Key Points
In patients receiving CAR-T cell therapy the development of CRS does not affect overall survival, progression-free survival, or response rate.
Decision to start additional lymphoma-directed therapy after CAR-T cell therapy should not be based on whether patients develop CRS.
Abstract
Chimeric antigen receptor (CAR) T-cell therapy has revolutionized the treatment of many patients with aggressive relapsed or refractory large B-cell lymphoma (LBCL). Treatment can be complicated by clinically evident cytokine release syndrome (CRS), which is characterized by the development of fever, hypoxia, and hypotension, and can be life-threatening. Most patients treated with CAR-T cells develop CRS, which is thought to represent an immune phenomenon. It was previously unknown whether patients who did not develop CRS had reduced CAR-T cell activity and were therefore likely to have worse outcomes. We conducted a multicenter retrospective analysis of 352 adult patients treated at 8 academic medical centers in the United States who received axicabtagene ciloleucel or tisagenlecleucel for the treatment of LBCL. The outcomes of interest included progression-free survival, overall survival, complete response rate, and overall response rate. Of the included patients, 262 (74.4%) developed CRS. There was no significant difference in progression-free survival (P = .99) or overall survival (P = .16) between patients who developed CRS and those who did not develop CRS. Peak ferritin levels >5000 ng/mL during treatment and lactate dehydrogenase levels greater than the institutional upper limit of normal before lymphodepleting chemotherapy were associated with significantly worse progression-free and overall survival in the multivariate analysis. There was no significant difference in the complete response or overall response rates between patients who did and did not develop CRS. In this retrospective analysis, we report that patients who develop CRS have clinical outcomes similar to those of patients without CRS treated with commercial anti-CD19 CAR-T cells.
Introduction
The emergence of chimeric antigen receptor (CAR) T-cell therapy for the treatment of aggressive large B-cell lymphoma (LBCL) has changed the management algorithms for this disease. Before CAR-T cell therapy, outcomes for patients with relapsed/refractory (r/r) LBCL were poor, with objective response rates (ORR) of 26% to subsequent lines of therapy and median overall survival (OS) of 6.3 months.1 In the trials that led to the approvals of various CAR-T cell products for r/r LBCL, ORR ranged from 52% to 82%, and median OS ranged from 11.1 months to 25.8 months.2-4 Real world experience has provided further evidence of the efficacy of these therapies with ORRs ranging between 59% and 73.3%, and 21-month OS reported in 1 study as 70.2%.5,6
Treatment with CAR-T cells is accompanied by a unique set of adverse events compared with traditional chemoimmunotherapy, characterized by the development of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). These clinical side effects occur when CAR-T cells bind to their targets and activate an intracellular cascade, leading to proliferation and expansion through the release of cytokines. These cytokines recruit immune cells to the tumor sites, but also have a physiological effect on fevers and capillary leak when exaggerated.
Although the rates of CRS vary in clinical trials, the development of severe (grade 3 or higher) CRS was low throughout all pivotal trials, ranging from 2% to 22%.2-4 Similar incidences of CRS have been noted in retrospective studies, with patients treated with axicabtagene ciloleucel (axi-cel) tending to develop CRS at higher rates than those treated with tisagenlecleucel (tisa-cel).5,7,8 CRS was initially proposed to be correlated with CAR-T cell expansion and thus with response. In patients who do not develop CRS, there is a general concern regarding the lack of expansion and efficacy. Data regarding CRS and outcomes are mixed: 1 retrospective study showed a non-significantly reduced response rate in patients who developed severe CRS whereas another study showed increased hematologic toxicity with severe CRS but did not report an association with response rate or survival.8,9
Several risk factors have been associated with higher incidences of CRS in patients undergoing CAR-T cell treatment, including higher tumor burden, higher CAR-T cell dose, and thrombocytopenia before initiation of lymphodepleting chemotherapy.10 Studies have also noted a possible association between grade of CRS and longer persistence of hematologic toxicity after treatment.11,12
The existing grading systems for CRS rely on clinically evident signs and symptoms including, fever, hypoxia, and hypotension. Although fever is a reliable measure of the onset of CRS, it becomes evident many days after CAR-T cells are infused and often resolves, while other parameters indicative of CAR-T cell activity are still present or rising, suggesting that CAR-T cell activity may not correlate with clinical CRS. Furthermore, the percentage of patients who experienced CRS is lower than the ORR in some clinical trials, further underscoring the need for studies to evaluate the association between CRS and clinical outcomes.
