Key Point
The combination of vemurafenib and intermediate-dose Ara-C and 2-CdA is feasible and effective for unmaintained remission in pediatric LCH
Abstract
Langerhans cell histiocytosis (LCH) is a disorder with a variety of clinical signs. The most severe forms affect risk organs (RO). The established role of the BRAF V600E mutation in LCH led to a targeted approach. However, targeted therapy cannot cure the disease, and cessation leads to quick relapses. Here, we combined cytosine-arabinoside (Ara-C) and 2'-chlorodeoxyadenosine (2-CdA) with targeted therapy to achieve stable remission. Nineteen children were enrolled in the study: 13 were RO-positive (RO+) and 6 RO-negative (RO–). Five patients received the therapy upfront, whereas the other 14 received it as a second or third line. The protocol starts with 28 days of vemurafenib (20 mg/kg), which is followed by 3 courses of Ara-C and 2-CdA (100 mg/m2 every 12 h, 6 mg/m2 per day, days 1-5) with concomitant vemurafenib therapy. After that, vemurafenib therapy was stopped, and 3 courses of mono 2-CdA followed. All patients rapidly responded to vemurafenib: the median disease activity score decreased from 13 to 2 points in the RO+ group and from 4.5 to 0 points in the RO– group on day 28. All patients except 1 received complete protocol treatment, and 15 of them did not have disease progression. The 2-year reactivation/progression-free survival (RFS) for RO+ was 76.9% with a median follow-up of 21 months and 83.3% with a median follow-up of 29 months for RO–. Overall survival is 100%. Importantly, 1 patient experienced secondary myelodysplastic syndrome after 14 months from vemurafenib cessation. Our study demonstrates that combined vemurafenib plus 2-CdA and Ara-C is effective in a cohort of children with LCH, and the toxicity is manageable. This trial is registered at www.clinicaltrials.gov as NCT03585686.
Introduction
Langerhans cell histiocytosis (LCH) is a disorder that arises from bone marrow (BM) early myeloid progenitors with an abnormal accumulation of myeloid cells that phenotypically resemble Langerhans cells, leading to a clinical presentation that varies from a single bone or skin lesion to severe multisystem disease. Although local forms can resolve without treatment, the involvement of risk organs (RO), which include hematopoietic involvement, the spleen, and the liver, is associated with an unfavorable prognosis.
The standard treatment approach is based on vinblastine and prednisone therapy, which lasts for at least 1 year. Although the overall outlook is good for RO- patients who receive vinblastine-prednisone therapy, up to 40% of the patients still experience relapses.1 The LCH-IV protocol is the established standard for second-line treatment,2 but there is no standard third-line therapy. 2’-Chlorodeoxyadenosine (2-CdA) showed good efficacy when used as monotherapy, but the cohort was diverse, and there was a significant number of nonresponders.3
The outcome in RO+ patients is worse, with a historical 5-year survival rate of ≤50% in patients refractory to first-line treatment using vinblastine and steroids.4 Salvage second-line therapy with 2-CdA at 9 mg/m2 per day and intermediate-dose (up to 500 mg/m2 every 12 hours) cytosine-arabinoside (Ara-C) is very effective and curative in most responders.5 This regimen provides an impressive response rate in RO+ patients but is associated with severe myelotoxicity and immune suppression.6 Reduced doses of Ara-C and 2-CdA were less toxic and more feasible, but still effective, with a 3-year overall survival (OS) of 73%.7 Alternative potentially curative therapies, such as allogeneic hematopoietic stem cell transplantation (allo-HSCT), have shown less promising results, with an OS of ∼50%.8
The discovery of the pivotal role of the BRAF V600E mutation in LCH provided a rationale for targeted therapy for patients with LCH.9 Notably, BRAF V600E-positive LCH showed a high incidence of relapse and risk organ involvement.10 Although aggressive, BRAF V600E histiocytosis is susceptible to targeted therapy. The activity of BRAF inhibition in BRAF V600E-positive histiocytic disorders was first demonstrated in adults with Erdheim-Chester disease (ECD)11 and LCH.12,13 Later, the safety and efficacy of BRAF inhibitors were shown in a cohort of children with BRAF V600E-positive LCH, including the most severe RO+ cohort.14
Although vemurafenib led to the immediate dramatic improvement of LCH symptoms and biological activity, the cessation of therapy resulted in a quick relapse in most cases. Thus, it is currently unknown whether targeted therapy can be withdrawn safely and what the potential predictive markers are for safe therapy cessation.
Based on the high activity of the targeted therapy with vemurafenib and the proven curative potential of the 2-CdA/Ara-C chemotherapy, we hypothesized that the 2 principles could be combined to create an effective and tolerable therapy without prolonged exposure to either of the components. The safety of the combination was observed in our previous retrospective study.15
Recently, a liquid biopsy approach with the detection of BRAF V600E in circulating cell-free DNA (cfDNA) using digital droplet polymerase chain reaction (ddPCR) was proposed as a way to monitor minimal residual disease (MRD) in BRAF + LCH.16,17 Later, this technology was suggested as a prognostic biomarker for patients with LCH.18 However, although negative cfDNA BRAF V600E is correlated with better progression-free survival, there is still a significant proportion of BRAF V600E cfDNA-negative patients who experience disease progression.19 Based on the biology of LCH as a clonal disorder of myeloid progenitors, we hypothesized that quantitative assessment of the sorted population of BM CD34+CD117+ cells for the BRAF V600E mutation could provide a new way to predict the long-term outcome of the novel combined therapy approach. Thus, to establish the origin and determine the potential for relapse, we applied the ddPCR method to previously sorted CD34+CD117+ myeloid progenitors at specific predetermined time points.
Based on the above, a prospective pilot clinical trial was initiated with the goal of testing the safety and efficacy of the consecutive use of targeted therapy with vemurafenib and intermediate-dose 2-CdA and Ara-C. Correlative research included monitoring of MRD in cfDNA and myeloid progenitors. This report summarizes the results of the trial.
Methods
The treatment regimen started with induction vemurafenib therapy for 28 days at a starting dose of 20 mg/kg per day, once a day, rounded up to half of the pill. Pills were taken orally as a whole or were crushed and diluted for the younger patients.
