TO THE EDITOR:
Lymphomas are a diverse group of hematopoietic neoplasms resulting from the malignant transformation of lymphoid cells and their precursors.1 If diagnosed early and precisely, most lymphomas in children and young adults have excellent cure rates of up to 90% using affordable treatments from the essential medicine list by the World Health Organization.2-6 In contrast, prolonged time-to-diagnosis and treatment usually results in poor treatment outcomes, especially in aggressive lymphoma types.7 In sub-Saharan Africa (SSA), cited causes of delay in diagnosis and treatment are low socioeconomic status, poor health-seeking behavior and access to health care, an inefficient health care referral system, a shortage of trained experts, and limited diagnostic capability.8,9 Studies analyzing delays in the diagnosis and treatment of lymphoma in SSA are limited. An objective assessment of the time to definitive care and associated factors is crucial in identifying intervention points along the pathway to care for patients with lymphoma in resource-restricted regions.
Here, we present the results of a prospective multicenter study involving 2 East African countries that evaluates both patient and health care–related delays among children and young adults diagnosed with lymphoma. The study was conducted at 3 tertiary cancer hospitals in Tanzania and 1 cancer center, St Mary's Hospital-Lacor Hospital, in Northern Uganda. The centers in Tanzania included Muhimbili National Hospital in Dar es Salaam, Kilimanjaro Christian Medical Centre in Kilimanjaro, and Bugando Medical Centre in the Mwanza region, north of Tanzania. Ethical approval was granted by the Oxford Tropical Research Ethics Committee, the National Institute of Medical Research in Tanzania, the Uganda National Council of Science and Technology, and the Lacor Hospital Institutional Research Ethics Committee in Uganda. The AI-REAL study enrolled children and young adults (aged 3-30 years) presenting with suspected lymphoma. Here, we focus on the subset of patients with a biopsy-confirmed lymphoma diagnosis. For all enrolled patients, demographic and baseline clinical data, including the date they first experienced symptoms, the date they first visited a local health care center, and the date the local health care center referred them to the tertiary cancer center, were recorded. Upon arrival at the tertiary cancer treatment centers, the dates and times of tissue sampling, arrival of tissue in the pathology laboratory for processing and pathology reports (with and without immunohistochemistry), and start of definitive cancer treatment were recorded. The primary outcome was the median total treatment delay and its individual components. Total treatment delay was defined as the time from the onset of symptoms to the start of definitive cancer treatment. The total treatment delay included the time-to-first health care contact (from the onset of symptoms) to contact with the first health care facility, excluding the visit to traditional healers, time-to-referral (from the first health care facility contact to arrival at a cancer treatment center), and time-to-treatment after arrival at a cancer center (time from arriving at a cancer treatment center to receiving definitive cancer treatment). Time-to-diagnosis was defined as the time from arrival at a cancer center to receiving a tissue diagnosis report, either a morphology report alone or a morphology with immunohistochemistry (IHC) report.
Between 1 May 2019 and 25 September 2022, 291 patients with suspected lymphoma were enrolled. Of these, 148 had a confirmed lymphoma diagnosis, completed follow-ups, and were included in the final analysis. The baseline characteristics of 148 patients with lymphoma who received cancer treatment are shown in Table 1. Most patients (74%) came from Tanzania cancer centers. The median age of patients was 12 years (interquartile range [IQR], 9-18), and 100 (68%) were males. Only 85 of 148 patients (57%) had visited a traditional healer. Seven of 111 patients (6.3%) were tested as HIV-positive, and 101 of 145 patients (70%) presented with advanced disease (stage III or IV disease).