In this retrospective multicenter study, we aimed to address 2 questions: whether the development of CRS is associated with improved or worsened survival and whether the absence of CRS reflects clinical efficacy in patients with LBCL undergoing treatment with CAR-T cells.
Methods
Study design
This retrospective, multicenter study included patients aged ≥18 years with aggressive LBCL who underwent apheresis between May 2018 and January 2021 for commercial CAR-T cell therapy at 8 academic medical centers in the United States. All patients had the option of prescribing either axi-cel or tisa-cel. Baseline clinical characteristics, laboratory and pathological data, CAR-T cell treatment course, toxicity, and outcomes for patients were recorded in a centralized research electronic data capture (REDCap) database. Institutional review board approval was obtained from all study centers. Patients were prescribed either axi-cel or tisa-cel, as chosen by the treatment center. Patient selection, use of bridging therapy, management of toxicity, timing of response assessments, and choice of inpatient or outpatient treatment was determined by individual institutions. CRS was graded using the American Society for Transplantation and Cellular Therapy (ASTCT) consensus criteria.13 Tumor response 90 days after CAR-T cell infusion was assessed per Lugano criteria by the treating clinician.14
Statistical analysis
The baseline patient and treatment characteristics were summarized using descriptive statistics and frequency tables. Comparisons between groups were performed using Pearson’s chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Kaplan-Meier methods and log-rank tests were applied to progression-free survival (PFS) and OS in all patients. PFS was defined as the time from CAR-T cell infusion to the earliest marker of progression, which included disease progression, initiation of subsequent anticancer therapy, or death. OS was defined as the time from CAR-T cell infusion to death from any cause.
Multivariate Cox regression modeling was performed for the outcomes of PFS and OS. The variables considered in the multivariate analysis are shown in Tables 1 and 2. The proportional hazard assumption was verified using the Schoenfeld residual. Variables with a significance level of 0.2 in the univariable analysis were considered for the multivariate model and subjected to a stepwise variable selection method with a significance level of 0.05 to enter the model and 0.1 for removal from the model. The variables of particular clinical interest were forced into the model despite being non-significance. A complete case analysis or list-wise deletion was implemented to handle missing data in the multivariate analysis. The hazard ratio (HR) and 95% confidence interval (CI) was calculated for all variables. All statistical analyses were performed using Stata version 17 (StataCorp, College Station, TX) and P < .05 was statistically significant.
Multivariate analysis of progression-free survival
| Effect . | P value . | Hazard ratio . | 95% confidence interval . |
|---|---|---|---|
| PFS | |||
| Developed CRS | .239 | 0.79 | 0.53-1.17 |
| Peak ferritin >5000 | <.001 | 2.61 | 1.71-3.98 |
| LDH>ULN | <.001 | 2.11 | 1.49-2.98 |
| Stage 3-4 | .333 | 1.21 | 0.82-1.80 |
| Bulky disease | .219 | 1.27 | 0.87-1.87 |
| Refractory to most recent chemo vs primary refractory | .427 | 0.86 | 0.60-1.24 |
| Relapsed vs primary refractory | .085 | 0.70 | 0.47-1.05 |
| Received steroids | .740 | 0.73 | 0.52-1.03 |
| Bridging therapy | .011 | 1.58 | 1.11-2.24 |
| Effect . | P value . | Hazard ratio . | 95% confidence interval . |
|---|---|---|---|
| PFS | |||
| Developed CRS | .239 | 0.79 | 0.53-1.17 |
| Peak ferritin >5000 | <.001 | 2.61 | 1.71-3.98 |
| LDH>ULN | <.001 | 2.11 | 1.49-2.98 |
| Stage 3-4 | .333 | 1.21 | 0.82-1.80 |
| Bulky disease | .219 | 1.27 | 0.87-1.87 |
| Refractory to most recent chemo vs primary refractory | .427 | 0.86 | 0.60-1.24 |
| Relapsed vs primary refractory | .085 | 0.70 | 0.47-1.05 |
| Received steroids | .740 | 0.73 | 0.52-1.03 |
| Bridging therapy | .011 | 1.58 | 1.11-2.24 |
Boldfaced values indicate factors that had a statistically significant impact on multivariate analysis.