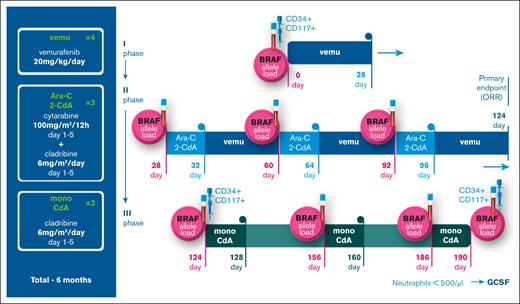
After that, 3 cycles of Ara-C plus 2-CdA (100 mg/m2 every 12 hours, days 1-5 and 6 mg/m2 per day, days 1-5, respectively) were conducted with 28-day intervals. Vemurafenib was interrupted during chemotherapy to prevent drug interactions. On the first day after each chemotherapy course, vemurafenib therapy was resumed. After 3 cycles of Ara-C plus 2-CdA, vemurafenib therapy was ceased, and 3 courses of 2-CdA monotherapy at 6 mg/m2 per day, days 1 to 5, were administered as maintenance therapy. The total planned duration of all therapies was 6 months. Granulocyte colony-stimulating factor (G-CSF) 10 mcg/kg IV was started on day +5 after each Ara-C+2-CdA course to shorten the duration of neutropenia. The patients were treated stationary during the first 3 courses of Ara-C+2-CdA, and 3 courses of mono 2-CdA were conducted in day hospital wards. Electrocardiogram and markers of myocardial injury were monitored once a week during the first 4 weeks and once a month during the following treatment period. Figure 1 represents the actual protocol scheme. The detailed protocol can be found in supplemental 1.
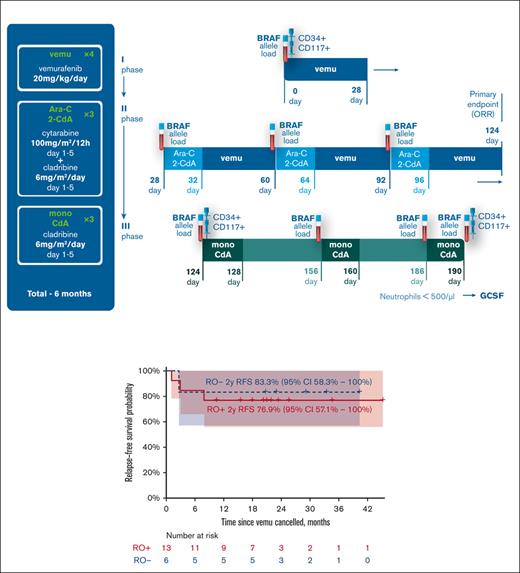
Protocol scheme, dosage, and schedule. The figure represents the scheme of the protocol, consisting of 3 phases: 28 days of mono vemurafenib induction, 3 courses of Ara-C + 2-CdA with continuous vemurafenib therapy and 3 courses of mono 2-CdA. The points of cfDNA and myeloid precursor population ddPCR analysis are also marked accordingly.
Protocol scheme, dosage, and schedule. The figure represents the scheme of the protocol, consisting of 3 phases: 28 days of mono vemurafenib induction, 3 courses of Ara-C + 2-CdA with continuous vemurafenib therapy and 3 courses of mono 2-CdA. The points of cfDNA and myeloid precursor population ddPCR analysis are also marked accordingly.
MRD was measured at specific predetermined points (initially, before the start of each course, and at the end of the therapy) using ddPCR on blood-derived cfDNA. Although we call these measurements “MRD,” this should be considered a simplification, as the role of BRAF V600E cfDNA measurement as MRD is not yet established in LCH.
At specific, predetermined points, we performed BM aspiration. Isolation of the CD34+CD117+ population was performed, and ddPCR was used to measure the BRAF V600E allelic load. Isolation of myeloid progenitor cells was performed on freshly collected BM samples.
The serum concentration of vemurafenib was quantified by high-performance liquid chromatography–mass spectrometry (HPLC–MS). More information about the specific methods of ddPCR, immunophenotyping, and vemurafenib serum concentration analysis can be found in supplemental 2.
This prospective, multicenter, nonrandomized study was approved by the local ethics committee (approval number 3e/1-18), and all patients’ legal representatives provided written informed consent according to the Declaration of Helsinki. The trial was registered on clinicaltrials.gov under NCT03585686. The first patient was enrolled on 22 June 2018, and the data cutoff date was 30 July 2022.
The inclusion criteria were age 0 to 18 years, immunohistochemistry (IHC) confirmed diagnosis of LCH, RO+ status irrespective of the previous therapy, RO– status with relapse/progression after at least 2 lines of previous therapy, confirmed BRAF V600E status, QTc interval <0.5 seconds, no previously documented heart disorders, and a signed informed consent form. The exclusion criteria were antiarrhythmic therapy, uncontrolled electrolyte disturbances, a QTc interval >0.5 seconds, and withdrawal of informed consent.
Disease activity was quantified using the disease activity score (DAS) described by Donadieu et al,20 the scoring scale used in the high-risk cohort in the international LCH-IV protocol.
According to the protocol, the primary end point was the overall response rate (ORR) proportion, which was defined as the sum of the complete response rate and partial response rate, which were defined as DAS scores 0 to 1 and 2 to 3, respectively, at day 124. The secondary end points were the reactivation/progression-free survival (RFS) from vemurafenib cessation to the last observation, the proportion of patients with severe adverse effects, and OS.
Adverse events were identified and graded according to version 5.0 of the Common Terminology Criteria for Adverse Events.
Statistical analysis was performed using XLSTAT 2021.2.2 software and R 4.0.2. Medians and ranges are used to describe continuous variables, and frequencies and proportions are used to describe categorical variables. The Kaplan–Meier method was used to evaluate OS, event-free survival (EFS), and relapse-free survival (RFS), and the median follow-up was measured using the reverse Kaplan–Meier method.21 Mann–Whitney U test was used to compare MRD between groups, and the Wilcoxon signed-rank test was used to compare MRD between time points. The study sample size was estimated to test the hypothesis that the ORR at day 124 is higher than the historical ORR with a power of 80% and a type 1 error of 10% using Simon’s 2-stage design22 with 5 patients in the first stage and 9 extra patients in the second stage.
Results
Patients
Our study included 19 patients (9 males, 10 females) with BRAF V600E-positive LCH. Thirteen patients had RO+ LCH, and 6 patients had RO– LCH. In all patients, the diagnosis was confirmed by either a surgical or punch biopsy with obligatory IHC staining for CD1a+/CD207+. The BRAF V600E mutation was detected with mutation-specific PCR and confirmed by Sanger sequencing.
