TO THE EDITOR:
The introduction of rituximab during the early 2000s was a paradigm-shifting event for the first-line treatment of patients with diffuse large B-cell lymphoma (DLBCL).1 Nevertheless, there is still some ambiguity regarding the prognostic effect of male sex on overall survival (OS) in older patients with DLBCL treated with rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).2-9
The post hoc analysis of the phase 3 RICOVER-60 trial initially sparked the notion that male sex is associated with poor OS among older (range, 61-80 years) R-CHOP14–treated patients with DLBCL.3 The phase 2 SEXIE-R-CHOP-14 trial investigated whether the poor prognostic effect of male sex could be abrogated by higher rituximab doses in older (range, 61-80 years) male patients with DLBCL.2 In a planned subgroup analysis of that trial, there was no difference in OS between males in the RICOVER-60 (375 mg/m2 rituximab) and those in the SEXIE-R-CHOP-14 trial (500 mg/m2 rituximab). However, progression-free survival (PFS) improved in the latter population. Furthermore, in the SEXIE-R-CHOP-14 trial, in which females were treated with standard-dose rituximab, there was no differential sex distribution in OS and PFS.2 In addition, other studies have shown that the difference in the sex distribution with regard to the OS diminished in adult patients with DLBCL treated with R-CHOP after receiving a higher rituximab dose.5-7 Collectively, the differential sex distribution in survival has been hypothesized to be associated with faster rituximab clearance in older males with DLBCL, resulting in lower rituximab blood levels in these individuals than in their female counterparts.3,10 Notwithstanding, other investigations on dose-augmented rituximab in older males with DLBCL have yielded opposing findings that offset this contentious prognostic effect.2,5,6 Therefore, rituximab dosing at 375 mg/m2 remains the standard.
The apparent differential sex distribution in OS might be partly attributed to the general comprehension that females ordinarily have greater longevity than males. Unlike OS, relative survival (RS) considers the expected survival of age-, sex-, and period-matched groups in the general population. Accordingly, the differential sex distribution in OS might not be entirely credited to insufficient rituximab dosing in older males. The influence of sex on the RS among older patients with DLBCL treated with R-CHOP at the population level has not been explicitly investigated thus far. Therefore, this nationwide, population-based study assessed the influence of sex on RS among older patients with DLBCL treated in The Netherlands using a 21-day R-CHOP schedule (R-CHOP21).
We selected all older (>60 years) patients diagnosed with primary DLBCL between 2014 and 2020 from the nationwide Netherlands Cancer Registry (NCR) using the International Classification of Diseases for Oncology morphology code 9680. We excluded patients diagnosed with primary central nervous system lymphoma, primary mediastinal B-cell lymphoma, leg-type DLBCL, posttransplantation lymphoproliferative disorders, and human immunodeficiency virus-associated DLBCL. In this study, we only included patients who completed ≥1 cycles of R-CHOP21.
Detailed information on disease characteristics (eg, parameters of the International Prognostic Index) and primary treatment (eg, specific therapeutic regimen, albeit no detailed information regarding dose intensity) was available in the NCR for patients diagnosed as of 2014.11 The vital status of the patients (ie, alive, dead, or emigrated) was obtained via the Nationwide Population Registries Network, with follow-up until 1 January 2022. Further details regarding the NCR have been described elsewhere.11
We calculated the RS by comparing the observed survival in the study population with the expected survival in the matched general population. This method estimates the disease-specific survival. Moreover, RS can hint toward excess mortality directly or indirectly attributed to DLBCL or its associated treatment, thereby offering some perspective without specific cause-of-death data, which are often unavailable in unreliable cancer registry data. Of note, RS should not be equated with disease-specific survival because of potential differences in mortality related to treatment toxicity. The calculation of RS involves calculating the ratio of the observed study survival of the population to the expected survival of the matched general population. The expected survival was estimated per the Ederer II method using age-, sex-, and period-stratified Dutch life tables and matched to the study population based on age, sex, and period. Sex-specific RS was computed for the overall cohort and stratified according to age (61-70, 71-80, and >80 years). RS was measured from the start of R-CHOP21 to death or the end of follow-up (1 January 2022). Poisson regression was used to assess (1) differences in RS across sex and (2) the effect of sex on the excess mortality rate ratio while simultaneously adjusting for years of follow-up and prognostic relevant covariates—that is, age, International Prognostic Index score, time from diagnosis to first-line treatment, and treatment at an academic center. P < .05 indicated statistical significance. Complete details of the statistical analyses are provided in the supplemental Methods.