Multivariate analysis of overall survival
| Effect . | P value . | Hazard ratio . | 95% confidence interval . |
|---|---|---|---|
| OS | |||
| Developed CRS | .549 | 0.87 | 0.54-1.39 |
| Peak ferritin>5000 | <.001 | 2.38 | 1.50-3.76 |
| LDH>ULN | <.001 | 2.34 | 1.55-3.53 |
| Stage 3-4 | .336 | 1.24 | 0.80-1.93 |
| Bulky disease | .005 | 1.81 | 1.20-2.75 |
| Refractory to most recent chemo vs primary refractory | .552 | 0.89 | 0.59-1.32 |
| Relapsed vs primary refractory | .173 | 0.72 | 0.44-1.16 |
| Received steroids | .775 | 1.06 | 0.73-1.53 |
| Bridging therapy | .300 | 1.23 | 0.83-1.84 |
| Effect . | P value . | Hazard ratio . | 95% confidence interval . |
|---|---|---|---|
| OS | |||
| Developed CRS | .549 | 0.87 | 0.54-1.39 |
| Peak ferritin>5000 | <.001 | 2.38 | 1.50-3.76 |
| LDH>ULN | <.001 | 2.34 | 1.55-3.53 |
| Stage 3-4 | .336 | 1.24 | 0.80-1.93 |
| Bulky disease | .005 | 1.81 | 1.20-2.75 |
| Refractory to most recent chemo vs primary refractory | .552 | 0.89 | 0.59-1.32 |
| Relapsed vs primary refractory | .173 | 0.72 | 0.44-1.16 |
| Received steroids | .775 | 1.06 | 0.73-1.53 |
| Bridging therapy | .300 | 1.23 | 0.83-1.84 |
Boldfaced values indicate factors that had a statistically significant impact on multivariate analysis.
Results
Patient and treatment characteristics
This study included 352 consecutive patients who underwent apheresis for commercial CAR-T cells. Baseline patient characteristics are outlined in Table 3. Of the included patients, 262 (74.4%) developed CRS, whereas 90 (25.5%) did not.
Baseline patient characteristics
| Characteristic . | Patients with CRS (n = 261) . | Patients without CRS (n = 90) . | P value . |
|---|---|---|---|
| Median age (range, y) | 61 (18-88) | 65 (37-85) | .005 |
| Gender | .895 | ||
| Male, n (%) | 172 (66) | 60 (66.7) | |
| Female, n (%) | 89 (34.1) | 30 (33.3) | |
| Stage | .893 | ||
| I-II, n (%) | 51 (19.5) | 17 (18.9) | |
| III-IV, n (%) | 210 (80.5) | 73 (81.1) | |
| IPI score | .226 | ||
| 1-2, n (%) | 110 (46.6) | 42 (54.5) | |
| ≥3, n (%) | 126 (53.4) | 35 (45.5) | |
| Product | <.001 | ||
| Axi-cel, n (%) | 182 (69.7) | 20 (22.2) | |
| Tisa-cel, n (%) | 79 (30.3) | 70 (77.8) | |
| Disease status | |||
| Primary refractory, n (%) | 92 (37.6) | 17 (21.8) | .014 |
| Refractory, n (%) | 83 (33.9) | 27 (34.6) | |
| Relapsed, n (%) | 70 (28.6) | 34 (43.6) | |
| Bulky disease | .330 | ||
| Yes, n (%) | 44 (18.4) | 10 (13.5) | |
| No, n (%) | 195 (81.6) | 64 (86.5) | |
| Peak ferritin >5000 | <.001 | ||
| Yes, n (%) | 43 (17.8) | 1 (1.3) | |
| No, n (%) | 199 (82.2) | 75 (98.7) | |
| LDH>ULN | <.001 | ||
| Yes, n (%) | 132 (62) | 24 (36.9) | |
| No, n (%) | 81 (32) | 41 (63.1) | |
| Received steroids | <.001 | ||
| Yes, n (%) | 116 (46.2) | 4 (4.8) | |
| No, n (%) | 135 (53.8) | 79 (95.1) | |
| Received tocilizumab | <.001 | ||
| Yes, n (%) | 156 (59.5) | 11 (12.4) | |
| No, n (%) | 106 (40.5) | 78 (87.6) | |
| Use of bridging therapy | .227 | ||