The median age at disease onset was 10 months (0-22 months) in the RO+ group and 24 months (18-43 months) in the RO- group. The median ages at enrollment in the protocol were 17 months (4-39 months) and 62 months (37-107 months) in the RO+ and RO– groups, respectively. RO+ patients received protocol therapy as their first (n = 6, 46.2%), second (n = 5, 38.5%) or further (n = 2, 14.4%) line of therapy. RO– patients received the protocol as the third (n = 5, 83.3%) or fourth (n = 1, 16.7%) line of therapy. No previous targeted therapy was conducted. In the RO+ group, the median DAS at protocol start was 13 points (6-20), whereas in the RO– group, the median DAS at protocol start was 4.5 points (1-6). The complete characteristics of the cohort are presented in Table 1, and the key features by cohort are presented in Table 2.
A characteristic of patients cohort
| MIS ID . | Sex . | Age Dx, mo . | Age Tx, mo . | Organs involved . | LoT . | Previous therapy . | DAS, points . | DAS after vemu, points . | cfDNA load initially, % . | cfDNA load vemu cess, % . | cfDNA load therapy end, % . | MP BRAF load initially, % . | MP load vemu cess, % . | MP BRAF load therapy end, % . | Relapse . | EFS/time to relapse, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 132056 | F | 15 | 27 | RO, skin | 2 | VBL + PRE | 20 | 2 | 14.08 | <LoD | 0.5 | 4.84 | <LoD | <LoD | No | 45 |
| 147958 | F | 0 | 4 | RO, skin, lungs | 1 | No | 8 | 0 | 1.41 | <LoD | n/d | <LoD | <LoD | n/d | Yes (skin) | 1 |
| 189102 | F | 15 | 17 | RO, skin | 1 | No | 16 | 0 | 15.88 | <LoD | <LoD | 0.87 | <LoD | 0.01 | No | 21 |
| 159833 | F | 22 | 25 | RO, skin | 1 | No | 15 | 0 | 5.25 | 0.33 | 0.24 | 0.6 | <LoD | <LoD | No | 34 |
| 153888 | M | 19 | 25 | RO, skin, lungs | 2 | VBL + PRED | 6 | 0 | 5.29 | <LoD | 0.86 | 0.1 | 0.03 | 0.07 | Yes (bone) | 8 |
| 187463 | F | 14 | 17 | RO | 1 | No | 13 | 0 | 12.69 | <LoD | <LoD | 0.79 | <LoD | <LoD | No | 25 |
| 190889 | M | 1 | 31 | RO, skin, MF bones | 4 | VBL + PRED, VBL + PRED + VP16, VCR/Ara-C/PRED | 8 | 2 | 3.48 | 0.51 | 1.55 | 1.13 | 0.02 | 0.03 | No | 18 |
| 186934 | M | 9 | 15 | RO, skin | 2 | VBL + PRED | 19 | 2 | 3.66 | 0.20 | 0.54 | n/d | 0.06 | 0.05 | No | 21 |
| 202807 | M | 5 | 7 | RO, MF bones, skin, l/n, lungs | 1 | No | 16 | 2 | 14.80 | 0.94 | 0.16 | 0.68 | <LoD | <LoD | No | 15 |
| 181102 | M | 3 | 8 | RO, skin | 1 | No | 16 | 0 | 12.41 | 1.18 | 2.33 | 1.81 | 0.20 | 1.03 | Yes (RO+) | 2 |
| 116953 | F | 10 | 39 | RO, MF bones, DI, skin | 3 | VBL + PRED, VCR/Ara-C/PRED | 10 | 2 | 30.91 | 0.34 | <LoD | 1.77 | <LoD | <LoD | No | 23 |
| 188277 | F | 10 | 15 | RO, MF bones | 2 | VBL + PRED | 13 | 0 | 8.32 | 0.12 | <LoD | 3.49 | <LoD | <LoD | No | 20 |
| 209525 | M | 4 | 8 | RO, MF bones, skin, l/n, gut | 2 | VBL + PRED | 13 | 2 | 12.7 | 0.39 | <LoD | n/d | 0.05 | <LoD | No | 10 |
| 23245 | F | 43 | 107 | MF bones, skin | 3 | VBL + PRED, VCR/Ara-C/PRED | 5 | 1 | 0.14 | n/d | n/d | <LoD | n/d | n/d | No | 29 |
| 71021 | M | 18 | 38 | MF bones, skin | 3 | VBL + PRED, VCR/Ara-C/PRED | 6 | 0 | 8.60 | 0.56 | <LoD | 0.98 | 0.10 | <LoD | No | 40 |
| 50799 | M | 35 | 73 | MF bones, skin | 3 | VBL + PRED, VCR/Ara-C/PRED | 4 | 0 | <LoD | <LoD | <LoD | <LoD | <LoD | <LoD | No | 20 |
| 143428 | F | 28 | 76 | MF bones, skin, lungs, l/n | 3 | VBL + PRED, VCR/Ara-C/PRED | 6 | 1 | 3.61 | 0.07 | 0.42 | 0.61 | 0.02 | 0.02 | No | 23 |
| 76298 | F | 20 | 51 | MF bones, skin, l/n, DI | 3 | VBL + PRED, VCR/Ara-C/PRED | 1 | 0 | 1.10 | <LoD | <LoD | 0.10 | <LoD | 0.02 | No | 33 |
| 127734 | M | 18 | 37 | MF bones, skin | 4 | VBL + PRED, VCR/Ara-C/PRED, 2-CdA | 3 | 0 | 1.43 | <LoD | <LoD | <LoD | <LoD | <LoD | Yes (bone) | 2 |
| MIS ID . | Sex . | Age Dx, mo . | Age Tx, mo . | Organs involved . | LoT . | Previous therapy . | DAS, points . | DAS after vemu, points . | cfDNA load initially, % . | cfDNA load vemu cess, % . | cfDNA load therapy end, % . | MP BRAF load initially, % . | MP load vemu cess, % . | MP BRAF load therapy end, % . | Relapse . | EFS/time to relapse, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 132056 | F | 15 | 27 | RO, skin | 2 | VBL + PRE | 20 | 2 | 14.08 | <LoD | 0.5 | 4.84 | <LoD | <LoD | No | 45 |
| 147958 | F | 0 | 4 | RO, skin, lungs | 1 | No | 8 | 0 | 1.41 | <LoD | n/d | <LoD | <LoD | n/d | Yes (skin) | 1 |
| 189102 | F | 15 | 17 | RO, skin | 1 | No | 16 | 0 | 15.88 | <LoD | <LoD | 0.87 | <LoD | 0.01 | No | 21 |
| 159833 | F | 22 | 25 | RO, skin | 1 | No | 15 | 0 | 5.25 | 0.33 | 0.24 | 0.6 | <LoD | <LoD | No | 34 |
| 153888 | M | 19 | 25 | RO, skin, lungs | 2 | VBL + PRED | 6 | 0 | 5.29 | <LoD | 0.86 | 0.1 | 0.03 | 0.07 | Yes (bone) | 8 |