The overall cohort included 5538 patients with DLBCL aged >60 years (55% males; median age, 74 years; interquartile age range, 68-80 years; and 64% with stage III-IV disease) diagnosed in The Netherlands between 2014 and 2020, of whom 4014 (72%) received R-CHOP21. Of these 4014 patients, 3781 completed ≥1 cycle of R-CHOP21 (42% females and 58% males). This population formed the basis of our analysis (Table 1). The remaining 1524 (29%) patients received either no therapy (61%) or treatment other than R-CHOP21 (39%; supplemental Tables 1-3).
Baseline patient and treatment characteristics of older patients (aged more than 60 years) with DLBCL treated with R-CHOP in The Netherlands according to sex, from 2014 to 2020
| Characteristics . | Male . | Female . | P . | Total . | |||
|---|---|---|---|---|---|---|---|
| N . | (%) . | N . | (%) . | N . | (%) . | ||
| Total no. of patients | 2121 | — | 1660 | — | 3781 | — | |
| Age at diagnosis, y | |||||||
| Median (IQR) | 72 (67-77) | 73 (68-78) | < .001 | 72 (67-77) | |||
| 61-70 | 909 | (43) | 626 | (38) | .001 | 1535 | (41) |
| 71-80 | 940 | (44) | 771 | (46) | 1711 | (45) | |
| >80 | 272 | (13) | 263 | (16) | 535 | (14) | |
| Stage at diagnosis∗ | .241 | ||||||
| I-II | 722 | (34) | 596 | (36) | 1318 | (35) | |
| III-IV | 1388 | (65) | 1057 | (64) | 2445 | (65) | |
| Unknown | 11 | (1) | 7 | (0) | 18 | (0) | |
| LDH > ULN∗ | .117 | ||||||
| No | 994 | (47) | 731 | (44) | 1725 | (46) | |
| Yes | 1093 | (52) | 892 | (54) | 1985 | (52) | |
| Unknown | 34 | (2) | 37 | (2) | 71 | (2) | |
| Extranodal sites | .332 | ||||||
| ≤1 | 1499 | (71) | 1149 | (69) | 2648 | (70) | |
| >1 | 622 | (29) | 511 | (31) | 1133 | (30) | |
| WHO performance score | .346 | ||||||
| 0 | 635 | (30) | 457 | (28) | 1092 | (29) | |
| 1 | 412 | (19) | 357 | (22) | 769 | (20) | |
| 2 | 129 | (6) | 118 | (7) | 247 | (7) | |
| 3 | 53 | (2) | 38 | (2) | 91 | (2) | |
| 4 | 7 | (0) | 6 | (0) | 13 | (0) | |
| Unknown | 878 | (41) | 679 | (41) | 1 557 | (41) | |
| IPI | .803 | ||||||
| Low (0-1) | 302 | (14) | 235 | (14) | 537 | (14) | |
| Low-int (2) | 276 | (13) | 218 | (13) | 494 | (13) | |
| High-int (3) | 337 | (16) | 254 | (15) | 591 | (16) | |
| High (4-5) | 480 | (23) | 403 | (24) | 883 | (23) | |
| Unclassifiable† | 726 | (34) | 550 | (33) | 1276 | (34) | |
| Time from diagnosis to treatment | |||||||
| Median (IQR), d | 24 (15-35) | 24 (15-36) | 0.892 | 24 (15-35) | |||
| 0-13 d | 433 | (20) | 321 | (19) | .708 | 754 | (20) |
| 14-27 d | 826 | (39) | 658 | (40) | 1484 | (39) | |
| ≥28 d | 862 | (41) | 681 | (41) | 1 543 | (41) | |
| Center of treatment | .403 | ||||||
| Nonacademic | 1911 | (90) | 1509 | (91) | 3420 | (90) | |
| Academic | 210 | (10) | 151 | (9) | 361 | (10) | |
| Best response | .184 | ||||||
