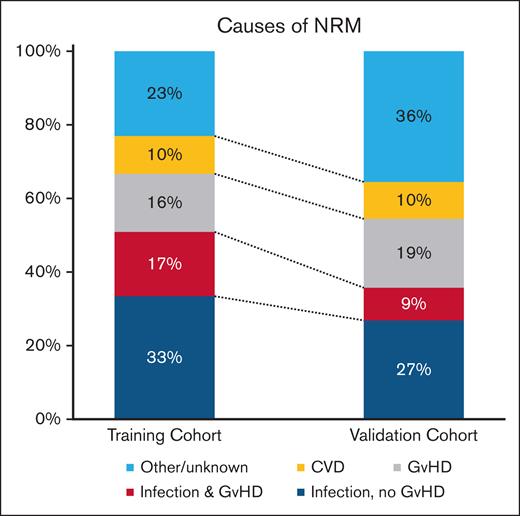
Principal causes of NRM in both training and validation cohorts were infections with and without cGVHD and cGVHD without infections but with cardiovascular complications. Specifically, infections with or without GVHD accounted for 51% and 36% of NRM events in the training and validation cohorts, respectively. Of note, 10% of NRM were directly attributed to cardiovascular diseases in both the training and the validation cohorts (Figure 2).
Causes of NRM in patients surviving 1 year without relapse after allo-SCT. CVD, cardiovascular disease.
Causes of NRM in patients surviving 1 year without relapse after allo-SCT. CVD, cardiovascular disease.
EASIX-1year in the context of cGVHD, age, and comorbidity
There was no significant interaction between EASIX-1year and history of cGVHD in training and validation cohorts (supplemental Table 3). Age and comorbidities are known additional principal causes of NRM. We, therefore, assessed the impact of EASIX-1year in patients above and below the age of 50 years and having above or below an HCT-CI score of 2. EASIX-1year had similar effects in all subgroups (supplemental Figure 3).
EASIX-pre and the EBMT scores are already validated predictors of NRM after allo-SCT, independent of HCT-CI. EASIX-pre and EASIX-1year correlated with a Spearman-ρ coefficient of 0.301 in the training cohort and 0.281 in the validation cohort. In contrast, there was no significant correlation between EASIX-1year and the HCT-CI score (Spearman-ρ coefficient of 0.016 in the training cohort and 0.233 in cohort 3) or the EBMT score (Spearman-ρ coefficient of 0.134 in the training cohort only). On multivariable Cox regression analysis adjusting for EASIX-pre, HCT-CI, and EBMT score, EASIX-1year remained a significant predictor of OS and NRM calculated from the 1-year landmark in all settings tested. In contrast, none of the other 3 scores showed consistent significant effects for NRM (supplemental Tables 4-6).
EASIX-1year and serum markers associating with endothelial complications and cGVHD
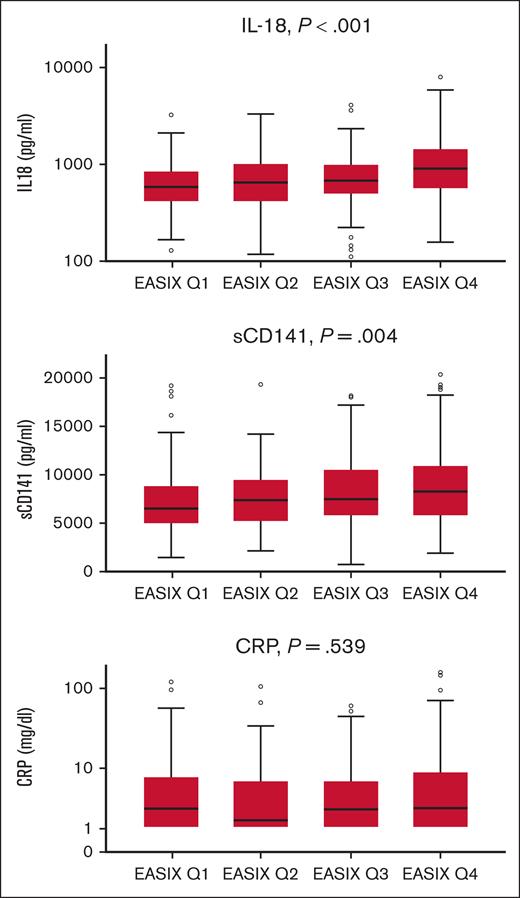
EASIX-1year correlated with IL-18 (Pearson correlation coefficient (r) = 0.236; 95% confidence interval, 0.139-0.328; P < .001) and sCD141 (r = 0.153; 0.07-0.245, P = 0.001). Similar results were obtained in subgroups of patients with and without severe cGVHD. In addition, EASIX and c-reactive protein (CRP) correlated in patients without severe cGVHD (r = 0.238; 0.027-0.414; P < .001; n = 382) but not in patients with severe cGVHD (r = 0.070; P = .481; n = 104). Figure 3 shows the association of EASIX-1year quartiles with IL-18, sCD141, and CRP using Jonckheere-Terpstra tests. No association was found between EASIX-1year and ST2 (P = .701), angiopoietin-2 (P = .508), CXCL8 (P = .707), and CXCL9 (P = .067). In addition, patients with severe cGVHD had higher serum levels of CRP (P = .029), CXCL9 (P = .007), and ST2 (P = .005 Kruskal-Wallis tests).
Correlation of EASIX-1year (quartiles) with IL-18, sCD141, and CRP in patients surviving 1 year without relapse after allo-SCT. Box plots: horizontal lines indicate the median. Dots represent individual patient samples. sCD141 (soluble thrombomodulin, n = 491), IL-18 (n = 494), and CRP (n = 508). P values were calculated using Jonckheere-Terpstra tests.