| 187463 | F | 14 | 17 | RO | 1 | No | 13 | 0 | 12.69 | <LoD | <LoD | 0.79 | <LoD | <LoD | No | 25 |
| 190889 | M | 1 | 31 | RO, skin, MF bones | 4 | VBL + PRED, VBL + PRED + VP16, VCR/Ara-C/PRED | 8 | 2 | 3.48 | 0.51 | 1.55 | 1.13 | 0.02 | 0.03 | No | 18 |
| 186934 | M | 9 | 15 | RO, skin | 2 | VBL + PRED | 19 | 2 | 3.66 | 0.20 | 0.54 | n/d | 0.06 | 0.05 | No | 21 |
| 202807 | M | 5 | 7 | RO, MF bones, skin, l/n, lungs | 1 | No | 16 | 2 | 14.80 | 0.94 | 0.16 | 0.68 | <LoD | <LoD | No | 15 |
| 181102 | M | 3 | 8 | RO, skin | 1 | No | 16 | 0 | 12.41 | 1.18 | 2.33 | 1.81 | 0.20 | 1.03 | Yes (RO+) | 2 |
| 116953 | F | 10 | 39 | RO, MF bones, DI, skin | 3 | VBL + PRED, VCR/Ara-C/PRED | 10 | 2 | 30.91 | 0.34 | <LoD | 1.77 | <LoD | <LoD | No | 23 |
| 188277 | F | 10 | 15 | RO, MF bones | 2 | VBL + PRED | 13 | 0 | 8.32 | 0.12 | <LoD | 3.49 | <LoD | <LoD | No | 20 |
| 209525 | M | 4 | 8 | RO, MF bones, skin, l/n, gut | 2 | VBL + PRED | 13 | 2 | 12.7 | 0.39 | <LoD | n/d | 0.05 | <LoD | No | 10 |
| 23245 | F | 43 | 107 | MF bones, skin | 3 | VBL + PRED, VCR/Ara-C/PRED | 5 | 1 | 0.14 | n/d | n/d | <LoD | n/d | n/d | No | 29 |
| 71021 | M | 18 | 38 | MF bones, skin | 3 | VBL + PRED, VCR/Ara-C/PRED | 6 | 0 | 8.60 | 0.56 | <LoD | 0.98 | 0.10 | <LoD | No | 40 |
| 50799 | M | 35 | 73 | MF bones, skin | 3 | VBL + PRED, VCR/Ara-C/PRED | 4 | 0 | <LoD | <LoD | <LoD | <LoD | <LoD | <LoD | No | 20 |
| 143428 | F | 28 | 76 | MF bones, skin, lungs, l/n | 3 | VBL + PRED, VCR/Ara-C/PRED | 6 | 1 | 3.61 | 0.07 | 0.42 | 0.61 | 0.02 | 0.02 | No | 23 |
| 76298 | F | 20 | 51 | MF bones, skin, l/n, DI | 3 | VBL + PRED, VCR/Ara-C/PRED | 1 | 0 | 1.10 | <LoD | <LoD | 0.10 | <LoD | 0.02 | No | 33 |
| 127734 | M | 18 | 37 | MF bones, skin | 4 | VBL + PRED, VCR/Ara-C/PRED, 2-CdA | 3 | 0 | 1.43 | <LoD | <LoD | <LoD | <LoD | <LoD | Yes (bone) | 2 |
Age Dx, age at diagnosis; Age Tx, age at protocol start; DI, diabetes insipidus; F, female; l/n, lymph nodes; <LoD, below level of detection; LoT, line of therapy; M, male; MF, multifocal; MP, myeloid progenitors; n/d, no data; PRED, prednisone; VBL, vinblastine; VP-16, etoposide.
The initial characteristics of RO+ and RO– groups
| . | RO+, median (range) . | RO–, median (range) . | P value, if applicable . |
|---|---|---|---|
| Number of patients (male:female) | 13 6:7 | 6 3:3 | |
| Age at manifestation, mo | 10 (0-22) | 24 (18-43) | 0.003 |
| Age at protocol start, mo | 17 (4-39) | 62 (37-107) | 0.001 |
| DAS, points | 13 (6-20) | 4.5 (1-6) | <0.001 |
| cfDNA load, % | 12.41 (1.4-30.9) | 1.3 (0-8.6) | 0.007 |
| CD34+CD117+ load, % | 0.87 (0-4.84) | 0.05 (0-0.98) | 0.038 |
| . | RO+, median (range) . | RO–, median (range) . | P value, if applicable . |
|---|---|---|---|
| Number of patients (male:female) | 13 6:7 | 6 3:3 | |
| Age at manifestation, mo | 10 (0-22) | 24 (18-43) | 0.003 |
| Age at protocol start, mo | 17 (4-39) | 62 (37-107) | 0.001 |
| DAS, points | 13 (6-20) | 4.5 (1-6) | <0.001 |
| cfDNA load, % | 12.41 (1.4-30.9) | 1.3 (0-8.6) | 0.007 |
| CD34+CD117+ load, % | 0.87 (0-4.84) | 0.05 (0-0.98) | 0.038 |
P value measured by Mann-Whitney U test.
Feasibility (toxicity) and therapeutic drug monitoring
The median time to the first 2-CdA + AraC course was 28 days (27-55), the median time to vemurafenib cessation was 135 days (125-161), and the median time to complete therapy was 202 days (188-283). Common adverse events of vemurafenib were skin rash (n = 7, 36.8%) and QTc elongation (n = 2, 10.5%). No events with grade 2 or more were observed while on vemurafenib single-agent therapy. All Ara-C+2-CdA courses were associated with grade 4 neutropenia and thrombocytopenia and grade 3 anemia in all cases. The common adverse events included grade 1 to 2 fever (n = 12, 63.2%) and skin rash (n = 7, 36.8%) after Ara-C infusion, cephalgias (n = 3, 15.8%), nausea (n = 6, 31.6%), and vomiting (n = 5, 26.3%). In addition, febrile neutropenia presented in 12 out of 19 patients after 19 out of 57 (33.3%) chemotherapy courses, but importantly, no episodes of microbiologically documented sepsis were observed. The median time of neutropenia (absolute neutrophil count < 500 cell/μL) was 8 (5-13) days. One grade 3 event (BCG-itis with lymphadenopathy) was documented, which led to a delay in therapy; thus, it was considered a preexisting condition before the protocol started. One patient (RO-) experienced secondary myelodysplastic syndrome with an acquired 7q- cytogenetic abnormality 14 months after vemurafenib therapy cessation. He is experiencing mild thrombocytopenia (∼80 000/μL) and is being evaluated as an HSCT candidate. A detailed account of the adverse effects is presented in Table 3. Thus, the proportion of patients with severe adverse effects is 5.3%.