| Yes, n (%) | 168 (65.3) | 63 (72.4) | |
| No, n (%) | 89 (34.6) | 24 (27.6) |
| Characteristic . | Patients with CRS (n = 261) . | Patients without CRS (n = 90) . | P value . |
|---|---|---|---|
| Median age (range, y) | 61 (18-88) | 65 (37-85) | .005 |
| Gender | .895 | ||
| Male, n (%) | 172 (66) | 60 (66.7) | |
| Female, n (%) | 89 (34.1) | 30 (33.3) | |
| Stage | .893 | ||
| I-II, n (%) | 51 (19.5) | 17 (18.9) | |
| III-IV, n (%) | 210 (80.5) | 73 (81.1) | |
| IPI score | .226 | ||
| 1-2, n (%) | 110 (46.6) | 42 (54.5) | |
| ≥3, n (%) | 126 (53.4) | 35 (45.5) | |
| Product | <.001 | ||
| Axi-cel, n (%) | 182 (69.7) | 20 (22.2) | |
| Tisa-cel, n (%) | 79 (30.3) | 70 (77.8) | |
| Disease status | |||
| Primary refractory, n (%) | 92 (37.6) | 17 (21.8) | .014 |
| Refractory, n (%) | 83 (33.9) | 27 (34.6) | |
| Relapsed, n (%) | 70 (28.6) | 34 (43.6) | |
| Bulky disease | .330 | ||
| Yes, n (%) | 44 (18.4) | 10 (13.5) | |
| No, n (%) | 195 (81.6) | 64 (86.5) | |
| Peak ferritin >5000 | <.001 | ||
| Yes, n (%) | 43 (17.8) | 1 (1.3) | |
| No, n (%) | 199 (82.2) | 75 (98.7) | |
| LDH>ULN | <.001 | ||
| Yes, n (%) | 132 (62) | 24 (36.9) | |
| No, n (%) | 81 (32) | 41 (63.1) | |
| Received steroids | <.001 | ||
| Yes, n (%) | 116 (46.2) | 4 (4.8) | |
| No, n (%) | 135 (53.8) | 79 (95.1) | |
| Received tocilizumab | <.001 | ||
| Yes, n (%) | 156 (59.5) | 11 (12.4) | |
| No, n (%) | 106 (40.5) | 78 (87.6) | |
| Use of bridging therapy | .227 | ||
| Yes, n (%) | 168 (65.3) | 63 (72.4) | |
| No, n (%) | 89 (34.6) | 24 (27.6) |
The median age at apheresis was 61 years (range, 18-88) among patients who developed CRS and 65 years (range, 37-85) among those who did not (P = .005). No significant differences in the incidence of CRS were noted when evaluated by gender, eastern cooperative oncology group (ECOG) performance status, disease type, disease stage, International Prognostic Index (IPI) score, presence of bulky disease (disease ≥10 cm), or administration of bridging therapy. As expected, significant differences were noted in the use of steroids and tocilizumab between the groups that did and did not develop CRS, respectively. No differences were noted between the number of lines of previous therapy and previous autologous or allogeneic stem cell transplant (data not shown).
Of the included patients, 202 (57.6%) received axi-cel and 149 (42.5%) received tisa-cel. Axi-cel recipients tended to be younger than tisa-cel recipients, with a median age of 59 years (range, 18-85) vs 67 years (range, 30-88); P < .001. Among patients who received axi-cel, 90.1% developed CRS, whereas 53.0% of tisa-cel recipients developed CRS (P = .001). Among patients with available data on disease status at the time of referral (n = 323), CRS occurred less frequently in patients with relapsed disease (n = 34, 43.6%) than in patients with primary refractory disease (n = 17, 21.8%) or disease refractory to the most recent therapy (n = 27, 34.6%) (P = .014).