| Complete remission | 1591 | (75) | 1286 | (77) | 2877 | (76) | |
| Partial remission | 14 | (1) | 9 | (1) | 23 | (1) | |
| Stable disease | 68 | (3) | 36 | (2) | 104 | (3) | |
| Progressive disease | 237 | (11) | 185 | (11) | 422 | (11) | |
| Unknown | 211 | (10) | 144 | (9) | 355 | (9) | |
| Characteristics . | Male . | Female . | P . | Total . | |||
|---|---|---|---|---|---|---|---|
| N . | (%) . | N . | (%) . | N . | (%) . | ||
| Total no. of patients | 2121 | — | 1660 | — | 3781 | — | |
| Age at diagnosis, y | |||||||
| Median (IQR) | 72 (67-77) | 73 (68-78) | < .001 | 72 (67-77) | |||
| 61-70 | 909 | (43) | 626 | (38) | .001 | 1535 | (41) |
| 71-80 | 940 | (44) | 771 | (46) | 1711 | (45) | |
| >80 | 272 | (13) | 263 | (16) | 535 | (14) | |
| Stage at diagnosis∗ | .241 | ||||||
| I-II | 722 | (34) | 596 | (36) | 1318 | (35) | |
| III-IV | 1388 | (65) | 1057 | (64) | 2445 | (65) | |
| Unknown | 11 | (1) | 7 | (0) | 18 | (0) | |
| LDH > ULN∗ | .117 | ||||||
| No | 994 | (47) | 731 | (44) | 1725 | (46) | |
| Yes | 1093 | (52) | 892 | (54) | 1985 | (52) | |
| Unknown | 34 | (2) | 37 | (2) | 71 | (2) | |
| Extranodal sites | .332 | ||||||
| ≤1 | 1499 | (71) | 1149 | (69) | 2648 | (70) | |
| >1 | 622 | (29) | 511 | (31) | 1133 | (30) | |
| WHO performance score | .346 | ||||||
| 0 | 635 | (30) | 457 | (28) | 1092 | (29) | |
| 1 | 412 | (19) | 357 | (22) | 769 | (20) | |
| 2 | 129 | (6) | 118 | (7) | 247 | (7) | |
| 3 | 53 | (2) | 38 | (2) | 91 | (2) | |
| 4 | 7 | (0) | 6 | (0) | 13 | (0) | |
| Unknown | 878 | (41) | 679 | (41) | 1 557 | (41) | |
| IPI | .803 | ||||||
| Low (0-1) | 302 | (14) | 235 | (14) | 537 | (14) | |
| Low-int (2) | 276 | (13) | 218 | (13) | 494 | (13) | |
| High-int (3) | 337 | (16) | 254 | (15) | 591 | (16) | |
| High (4-5) | 480 | (23) | 403 | (24) | 883 | (23) | |
| Unclassifiable† | 726 | (34) | 550 | (33) | 1276 | (34) | |
| Time from diagnosis to treatment | |||||||
| Median (IQR), d | 24 (15-35) | 24 (15-36) | 0.892 | 24 (15-35) | |||
| 0-13 d | 433 | (20) | 321 | (19) | .708 | 754 | (20) |
| 14-27 d | 826 | (39) | 658 | (40) | 1484 | (39) | |
| ≥28 d | 862 | (41) | 681 | (41) | 1 543 | (41) | |
| Center of treatment | .403 | ||||||
| Nonacademic | 1911 | (90) | 1509 | (91) | 3420 | (90) | |
| Academic | 210 | (10) | 151 | (9) | 361 | (10) | |
| Best response | .184 | ||||||
| Complete remission | 1591 | (75) | 1286 | (77) | 2877 | (76) | |
| Partial remission | 14 | (1) | 9 | (1) | 23 | (1) | |
| Stable disease | 68 | (3) | 36 | (2) | 104 | (3) | |
| Progressive disease | 237 | (11) | 185 | (11) | 422 | (11) | |
| Unknown | 211 | (10) | 144 | (9) | 355 | (9) | |
int, intermediate; IPI, International Prognostic Index; IQR, interquartile range; LDH, lactate dehydrogenase; ULN, upper limit of normal; WHO, World Health Organization.