Adverse events (according to CTCAE v.5.0)
| . | Neutropenia . | Febrile neutropenia . | Thrombocytopenia . | Anemia . | Fever (after Ara-C infusion) . | Rash maculo-papular (after Ara-C infusion) . | Rash maculo-papular (vemurafenib-associated) . | Electrocardiogram QT corrected interval prolonged . | Headache . | Nausea . | Vomiting . | Other complications∗ . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | 0 | 0 | 0 | 0 | 9 (47.4%) | 5 (26.3%) | 7 (36.8%) | 2 (10.5%) | 0 | 2 (10.5%) | 4 (21%) | 3 (15.8%) |
| Grade 2 | 0 | 0 | 0 | 0 | 3 (15.8%) | 2 (10.5%) | 0 | 0 | 3 (15.8%) | 4 (21%) | 1 (5.3%) | 3 (1.8%) |
| Grade 3 | 0 | 12 (63.2%) | 0 | 19 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5.3%) |
| Grade 4 | 19 (100%) | 0 | 19 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5.3%) |
| Grade 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| . | Neutropenia . | Febrile neutropenia . | Thrombocytopenia . | Anemia . | Fever (after Ara-C infusion) . | Rash maculo-papular (after Ara-C infusion) . | Rash maculo-papular (vemurafenib-associated) . | Electrocardiogram QT corrected interval prolonged . | Headache . | Nausea . | Vomiting . | Other complications∗ . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | 0 | 0 | 0 | 0 | 9 (47.4%) | 5 (26.3%) | 7 (36.8%) | 2 (10.5%) | 0 | 2 (10.5%) | 4 (21%) | 3 (15.8%) |
| Grade 2 | 0 | 0 | 0 | 0 | 3 (15.8%) | 2 (10.5%) | 0 | 0 | 3 (15.8%) | 4 (21%) | 1 (5.3%) | 3 (1.8%) |
| Grade 3 | 0 | 12 (63.2%) | 0 | 19 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5.3%) |
| Grade 4 | 19 (100%) | 0 | 19 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5.3%) |
| Grade 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
CTCAE,.
Infectious complications included: Herpes zoster infection, external otitis, earlobe abscess, BCGitis with lymphadenopathy, and meibomian cyst; grade 4 noninfectious complication is a secondary myelodysplastic syndrome.
Vemurafenib concentration measurements in a steady-state were available for 12 out of 19 patients. The median peak concentration was 28.42 μg/ml (11.33-45.37 μg/mL), which was higher than that previously reported.14 Only 1 patient had a peak concentration above 40 μg/mL; in 3 patients, it ranged from 30 μg/mL to 40 μg/mL. Grade 1 toxicity was observed in 2 out of 4 patients with vemurafenib concentrations >40 μg/ml; no dose modifications were needed.
Response
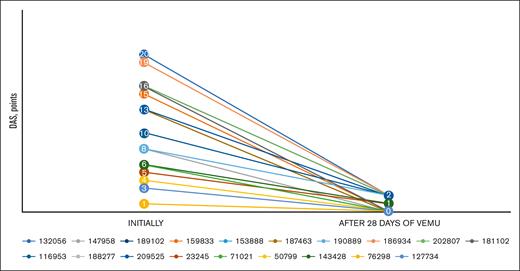
All patients rapidly responded to vemurafenib induction therapy. The median DAS decreased from 13 to 2 points (0-2 points) in the RO+ group and from 4.5 to 0 points (0-1 point) in the RO- group on day 28 from the start of vemurafenib therapy.
After 3 courses of Ara-C + 2-CdA, 1 patient violated the protocol and voluntarily discontinued the treatment, omitting 2-CdA monotherapy. Nevertheless, the follow-up was possible, and the patient stayed in complete remission (CR) without additional therapy.
By the end of vemurafenib therapy (before mono 2-CdA course number 1), all patients responded to the therapy both according to the DAS scoring system (0 points in all patients) and to the standard Histiocyte Society Scoring system23 (nonactive disease in all patients). The estimated ORR at vemurafenib cessation is 100% (100% of CR; the lower bound of the 1-sided 95% confidence interval [CI] is 81%), which proves the study hypothesis (P < .001).
LCH relapse
Vemurafenib cessation was attempted in all patients. Four relapses occurred in 4 patients (3 RO+, 1 RO–) at a median of 2 months (1-8 months) after vemurafenib withdrawal. In 3 patients, the relapse occurred during or right after mono 2-CdA therapy, and in 1 patient, it occurred 4 months after the full completion of the protocol. In 1 patient, the relapse occurred in all RO. Other sites were bone (2 patients) and skin (1 patient).
EFS and OS
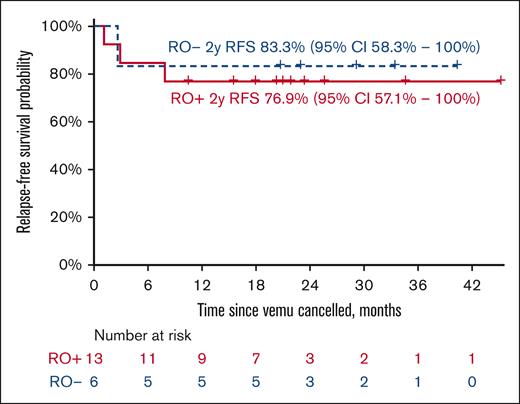
All enrolled patients were alive at the data cutoff, and 15 of them (10 RO+, 5 RO–) did not have disease progression. The 2-year EFS for the RO+ group was 76.9% (95% CI, 57.1-100) with a median follow-up of 21 months (maximum follow-up of 45 months). The 2-year EFS for the RO– group was 83.3% (95% CI, 58.3-100) with a median follow-up of 29 months (maximum follow-up of 40 months) (Figure 5). The OS in both groups was 100%. For detailed swimmer plot see Figure 6.
DAS pattern after vemurafenib induction. The figure represents the dramatic decrease in DAS after 28 days of mono vemurafenib therapy. Each line represents the individual DAS trend of each protocol patient, labeled by their IDs.
DAS pattern after vemurafenib induction. The figure represents the dramatic decrease in DAS after 28 days of mono vemurafenib therapy. Each line represents the individual DAS trend of each protocol patient, labeled by their IDs.