Baseline characteristics of study participants
| Characteristic . | Overall, N = 148 . | BL, N = 65 . | DLBCL, N = 37 . | HL, N = 46 . | P value∗ . |
|---|---|---|---|---|---|
| Treatment center, no. (%) | < .001 | ||||
| Rural Uganda | 39 (26) | 30 (46) | 4 (11) | 5 (11) | |
| Tanzania | 109 (74) | 35 (54) | 33 (89) | 41 (89) | |
| Age in y, median (IQR) | 12 (9-18) | 11 (7-14) | 15 (10-19) | 16 (9-20) | .003 |
| Sex, no. (%) | .10 | ||||
| Female | 48 (32) | 15 (23) | 15 (41) | 18 (39) | |
| Male | 100 (68) | 50 (77) | 22 (59) | 28 (61) | |
| Distance (region) from the cancer center, no. (%) | .11 | ||||
| Far | 68 of 144 (47) | 36/63 (57) | 15/37 (41) | 17/44 (39) | |
| Near | 76 of 144 (53) | 27/63 (43) | 22/37 (59) | 27/44 (61) | |
| A prior visit to the local healer, no. (%) | 85 (57) | 37 (57) | 21 (57) | 27 (59) | > .9 |
| HIV-positive, no. (%) | 7 of 111 (6.3) | 3/51 (5.9) | 4/27 (15) | 0/33 (0) | .053 |
| B-symptoms, no. (%) | 128 of 145 (88) | 56/64 (88) | 32/36 (89) | 40/45 (89) | > .9 |
| LDH levels (IU/L), median (IQR) | 700 (396-1156) | 960 (678-1587) | 613 (387-1436) | 549 (336-700) | < .001 |
| Cytopenias, no. (%) | 110 of 129 (85) | 50 of 60 (83) | 25 of 32 (78) | 35 of 37 (95) | .11 |
| Peripheral LAD, no. (%) | 101 of 143 (71) | 36 of 60 (60) | 23 of 37 (62) | 42 of 46 (91) | < .001 |
| Jaw mass, no. (%) | 48 of 147 (33) | 32 of 64 (50) | 10 of 37 (27) | 6 of 46 (13) | < .001 |
| Mediastinal mass, no. (%) | 24 of 105 (23) | 3 of 43 (7.0) | 6 of 24 (25) | 15 of 38 (39) | .002 |
| Abdominal mass, no. (%) | 65 of 123 (53) | 31 of 53 (58) | 10 of 29 (34) | 24 of 41 (59) | .077 |
| Site of biopsy, no. (%) | < .001 | ||||
| Abdomen | 40 of 138 (29) | 26 of 62 (42) | 14 of 34 (41) | 0 of 42 (0) | |
| Peripheral node | 90 of 138 (65) | 32 of 62 (52) | 17 of 34 (50) | 41 of 42 (98) | |
| Other deeper tissue regions | 8 of 138 (5.8) | 4 of 62 (6.5) | 3 of 34 (8.8) | 1 of 42 (2.4) | |
| Clinical stage, no. (%) | .4 | ||||
| Stage I/II | 44 of 145 (30) | 23 of 63 (37) | 11 of 36 (31) | 10 of 46 (22) | |
| Stage III | 65 of 145 (45) | 24 of 63 (38) | 15 of 36 (42) | 26 of 46 (57) | |
| Stage IV | 36 of 145 (25) | 16 of 63 (25) | 10 of 36 (28) | 10 of 46 (22) | |
| Change in initial morphology-only diagnosis after IHC, no. (%) | 24 of 116 (20.7) | 12 of 53 (22.6) | 8 of 28 (28.6) | 4 of 35 (11.4) | .222 |
| Characteristic . | Overall, N = 148 . | BL, N = 65 . | DLBCL, N = 37 . | HL, N = 46 . | P value∗ . |