Correlation of EASIX-1year (quartiles) with IL-18, sCD141, and CRP in patients surviving 1 year without relapse after allo-SCT. Box plots: horizontal lines indicate the median. Dots represent individual patient samples. sCD141 (soluble thrombomodulin, n = 491), IL-18 (n = 494), and CRP (n = 508). P values were calculated using Jonckheere-Terpstra tests.
Discussion
In this study, we report that EASIX-1year in patients who are disease free is a predictor of overall mortality and NRM, measured since the 1-year landmark.
The mortality rate of patients after allo-SCT remains increased for many years compared with that of the general population.1 Several studies underline the importance of regular assessment and screening for secondary malignancies, infectious complications, and organ dysfunctions.21 In particular, patients with cGVHD require close monitoring for an extended time.1,6 The necessity of continuing anti-infective prophylaxis and vaccination in patients with cGVHD has to be emphasized.21 Moreover, patients suffering from cGVHD require a close follow-up for cardiovascular risk factors, renal function, and other organ damages.21
In recent years, growing evidence emerged that endothelial dysfunction plays a central role in the pathophysiology of severe complications after allo-SCT, such as acute GVHD, transplant-associated thrombotic microangiopathy, and SOS/VOD.9,10,22,23 Similarly, the endothelium may affect the long-term outcome of survivors of allo-SCT; chronic skin GVHD was reported to be associated with loss of capillaries,24,25 and high CXCL9 has been found in cGVHD26 and may predict severity of the disease.27 CXCL9 binds to CXCR3, a receptor expressed on both T cells and endothelial cells, thus representing a possible link between immunity and endothelial cell dysfunction. Indeed, increased CXCL9 serum levels were observed in our training cohort in patients with severe cGVHD 1 year after allo-SCT.
The role of endothelial cell biology in late survivors is further emphasized by the observation that these patients become a cardiovascular high-risk cohort after allo-SCT.17 EASIX was initially developed as a marker to predict TMA-like complications in patients who received allografts. Accumulating experience with this score suggests a more extended applicability including the prediction of SOS/VOD and early fluid retention after transplantation28,29 and even prediction of mortality of patients with lower-risk MDS not receiving allo-SCT.14 EASIX-1year predicted late NRM in both training and validation cohorts. Major causes of NRM were infections with or without GVHD, GVHD without infections, and cardiovascular events. Clearly, risk of infections is associated with prolonged immunosuppression and/or immunodeficiency, particularly, in the context of GVHD. However, death from infections, for example, in sepsis, may be related to endothelial dysfunction leading to impaired microcirculation and organ damage.30,31 The prediction of mortality via EASIX-1year, including a major proportion of lethal infectious complications, supports the hypothesis of a link between resilience against infections and the endothelium.