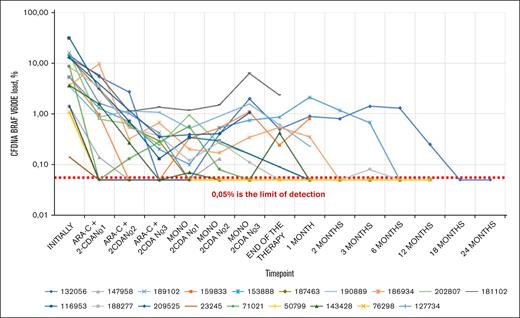
cfDNA BRAF V600E load in peripheral blood during the therapy. The figure represents the changes in cfDNA BRAF V600E allelic load in peripheral blood during various timepoints on protocol therapy and during the follow-up. The Y-axis is logarithmic, and each line represents a single patient allelic load trend, labeled by the respective IDs. The lowest limit of detection is represented by 0.05%, however, they were detected individually, based on the DNA concentration. Certain fluctuations can be observed after vemurafenib therapy cessation and during the follow-up period.
cfDNA BRAF V600E load in peripheral blood during the therapy. The figure represents the changes in cfDNA BRAF V600E allelic load in peripheral blood during various timepoints on protocol therapy and during the follow-up. The Y-axis is logarithmic, and each line represents a single patient allelic load trend, labeled by the respective IDs. The lowest limit of detection is represented by 0.05%, however, they were detected individually, based on the DNA concentration. Certain fluctuations can be observed after vemurafenib therapy cessation and during the follow-up period.
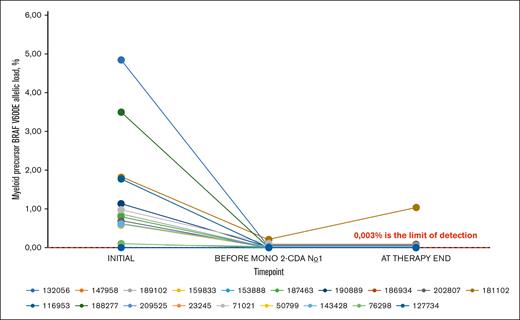
Myeloid precursor BRAF V600E allelic load during the therapy. The figure represents the changes in myeloid precursor BRAF V600E allelic load during various time points on protocol therapy. Each line represents a single patient allelic load trend, labeled by the respective IDs. The value 0.014% represents the lowest limit of detection, however, they were detected individually based on the DNA concentration. No similar fluctuation pattern as in Figure 3 is observed.
Myeloid precursor BRAF V600E allelic load during the therapy. The figure represents the changes in myeloid precursor BRAF V600E allelic load during various time points on protocol therapy. Each line represents a single patient allelic load trend, labeled by the respective IDs. The value 0.014% represents the lowest limit of detection, however, they were detected individually based on the DNA concentration. No similar fluctuation pattern as in Figure 3 is observed.
RFS probability by the group. The figure represents the RFS probability by group, measured from the cessation of vemurafenib treatment (D124) with respective confidence intervals.
RFS probability by the group. The figure represents the RFS probability by group, measured from the cessation of vemurafenib treatment (D124) with respective confidence intervals.
Swimmer plot representing all protocol patients. Each bar represents the treatment and follow-up of a single respective patient. All the events are represented with special symbols.
Swimmer plot representing all protocol patients. Each bar represents the treatment and follow-up of a single respective patient. All the events are represented with special symbols.
MRD and MRD-outcome correlation
Pretreatment mutant BRAF status was assessed in all patients (allelic load in cfDNA in blood, n = 19, allelic load in CD34+CD117+ cells, n = 17). The median cfDNA allelic load on day 0 in the RO+ group was 12.41% (1.41-30.91) and the median allelic load in CD34+CD117+ was 0.79% (0-4.84). Both cfDNA (1.27% [0-8.60]) and CD34+CD117+ allelic load (0.05% [0-0.98]) in RO– were lower (P < .05 and P = .1, respectively). The cfDNA allelic load reflected the efficacy of vemurafenib induction: in the RO+ group, the median absolute decrease from baseline was 6.63% (−5.9 to 27.74; P < .005), and in the RO– group, the median absolute decrease was 1.27% (0-5.82; P = .0625). Thus, the median cfDNA allelic load on day 28 was 1.63% (0.14-9.56) and 0% (0-2.78) in the RO+ group and in the RO– group, respectively.
After 3 courses of Ara-C + 2-CdA, MRD measurement was performed in cfDNA and the CD34+CD117+ cell line. Compared with the initial values, the load decreased dramatically in all patients: in the RO+ group, the median absolute decrease of allelic load in cfDNA was 11.23% (1.41-30.57; P < .001), and the median absolute decrease of allelic load in CD34+CD117+ was 0.79% (0-4.84; P = .002); in the RO- group, the median decrease of allelic load in cfDNA was 1.43% (0-8.04; P=.1), and the median decrease of allelic load in CD34+CD117+ was 0.10% (0-0.88; P = .18). Thus, at the end of intensive chemotherapy, in the RO+ group, the median allelic load in cfDNA was 0.2% (0-1.18), and the median allelic load in CD34+CD117+ was 0% (0-0.2); in the RO– group, the median allelic load in cfDNA was 0% (0-0.56), and the median allelic load in CD34+CD117+ was 0% (0-0.1). No significant differences between the RO+ and RO– groups were found (P = .3 and P = .9, respectively) (Figures 2, 3 and 4).
Relapsed patient follow-up and therapy
Vemurafenib was reintroduced for all relapsed patients, and we closely monitored their MRD loads (in both cfDNA and CD34+CD117+). Both cfDNA and MP allelic loads converted to negative in 2 of 4 patients. After all the measurements became negative, the second attempt to cease vemurafenib was performed in the 2 cases with MRD negativity. It was successful in both patients (1 RO+, 1 RO–) with EFS times of 25 and 9 months from the second attempt, respectively. Two patients remain on prolonged vemurafenib therapy.