|---|---|---|---|---|---|
| Treatment center, no. (%) | < .001 | ||||
| Rural Uganda | 39 (26) | 30 (46) | 4 (11) | 5 (11) | |
| Tanzania | 109 (74) | 35 (54) | 33 (89) | 41 (89) | |
| Age in y, median (IQR) | 12 (9-18) | 11 (7-14) | 15 (10-19) | 16 (9-20) | .003 |
| Sex, no. (%) | .10 | ||||
| Female | 48 (32) | 15 (23) | 15 (41) | 18 (39) | |
| Male | 100 (68) | 50 (77) | 22 (59) | 28 (61) | |
| Distance (region) from the cancer center, no. (%) | .11 | ||||
| Far | 68 of 144 (47) | 36/63 (57) | 15/37 (41) | 17/44 (39) | |
| Near | 76 of 144 (53) | 27/63 (43) | 22/37 (59) | 27/44 (61) | |
| A prior visit to the local healer, no. (%) | 85 (57) | 37 (57) | 21 (57) | 27 (59) | > .9 |
| HIV-positive, no. (%) | 7 of 111 (6.3) | 3/51 (5.9) | 4/27 (15) | 0/33 (0) | .053 |
| B-symptoms, no. (%) | 128 of 145 (88) | 56/64 (88) | 32/36 (89) | 40/45 (89) | > .9 |
| LDH levels (IU/L), median (IQR) | 700 (396-1156) | 960 (678-1587) | 613 (387-1436) | 549 (336-700) | < .001 |
| Cytopenias, no. (%) | 110 of 129 (85) | 50 of 60 (83) | 25 of 32 (78) | 35 of 37 (95) | .11 |
| Peripheral LAD, no. (%) | 101 of 143 (71) | 36 of 60 (60) | 23 of 37 (62) | 42 of 46 (91) | < .001 |
| Jaw mass, no. (%) | 48 of 147 (33) | 32 of 64 (50) | 10 of 37 (27) | 6 of 46 (13) | < .001 |
| Mediastinal mass, no. (%) | 24 of 105 (23) | 3 of 43 (7.0) | 6 of 24 (25) | 15 of 38 (39) | .002 |
| Abdominal mass, no. (%) | 65 of 123 (53) | 31 of 53 (58) | 10 of 29 (34) | 24 of 41 (59) | .077 |
| Site of biopsy, no. (%) | < .001 | ||||
| Abdomen | 40 of 138 (29) | 26 of 62 (42) | 14 of 34 (41) | 0 of 42 (0) | |
| Peripheral node | 90 of 138 (65) | 32 of 62 (52) | 17 of 34 (50) | 41 of 42 (98) | |
| Other deeper tissue regions | 8 of 138 (5.8) | 4 of 62 (6.5) | 3 of 34 (8.8) | 1 of 42 (2.4) | |
| Clinical stage, no. (%) | .4 | ||||
| Stage I/II | 44 of 145 (30) | 23 of 63 (37) | 11 of 36 (31) | 10 of 46 (22) | |
| Stage III | 65 of 145 (45) | 24 of 63 (38) | 15 of 36 (42) | 26 of 46 (57) | |
| Stage IV | 36 of 145 (25) | 16 of 63 (25) | 10 of 36 (28) | 10 of 46 (22) | |
| Change in initial morphology-only diagnosis after IHC, no. (%) | 24 of 116 (20.7) | 12 of 53 (22.6) | 8 of 28 (28.6) | 4 of 35 (11.4) | .222 |
P value < 0.05 was considered statistically significant and is indicated in bold.
Pearson χ2 test; Kruskal-Wallis rank sum test; Fisher exact test; LDH, lactate dehydrogenase; peripheral LAD, peripheral lymphadenopathy.