Survival outcomes
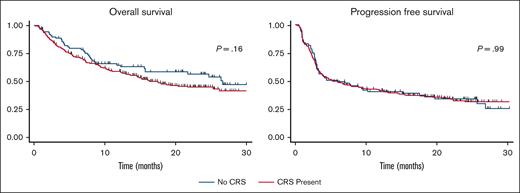
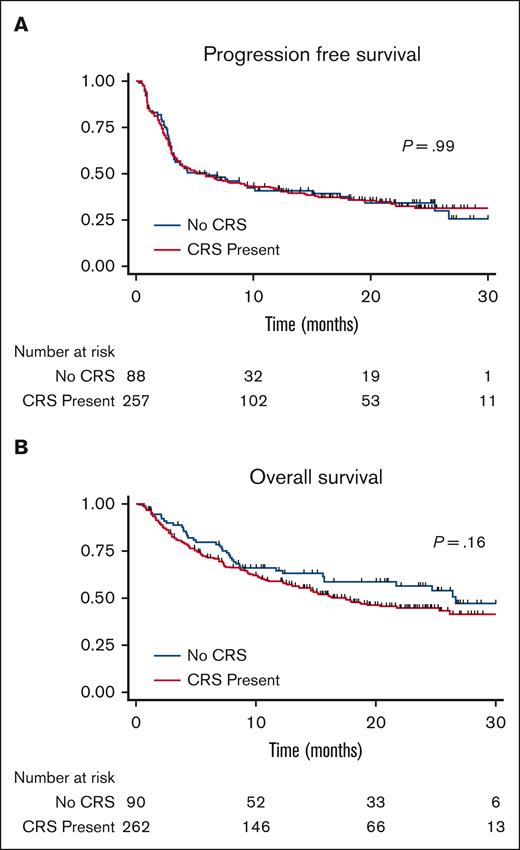
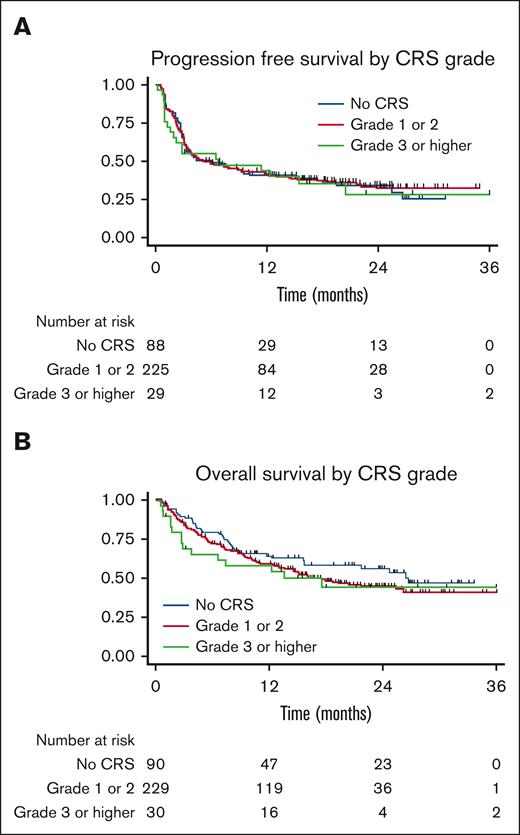
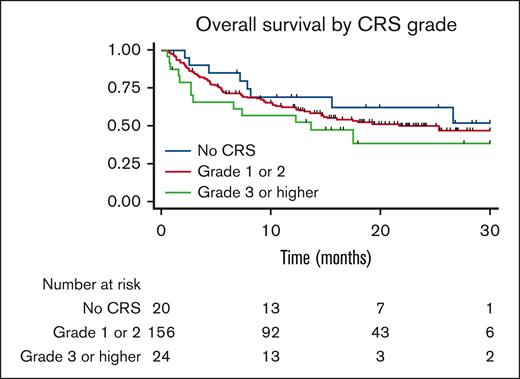
The PFS and OS curves are shown in Figure 1. With a median follow-up of 30 months, there were no significant differences in PFS or OS between patients who developed CRS and those who did not. The median PFS in patients who did not develop CRS was 5.99 months and that in patients who developed CRS was 5.89 months, P = .99. The median OS in patients who did not develop CRS was 26.6 months and was 17.5 months in patients who developed CRS, P = .16. For patients who developed CRS, univariate analysis showed that when comparing patients who did not develop CRS, patients who developed grade 1 to 2 CRS, and patients who developed grade 3 to 4 CRS, grade did not influence PFS, P = .36 or OS, P = .92 (Figure 2). No CRS-related deaths occurred in the study population. When these results were stratified by axi-cel, there was a trend of worse OS with higher-grade CRS (Figure 3).
Progression-free survival. Kaplan-Meier curve showing progression-free survival in patients treated with CD19-directed CAR-T cells showing similar curves for those who did and did not develop cytokine release syndrome.