Discussion
The standard approach for LCH treatment includes the combination of vinblastine and prednisone applied for at least 1 year.24 Unfortunately, although effective for most patients, it still cannot prevent the high incidence of relapses among RO– patients and is insufficient among RO+ patients. The Ara-C and 2-CdA combination used at high doses is highly active and curative in a significant proportion of RO+ patients who are refractory to vinblastine + prednisone. Major myelo- and immunotoxicity of the regimen precludes its wider use.5 In a study by Rosso et al, intermediate doses of Ara-C and 2-CdA among 9 patients with RO+ resulted in a 3-year OS of 76%.7
The true neoplastic nature of LCH was definitely established in 2010 when Badalian-Very et al proved the role of the BRAF V600E mutation.9 Currently, the predominant model that explains the heterogeneity of LCH lesions is a misguided myeloid precursor model. If the mutation event affects early myeloid progenitors, it leads to an extended disease, whereas a mutation in more differentiated dendritic cells results in a limited single-system lesion. The theory was suggested by Marie Louise-Berres et al as myeloid progenitors showed BRAF-positivity in RO+ patients.25 Nevertheless, Xiao et al later found that RO– patients with LCH also have BRAF-positive myeloid progenitors, but their quantity is much lower.26
These discoveries led to a breakthrough in the targeted therapy for LCH. BRAF inhibitors are very effective in patients with the BRAF V600E mutation, and vemurafenib monotherapy therapy appears safe in the short-term and midterm. However, although outstandingly effective, BRAF inhibitors do not solve the LCH problem entirely. The rate of successful cessation of vemurafenib therapy in patients with LCH is <7%, with abysmal rates in RO+ patients of 4.5%.14,15,27-29
Meanwhile, concerns are growing about the future of patients with LCH receiving vemurafenib therapy for a long time. There are scattered reports on the clonal and biological evolution of the disease,30,31 which are the first signs of the problems we might face in the future. Clonal proliferation, lineage switching, or secondary transformation still seem possible during prolonged BRAF inhibition.
Considering all the above, we hypothesized that the curative potential of chemotherapy and the efficacy and safety of BRAF inhibitors could be combined to create an effective, shortened, less toxic, and curative regimen. Based on our previous research and published evidence,15,32 we combined vemurafenib with intermediate doses of Ara-C and 2-CdA and tested this approach in a prospective trial.
Vemurafenib induction, followed by 3 courses of intermediate-dose Ara-C and 2-CdA with intermittent vemurafenib, followed by 3 courses of 2-CdA monotherapy, was highly feasible. All patients except 1 completed the prescribed course. Remarkably, the only patient who voluntarily skipped maintenance therapy remained in CR. The toxicity of vemurafenib was minor, whereas chemotherapy was associated with the expected degree of myelosuppression. The burden of the infectious complications and the required supportive care were significantly lower than the published data on high-dose Ara-C + 2-CdA and our own experience, and the median neutropenia time was about 3 times shorter than in the Donadieu et al study (8 vs 23 days, respectively).5 We attribute this improved safety partly to the lowered doses and, importantly, to the timing of chemotherapy administration, because in all patients with RO+ disease, vemurafenib induction resulted in the rapid and complete recovery of BM and liver function. Vemurafenib administration was effective, with therapeutic concentrations achieved in all patients, and there was no need for dose modifications.
Vemurafenib induction was highly effective, confirming previous reports. Based on the ongoing unmaintained remission of ∼80% of the patients, which is much higher, than in previous reports,14,15 our results suggest that the proposed regimen might be curative in a significant proportion of patients. Despite the relatively short follow-up, all patients were followed for a median of 23 months after vemurafenib cessation. Of note, LCH relapses were reported to develop at a median of 1 month after vemurafenib monotherapy withdrawal, according to recent publications14,15 and at a median of 2 months in our current study. The overall duration of the therapy was shorter than of standard-of-care LCH therapy. The demonstrated ability to withdraw vemurafenib contrasts with previous reports, including our data.14,15 This could be due to several reasons (eg, limited cohort, more prospective application of drugs), but we assume that the main reason is that our previous experience was limited to a pretreated cohort: 4 out of 7 patients received similar concomitant chemotherapy as the second line, 2 as the third line, and 1 as the fourth line; 2 patients failed to respond to higher doses of Ara-C and 2-CdA before the concomitant therapy. Of the 5 remaining patients, who have not previously received Ara-C + 2-CdA courses, 1 died from unexpected toxicity, 1 stayed in partial remission after vemurafenib withdrawal, and 3 patients relapsed. These extremely low numbers do not let us shed any light on the reasons for the limited success of the previous study.
The possible mechanism of the cure provided by the tested combination regimen remains unknown. It is improbable that 2-CdA and Ara-C at the doses used in this trial can eradicate the population of the mutant myeloid progenitors, especially considering that even HSCT conditioning often fails to do that.8 Lymphodepletion by chemotherapy and the reset of local immune environment should be studied further as potential mechanisms of immune control over mutant LCH cells.
Our data confirm that monitoring mutant BRAF by ddPCR in cfDNA is a valuable method of assessing disease burden in BRAF+ LCH. The cfDNA BRAF V600E allelic load was correlated with disease severity and clinical response to therapy. We believe that it is too early to use this method as a predictor of LCH relapse or refractoriness because 2 out of 4 patients relapsed before therapy stopped, whereas the other 2 had higher levels compared with the nonrelapsed cohort (Table 4). This finding is consistent with the findings published by Heritier et al, where the 2-year risk of reactivation in patients with negative values of cfDNA was as high as 34%.18 In the meantime, 6 out of 14 patients in CR maintained detectable levels of BRAF V600E load in cfDNA even after the end of the treatment. The finding also suggests other mechanisms involved in the maintenance of stable remission.
CfDNA allelic load levels and the incidence of relapses
| Patient (MIS ID) . | cfDNA allelic load level at vemu cessation, % . | cfDNA allelic load level at therapy end, % . | Relapse . |
|---|---|---|---|
| 147958 (RO+) | <LoD | Relapse | Yes (RO–) |
| 153888 (RO+) | <LoD | 0.86 | Yes (RO–) |
| 127734 (RO–) | <LoD | Relapse | Yes (RO–) |
| 181102 (RO+) | 1.18 | 2.33 | Yes (RO+) |
| 159833 (RO+) | 0.33 | 0.24 | No |
| 187463 (RO+) | <LoD | <LoD | No |
| 190889 (RO+) | 0.51 | 1.55 | No |
| 186934 (RO+) | 0.2 | 0.54 | No |
| 202807 (RO+) | 0.94 | 0.16 | No |
| 132056 (RO+) | <LoD | 0.5 | No |
| 189102 (RO+) | <LoD | <LoD | No |
| 116953 (RO+) | 0.34 | <LoD | No |
| 188277 (RO+) | 0.12 | <LoD | No |
| 209525 (RO+) | 0.39 | <LoD | No |
| 23245 (RO–) | n/d | n/d | No |
| 71021 (RO–) | 0.56 | <LoD | No |
| 50799 (RO–) | <LoD | <LoD | No |
| 143428 (RO–) | 0.07 | 0.42 | No |
| 76298 (RO–) | <LoD | <LoD | No |
| Patient (MIS ID) . | cfDNA allelic load level at vemu cessation, % . | cfDNA allelic load level at therapy end, % . | Relapse . |
|---|---|---|---|
| 147958 (RO+) | <LoD | Relapse | Yes (RO–) |
| 153888 (RO+) | <LoD | 0.86 | Yes (RO–) |
| 127734 (RO–) | <LoD | Relapse | Yes (RO–) |
| 181102 (RO+) | 1.18 | 2.33 | Yes (RO+) |
| 159833 (RO+) | 0.33 | 0.24 | No |
| 187463 (RO+) | <LoD | <LoD | No |
| 190889 (RO+) | 0.51 | 1.55 | No |
| 186934 (RO+) | 0.2 | 0.54 | No |
| 202807 (RO+) | 0.94 | 0.16 | No |
| 132056 (RO+) | <LoD | 0.5 | No |
| 189102 (RO+) | <LoD | <LoD | No |
| 116953 (RO+) | 0.34 | <LoD | No |
| 188277 (RO+) | 0.12 | <LoD | No |
| 209525 (RO+) | 0.39 | <LoD | No |
| 23245 (RO–) | n/d | n/d | No |
| 71021 (RO–) | 0.56 | <LoD | No |
| 50799 (RO–) | <LoD | <LoD | No |
| 143428 (RO–) | 0.07 | 0.42 | No |
| 76298 (RO–) | <LoD | <LoD | No |
LoD, limit of detection; n/d, no data.