P value computed without the missing values.
Primairly owing to a missing WHO performance score.
Virtually, all baseline and treatment characteristics of our study cohort were well-balanced according to sex (Table 1). However, there was a 1-year age difference in the median age between male and female patients (72 vs 73 years, respectively; P < .001).
More than half of the patients received 6 cycles of R-CHOP21 with or without 2 additional cycles of rituximab (56%). The remaining patients received 8 cycles of R-CHOP21 (16%), 3 cycles of R-CHOP21 with radiotherapy (10%), or other number of cycles of R-CHOP21 (18%).
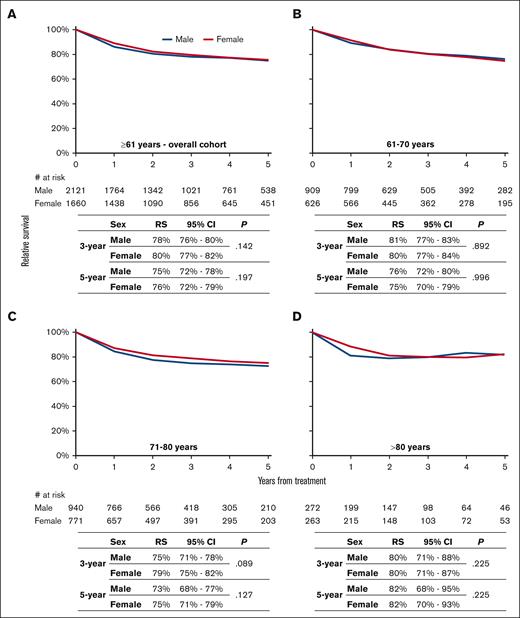
At a median follow-up of 35.6 months (interquartile range, 17.2-61.3 months), the 3-year RS was not significantly different across sex for the overall cohort (Figure 1A) and according to the 3 age groups (Figures 1B-D). Multivariable analysis confirmed that RS was not significantly different across sex for the overall cohort (excess mortality rate ratio, 0.90; 95% confidence interval, 0.77-1.05; P = .177; supplemental Table 4). Furthermore, multivariable analysis revealed no evidence that the effect of sex was different across the 3 age groups (P for interaction = 0.808).
The sex-specific relative survival (RS) of R-CHOP21-treated older (>60 years) patients with DLBCL in The Netherlands, from 2014 to 2020. Sex-specific RS is shown for the following age categories at diagnosis: (A) >60 years, (B) from 61 to 70 years, (C) from 71 to 80 years, and (D) >80 years. The tables present the projected 3- and 5-year RS with associated 95% confidence intervals and the number at risk according to sex. The P value is derived from the likelihood ratio test that compares the model with years of follow-up and sex with the model containing only years of follow-up. The median follow-up for 2115 (66%) patients alive at the end of follow-up was 47.3 months (interquartile range, 28.2-69.5 months). CI, confidence interval.
The sex-specific relative survival (RS) of R-CHOP21-treated older (>60 years) patients with DLBCL in The Netherlands, from 2014 to 2020. Sex-specific RS is shown for the following age categories at diagnosis: (A) >60 years, (B) from 61 to 70 years, (C) from 71 to 80 years, and (D) >80 years. The tables present the projected 3- and 5-year RS with associated 95% confidence intervals and the number at risk according to sex. The P value is derived from the likelihood ratio test that compares the model with years of follow-up and sex with the model containing only years of follow-up. The median follow-up for 2115 (66%) patients alive at the end of follow-up was 47.3 months (interquartile range, 28.2-69.5 months). CI, confidence interval.