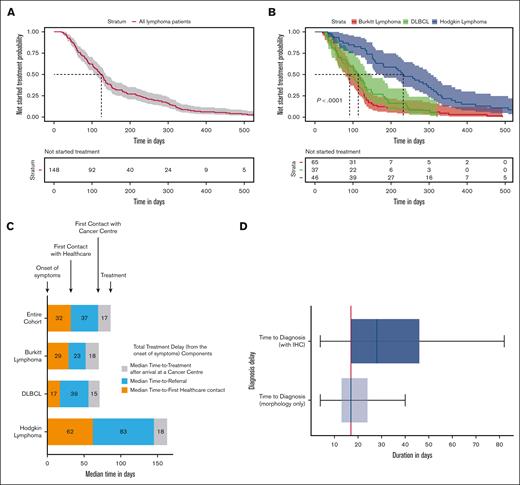
The median total treatment delay for the entire cohort was 124 days (95% confidence interval [95% CI], 107-136; Figure 1A). The probability of treatment not started for the entire cohort was 64% (95% CI, 56-72) at 90 days and 30% (95% CI, 24-39) at 180 days. The median total treatment delay for those with Burkitt lymphoma (BL) was 91 days (95% CI, 80-115), whereas for diffuse large B-cell lymphoma (DLBCL) and Hodgkin’s lymphoma (HL), it was 114 days (95% CI, 84-148) and 232 days (95% CI, 179-305), respectively (P value < .0001; Figure 1B). The probability of treatment not started for BL was 51% (95% CI, 40-65) at 90 days and 12% (95% CI, 6.4-24) at 180 days. For DLBCL, the probability of treatment not started was 59% (95% CI, 46-78) at 90 days and 24% (95% CI, 14-43) at 180 days; whereas for HL, it was 85% (95% CI, 75-95) at 90 days and 61% (95% CI, 48-77) at 180 days.
Total treatment delay (total time-to-treatment from the onset of symptoms) and diagnosis delay after arrival to cancer centers. (A) Total treatment delay across all lymphoma types and (B) for individual types of lymphoma. The shaded region shows 95% CIs. (C) Total treatment delay (from the onset of symptoms) components. (D) Boxplots of the time-to-diagnosis for morphology-only and IHC reports after the arrival to cancer centers for the entire cohort. The vertical red line indicates the median time-to-treatment after arrival at a cancer center (17 days).
Total treatment delay (total time-to-treatment from the onset of symptoms) and diagnosis delay after arrival to cancer centers. (A) Total treatment delay across all lymphoma types and (B) for individual types of lymphoma. The shaded region shows 95% CIs. (C) Total treatment delay (from the onset of symptoms) components. (D) Boxplots of the time-to-diagnosis for morphology-only and IHC reports after the arrival to cancer centers for the entire cohort. The vertical red line indicates the median time-to-treatment after arrival at a cancer center (17 days).
Analysis of the different components of total treatment delay (Figure 1C) showed a median time-to-first health care facility contact of 32 days (IQR, 12-65), a median time-to-referral of 37 days (IQR, 14-83), and a median time-to-treatment after arrival to the cancer center of 17 days (IQR, 9-28). With respect to delay in secondary and tertiary care, the median time-to-diagnosis was 17 days without IHC and 28 days for cases with IHC (Figure 1D).
The nature of treatment delay and its components in SSA have not been well described. This prospective multicenter study found that patients presenting with signs of typical BL had a significantly shorter time-to-treatment (median, 91 days) compared with those with DLBCL (114 days) and HL (232 days). However, this delay is still far beyond the recommended time of 5 days for BL. We attribute these differences in total treatment delay between BL, DLBCL, and HL to the characteristic jaw mass presentation and rapid increase in tumor size seen among patients with BL, contrary to the presence of slowly progressive peripheral lymphadenopathy or mediastinal mass in patients with HL, which can be wrongly diagnosed as tuberculosis. In addition, BL is 1 of the region's most studied childhood cancers, and there is awareness among the public and health care workers of the need for rapid referral.10 Importantly, health care–related delays (time-to-referral and time-to-treatment after arrival at the cancer center) make up two-thirds of the total treatment delay, with limited diagnostic capacity at primary-level facilities significantly contributing to the observed referral delay. Furthermore, we observed that the median time-to-treatment after arrival at the cancer center (17 days) corresponded to the time-to-diagnosis (morphology only), which was also 17 days. By contrast, the time to World Health Organization gold standard diagnosis for lymphoma, the time-to-diagnosis (with IHC) was 28 days for the entire cohort. Importantly, the diagnosis had changed among 20.7% of patients after review of IHC (Table 1). These results show that even in a research setting where automated IHC was made available, treatment often had to be instituted based on morphological diagnosis alone because of a combination of long turnaround times for IHC and clinical urgency, thus increasing the risk of misdiagnosis.