Progression-free survival. Kaplan-Meier curve showing progression-free survival in patients treated with CD19-directed CAR-T cells showing similar curves for those who did and did not develop cytokine release syndrome.
Overall survival. Kaplan-Meier curve showing overall survival in patients treated with CD19-directed CAR-T cells showing similar curves for patients who did and did not develop cytokine release syndrome.
Overall survival. Kaplan-Meier curve showing overall survival in patients treated with CD19-directed CAR-T cells showing similar curves for patients who did and did not develop cytokine release syndrome.
Overall survival stratified by CRS grade. Kaplan-Meier curve showing overall survival for patients who received axicabtagene ciloleucel, showing no significant difference in survival when stratified by CRS grade.
Overall survival stratified by CRS grade. Kaplan-Meier curve showing overall survival for patients who received axicabtagene ciloleucel, showing no significant difference in survival when stratified by CRS grade.
Multivariate analysis
Several variables were included in multivariate analysis to further elucidate the impact of CRS on survival. Patient-related variables included age at apheresis, sex, ECOG performance status, Charlson Comorbidity Index (CCI) score, and IPI score. Disease-related variables included lactate dehydrogenase (LDH), platelet count before administration of lymphodepleting chemotherapy, disease stage at apheresis, presence of bulky disease (defined as disease ≥10 cm), prior autologous stem cell transplant, prior allogeneic stem cell transplant, use of steroids during the treatment course, use of bridging therapy, and disease status at referral. Therapy-related characteristics included product type (axi-cel or tisa-cel) and peak ferritin. The variables that were significant and several non-significant but clinically relevant variables included in the analysis are shown in Table 1 and 2. Other nonsignificant variables were not included in the final analysis.
The development of CRS did not have a significant impact on PFS or OS, with HR of 0.79 (95% CI, 0.53-1.17; P = .239) and 0.87 (95% CI, 0.54-1.39; P = .549), respectively. A peak ferritin level of greater than 5000 within 28-days following CAR-T cell infusion was associated with significantly worse PFS and OS, with HR of 2.61 (95% CI, 1.71-3.98; P < .001) and 2.38 (95% CI, 1.50-3.76; P < .001), respectively. LDH before the start of lymphodepleting chemotherapy that was greater than the institutional upper limit of normal was associated with worse PFS and OS, with HR 2.11 (95% CI, 1.49-2.98; P < .001) and HR 2.34 (95% CI, 1.55-3.53; P < .001), respectively. The administration of steroids during treatment had no significant impact on PFS or OS when compared with patients who did not receive steroids.
There were no significant differences in PFS or OS based on disease stage (stage III-IV vs stage I-II) or disease status at the time of referral. The presence of bulky disease had no significant impact on PFS, but it was associated with worse OS with HR 1.81 (95% CI, 1.29-2.75; P = .005).
The relationship between receipt of bridging therapy and PFS and OS was also evaluated. When compared with patients who did not receive bridging therapy, receipt of bridging therapy was associated with significantly worse PFS (HR, 1.58; 95% CI, 1.11-2.24; P = .011), though no difference in OS.
Cytopenia analysis
Analysis was performed to evaluate the relationship between the development of CRS and the presence of cytopenia (defined as absolute neutrophil count [ANC], <1000/mm3; or platelets, <50x103/mcL) at day 30, 3 months, 6 months, 9 months, and 1 year after CAR-T cell infusion. Patients with relapsed disease or those who received subsequent anticancer therapy were censored. Data are presented in Table 4. The occurrence of CRS within 28 days of treatment was associated with cytopenia at day 30 (P = .001), but not associated with cytopenias at any other time point. Exploratory analyses were conducted in 30 patients who developed severe CRS. Among patients with grade 3 or higher CRS, 63% had cytopenia at day 30, compared with 47% among patients who did not have CRS. CRS grade 3 or higher was not significantly associated with persistence of cytopenia after day 30.
Response rate analysis
Analysis was performed to evaluate the relationship between the development of CRS, ORR, and complete response (CR) rate. At 30 days post-treatment, there was no difference in ORR (P = .081) or CR rate (P = .155) between patients who developed CRS and those who did not (Table 5). When stratified by severity of CRS there was also no difference in ORR (P = .187) or CR rate (P = .286) among patients with no CRS, those with grade 1 to 2 CRS, and those with grade 3 or higher CRS (Table 6).