This nationwide, population-based study suggests that there is no sex difference in RS among older (>60 years) patients with DLBCL treated with R-CHOP21 in an era in which rituximab dosing at 375 mg/m2 is the standard. Although this study is the first of its kind, to our knowledge, our findings evoke a brief reflection on the scant literature addressing sex-based survival differences in patients with DLBCL treated with R-CHOP. Initially, we briefly touched upon studies invigorating the differential sex distribution in survival among older patients with DLBCL treated with R-CHOP. On the other side of the pendulum, our population-based study corroborates the sex-specific findings of the phase 3 HOVON-84 trial, showing that R-CHOP14 with early rituximab intensification (as compared with standard R-CHOP14) does not improve outcomes in adult (≥18 years) patients with DLBCL, irrespective of sex.8 In addition, a meta-analysis of 3 phase 3 GELA trials, in which adult (≥18 years) patients with DLBCL treated with R-CHOP received standard-dose rituximab, showed no association between sex and OS.9
The strengths of our study include the use of RS over OS and a nationwide, population-based cohort with large patient numbers and details of primary treatment. The limitations of our study mainly relate to its retrospective and uncontrolled nature, lack of data on the cause of death and PFS, and lack of detailed information on dose intensity (eg, R-mini-CHOP). The former is particularly noteworthy because the Dutch DLBCL guidelines recommend R-mini-CHOP for older, frail patients.12 However, because of this data gap, our analysis could not account for potential variations in the treatment intensity. Furthermore, the duration of our follow-up could potentially miss late relapses of DLBCL or late effects of treatment that may occur after the end of our follow-up period. These late effects, which may vary between the sexes, could potentially influence long-term survival outcomes. Collectively, future research might benefit from considering these factors more explicitly. Notwithstanding these limitations, our nationwide, population-based study adds to the ambivalence regarding the differential sex distribution in various survival outcomes among older patients with DLBCL treated with R-CHOP. This ambivalence is driven by differences across various studies in terms of patient inclusion criteria (eg, age and type of aggressive B-cell lymphoma diagnosis), study design (ie, phase 2 vs 3 clinical trials, historical comparisons, and prospective vs observational studies), rituximab dosing strategies (ie, increasing the standard rituximab dose, extending rituximab exposure, and applying an upfront dose-dense schedule of rituximab), and survival measures (ie, OS, PFS, and RS).2-9 The phase 3 OPTIMAL >60 trial (#NCT01478542) will hopefully confirm the optimal dosage of rituximab in older (range, 61-80 years) patients with DLBCL.
Acknowledgments: The authors thank the registration clerks of the NCR for their dedicated data collection. The nationwide, population-based NCR is maintained and hosted by The Netherlands Comprehensive Cancer Organisation.
Contribution: A.G.D. designed the study and supervised the data analyses; E.E.G. and M.D. analyzed the data; O.V. was responsible for data collection; E.E.G. wrote the manuscript, with contributions from all authors; and all authors interpreted the data, read and commented on, and approved the final version of the manuscript.
Conflict-of-interest disclosure: M.J.K. reports honoraria and research funding from Roche to the institution, where she is employed. The remaining authors declare no competing financial interests.
Correspondence: Avinash G. Dinmohamed, Department of Research and Development, Netherlands Comprehensive Cancer Organisation, Godebaldkwartier 419, 3511 DT Utrecht, The Netherlands; e-mail: a.dinmohamed@iknl.nl.
References
Author notes
∗E.E.G. and M.D. contributed equally to this work.
Data from The Netherlands Cancer Registry have been available since 1989 and are widely used in population-based cancer research.
Data are available on request from the corresponding author, Avinash G. Dinmohamed (a.dinmohamed@iknl.nl).
The full-text version of this article contains a data supplement.