In conclusion, significant treatment delays for patients with lymphoma emanate from health care system–related factors. Because of delays in referrals from primary care and a lack of capacity for pathology in secondary care, initial treatment decisions are often based on clinical suspicion, urgency, and morphology alone. New diagnostic approaches for BL that overcome these health care–related delays are urgently needed to improve the outcome of children with this highly curable disease.
The study received an institutional review board approval from the Oxford Tropical Research Ethics Committee (OxTREC reference, 15-19), National Institute of Medical Research (NIMR registration number NIMR/HQ/R.8a/Vol.IX/3408) in Tanzania, Uganda National Council of Science and Technology (UNCST registration number HS529ES), and the Lacor Hospital Institutional Research Ethics Committee (LHIREC number 074/05/19) in Uganda. Initial research ethics committee approval was given on 6 February 2019 (protocol version 3.1), and the current protocol version 3.3 was approved on 4 April 2021 via substantial amendment. Participants were enrolled in the study only after written informed consent or assent had been obtained from the patient as per the institutional review board guidelines.
Acknowledgments: The authors are grateful to the patients and their families for their participation. The authors also thank biotech companies IlluminaTM, NanoporeTM, and AlexapathTM for their in-kind support in the form of equipment and reagent supplies. The authors express sincere gratitude to their collaborators in East Africa (AFENET, MUHAS, CPHL, MNH, KCMC, TLM, St. Mary's Hospital Lacor, AFRON, and Soleterre) for supporting the study. The authors acknowledge the contribution of each of the following members of the research consortium: Claire El Moulden, Faraja Chiwanga, Kristin Schroeder, Erick Marogosa, Leah Mnango, Alex Mremi, Emmanuel Josephat, Oliver Henke, Patricia Scanlan, Priscus Mapendo, Martin D. Ogwang, Isaac Otim, Ismail D. Legason, Kieran Howard, Adam Burns, Helene Dreau, Daisy Jennings, Laura Lopez Pascua, Kate Ridout, Anthony Cutts, Sarah Wordsworth, Sam M. Mbulaiteye, George Ruhago, and Malale Tungu. The authors also thank the following members of the Aggressive Infection-Related East Africa Lymphoma scientific advisory board: Reiner Siebert, Ming-Qing Du, Satish Gopal, Dennis Lo, Lorenzo Leoncini, Kikkeri N Naresh, and David Kurtz, for their contributions.
This study was fully funded by the UK Government through the National Institute for Health and Care Research and Innovation for Global Health Transformation grant.
The views expressed in this publication are those of the authors and not necessarily those of the UK Department of Health.
Contribution: W.F.M., L.C., H.M., and A.S. contributed to the conception and design of the study; C.A., G.S., S.M., A.K., P.N., N.H., E.M., and C.C. participated in data collection and analysis; W.F.M. wrote the first draft of the manuscript; L.M., D.V., and A.S. reviewed the manuscript critically; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: A.S. has received honoraria from Oxford Nanopore Technology, Illumina, Exact Sciences, AbbVie, AstraZeneca, BeiGene, and Janssen; is a director and shareholder of SERENOx, an Oxford University social enterprise spin-off; and receives unrestricted research grants from AstraZeneca and Janssen. W.F.M. is a shareholder of SERENOx and a director and shareholder of SERENOx Africa Limited, a SERENOx partner company in Tanzania. C.C. is a director and shareholder of SERENOx Africa Limited. The remaining authors declare no competing financial interests.
Correspondence: William Frank Mawalla, Department of Haematology and Blood Transfusion, Muhimbili University of Health and Allied Science (MUHAS), PO Box 65001, Upanga, Dar es Salaam, Tanzania; e-mail: mawallawf@ymail.com.
References
Author notes
Data are available on request from the corresponding author, William Frank Mawalla (mawallawf@ymail.com).