Although we have reported previously that EASIX indicates an increased risk of NRM after cGVHD onset, it was not correlated with cGVHD severity.27 In this analysis, a significant interaction between EASIX and cGVHD for the end point OS did not emerge, and EASIX-1year had similarly increased HRs of NRM in patients with cGVHD (ongoing or cleared) and without cGVHD in both cohorts. Accordingly, EASIX correlated with sCD141, an endothelial marker shed by stressed endothelial cells,32 and IL-18, both in patients with and without severe cGVHD. EASIX-pre strongly correlated with IL-18 before conditioning therapy, and our group suggested a direct inhibitory effect of IL-18 on platelet production.33,34 In contrast, CXCL9, CRP, and ST2, although increased in this subgroup of patients with severe cGVHD, were not associated with EASIX-1year, underlining the heterogeneity of endothelial derived serum markers.
Other possible causes of late endothelial complications could be age and preexisting endothelial dysfunction, as indicated via EASIX-pre.13,35 Although EASIX-1year appears to be equally predictive in younger and older patients, there was a weak but significant correlation between EASIX-pre and EASIX-1year. However, in contrast to EASIX-1year, the predictive capacity of EASIX-pre for outcomes measured from the 1-year landmark was only marginal and inconsistent, suggesting that the baseline endothelial dysfunction indicated by EASIX-pre is not a major contributor to the endothelial risk of survivors who were 1-year disease free, as detected via EASIX-1year.
Although this is a retrospective study, the results have been analyzed in a large training and confirmed in several, equally large validation cohorts in institutions in Germany and the United States. Shortcomings are the lack of immune reconstitution data and the lack of data on early endothelial complications (sepsis, TA-TMA, and SOS/VOD) in our cohorts. The advantage of EASIX-1year is the simplicity of the approach. The data required for EASIX are laboratory parameters, which are inexpensive and routinely assessed.
In summary, EASIX assessed 1 year after allo-SCT identifies patients at high risk for late NRM, and it may be a valuable tool for stratifying surveillance intensity in surviving recipients of allo-SCT who were disease-free long term. Moreover, these findings back up clinical observations suggesting an important role of the endothelium for late NRM, highlighting the importance of endothelium-protective measures during the long-term care of survivors of transplantation, such as optimized management of infections, diabetes, and hypertension, or even more aggressive interventions.
Acknowledgments
The authors thank the following funding agencies for supporting this work: Deutsche Forschungsgemeinschaft (TL820.8-1), Wilhelm Sander-Stiftung (2016.077.1), Deutsche Krebshilfe (70113519), and EU306240 (T.L.); José Carreras Leukämie-Stiftung (3R/2019), Deutsche Krebshilfe (70113519), and Deutsche Forschungsgemeinschaft (PE 1450/7-1) (O.P.); CA 78902, CA 18029, CA 15704, and HL 122173 (M.S., T.G., and B.M.S.).
There was no external funding source for this study. All authors had access to the raw data. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Authorship
Contribution: All authors contributed substantially to the manuscript. T.L., L.K., T.G., O.P., and P.D. contributed to the study conception and design; L.K., T.L., and P.D. drafted the manuscript; and all authors critically revised and approved the manuscript, contributed to data acquisition, analysis, or interpretation and agree that they are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Luft, Medicine V, University Hospital Heidelberg, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany; e-mail: thomas.luft@med.uni-heidelberg.de.
References
Author notes
∗L.K. and T.T. are joint authors.
Data are available on request from the corresponding author, Thomas Luft (thomas.luft@med.uni-heidelberg.de).
The full-text version of this article contains a data supplement.