Relapsed patients and allelic load at therapy end: 2/4 n/d, 2/4 >LoD (2.33% RO+, 0.86% RO–).
Patients in remission and allelic load at therapy end: 8/14 <LoD, 6/14 >LoD (median at therapy end, 0.46%; range, 0.16%-1.55%).
The prognostic value of allelic load has not been studied before. Generally, the BRAF V600E allelic load is correlated with the initial disease burden and decreases significantly during therapy. By the end of therapy, 6 of the 17 tested patients remained BRAF V600E+ in the MP progenitor compartment, although at a low level in most cases. Of note, the only patient with a relapse involving the RO had the highest level of MP allelic load of all the patients (0.20% at the time of vemurafenib therapy cessation and 1% at relapse). The data are insufficient to draw a final conclusion. Nevertheless, we believe that further research on the prognostic value of mutant allelic load in the BM might provide important biological and clinical clues. Another remarkable point is that in 6 patients, the levels of cfDNA BRAF load went up during the maintenance therapy (Table 4). Although not clearly influencing the relapse incidence, this is a concerning sign. The possible reason for it may be the persistence of BRAF-positive cell populations, which was recently shown in patients being treated with BRAFi for a long period.31 Because of the unequal distribution of the chemotherapy, the pathological cells can also accumulate in other tissues, like the skin. Although they are able to influence the cfDNA fluctuations, they seem not to be potent for relapse, at least not during the study period.
The low but increasing levels of cfDNA during 2-CdA maintenance may question the value of this therapy, but the lack of overt clinical relapse may also suggest that the maintenance therapy actually had a value in reducing disease activity. Future studies should critically analyze the need for 2-CdA maintenance, considering the unclear efficacy and potential toxicity, especially in the RO- population.
Our study has certain limitations that should be considered. First, the cohort was relatively small, even considering the rarity of RO+ lesions in LCH. Second, although ddPCR in cfDNA showed promising results, the nature of the method could be affected by many processes that are not relevant to the course of the disease (eg, nonspecific probe decay), leading to the misinterpretation of the quantitative results. Finally, we did not include any patients with central nervous system lesions or sclerosing cholangitis. Because of the different nature of these lesions, we suppose that these cohorts would benefit more from different approaches to therapy.
To our knowledge, this study provides the first prospective evidence for a potentially curative approach for high-risk and relapsed LCH. We believe that among the RO+ cohort, the risk/benefit ratio of the approach is in favor of testing the therapy as the front line. The use of Ara-C and 2-CdA is disputable in RO- patients. Although the hematologic toxicity of combined therapy is moderate, it is still present, which might put RO- patients at unnecessary risks, including the risk of late clonal events. In this cohort, single-agent 2-CdA was an effective therapy for ∼70% of relapsed patients and could be tested in combination with a BRAF inhibitor, administered on a schedule similar to the proposed.33 In contrast, the presence of a CD34+CD117+ BRAF V600E+ population in RO- patients could be considered a reason for a more intensive approach. Thus, the molecular basis could act as a division rationale for the stratification. Future studies can include an “adaptive schedule” with more prolonged vemurafenib therapy or more intensive chemotherapy courses for MRD-refractory patients. Several questions remain unanswered, including the mechanistic basis of the clinical synergy of chemotherapy and vemurafenib, the relative role of germ line genetic variations and chemotherapy in developing secondary myelodysplastic syndrome in patients with LCH, and the optimal therapy duration.
In conclusion, our pilot study demonstrates that combined therapy with vemurafenib and intermediate-dose 2-CdA and Ara-C is safe in a cohort of children with RO+ LCH and relapsed RO– LCH. Unmaintained remission was achieved in a significant majority of the patients. If confirmed in a larger study with an extended follow-up, this approach may become the foundation for a safe and curative therapy for LCH.
Acknowledgments
This study was supported (in part, financing provided for molecular genetic studies) by research funding from the Russian Science Foundation (RSCF) number 22-15-00450 to M.M., D.E., E.R., and D.O. The authors thank the “Gift of Life” charity fund for financial support in providing vemurafenib to the patients and with the help in logistics.
Authorship
Contribution: M.M., A.M., D.E., D.O., and E.R. designed the study; D.E., I.K., E.L., D.B., N.K., E.C., A.I., E.B., G.B., I.V., B.P., O.F., E.P., I.D., and E.E. collected and aggregated the data; E.R. and D.O. did the molecular analysis and interpreted the data; A.P., A.S., and V.Z. did the cell sorting; E.L. did the concentration analysis; K.V. did the statistical editing; G.N., M.M., A.M., D.E., D.O., and A.I. analyzed the data; D.E. wrote the article; and all authors critically revised the manuscript.
Conflict-of-interest disclosure: A.M. received lecturer’s fees from Novartis. M.M. received lecturer’s fees from Miltenyi Biotec. The remaining authors declare no competing financial interests.
Correspondence: Dmitry Evseev, Dmitry Rogachev National Medical Research Center Of Pediatric Hematology, Oncology, and Immunology, 1 Samory Mashela St, 117997, Moscow, Russia;; and Michael Maschan, Dmitry Rogachev National Medical Research Center Of Pediatric Hematology, Oncology, and Immunology, 1 Samory Mashela St, 117997, Moscow, Russia; e-mail: mmaschan@yandex.ru.
References
Author notes
Data are available on request from the corresponding author, Michael Maschan (mmaschan@yandex.ru) and Dmitry Evseev (dmitryevseev1991@gmail.com).
The full-text version of this article contains a data supplement.