Response rate analysis
| Characteristic . | Patients with CRS (n = 261) . | Patients without CRS (n = 90) . | P value . |
|---|---|---|---|
| Overall response | .081 | ||
| Yes, n (%) | 137 (66.2) | 23 (52.3) | |
| No, n (%) | 70 (33.8) | 21 (47.7) | |
| Complete response | .155 | ||
| Yes, n (%) | 80 (38.7) | 12 (27.3) | |
| No, n (%) | 127 (61.4) | 32 (72.7) |
| Characteristic . | Patients with CRS (n = 261) . | Patients without CRS (n = 90) . | P value . |
|---|---|---|---|
| Overall response | .081 | ||
| Yes, n (%) | 137 (66.2) | 23 (52.3) | |
| No, n (%) | 70 (33.8) | 21 (47.7) | |
| Complete response | .155 | ||
| Yes, n (%) | 80 (38.7) | 12 (27.3) | |
| No, n (%) | 127 (61.4) | 32 (72.7) |
Response rate analysis, stratified by degree of CRS
| Characteristic . | Patients without CRS (n = 44) . | Patients with grade 1-2 CRS (n = 185) . | Patients with grade 3 or higher CRS (n = 19) . | P value . |
|---|---|---|---|---|
| Overall response | ||||
| Yes, n (%) | 23 (52.3) | 123 (66.5) | 11 (57.9) | |
| No, n (%) | 21 (47.7) | 62 (33.5) | 8 (42.1) | |
| Complete response | .286 | |||
| Yes, n (%) | 12 (27.3) | 73 (39.5) | 6 (31.6) | |
| No, n (%) | 32 (72.7) | 112 (60.5) | 13 (68.4) |
| Characteristic . | Patients without CRS (n = 44) . | Patients with grade 1-2 CRS (n = 185) . | Patients with grade 3 or higher CRS (n = 19) . | P value . |
|---|---|---|---|---|
| Overall response | ||||
| Yes, n (%) | 23 (52.3) | 123 (66.5) | 11 (57.9) | |
| No, n (%) | 21 (47.7) | 62 (33.5) | 8 (42.1) | |
| Complete response | .286 | |||
| Yes, n (%) | 12 (27.3) | 73 (39.5) | 6 (31.6) | |
| No, n (%) | 32 (72.7) | 112 (60.5) | 13 (68.4) |
Discussion
The development of clinical CRS is one of the hallmarks of CAR-T cell therapy, and there has been a theoretical concern that patients who do not experience CRS will not respond to this treatment. In this retrospective analysis, we compared the outcomes of patients with advanced LBCL treated with CAR-T cell therapy who did and did not develop CRS. The baseline characteristics in this study were generally similar between patients with CRS and those without CRS, except for the type of product received and disease status at the time of therapy. Patients who received axi-cel developed CRS more frequently than those who received tisa-cel, which is in line with data from clinical trials and other published retrospective series.2,3,6,15,16 A few retrospective studies have shown inferior survival in patients receiving tisa-cel compared with those receiving axi-cel.17,18 However, our study was not designed to evaluate these outcomes.
When evaluated by disease status at the time of referral, patients with relapsed disease tended to develop CRS less frequently than patients with primary refractory disease or those with disease refractory to the most recent line of therapy. These observations could be explained by differences in disease burden, type of lymphodepleting therapy, or elevated baseline markers of inflammation including CRP, LDH, and ferritin, all of which have been shown to be associated with incidence of CRS.10,19-21
In the multivariate analysis, CRS was not associated with a difference in survival. This finding remained consistent when comparing patients with grade 1 to 2 CRS with those with grade 3 to 4 CRS. Notably, the median PFS in our study was less than 6 months, which is consistent with other real-world studies and may be reflective of an overall less healthy patient population than was included in clinical trials.22,23 Peak ferritin >5000 following cellular therapy infusion was associated with shorter PFS and OS. Elevated ferritin levels during treatment have been shown to be related to CAR-T cell activation and expansion.24 Elevated ferritin may also be correlated with a higher disease burden or worse overall clinical status, although this relationship is not clear. Ferritin may also be a marker of severity of CRS, although development of CRS did not have an impact on PFS or OS in this analysis. Further study of this finding is warranted to determine whether the timing of ferritin peak and pretreatment ferritin levels, which were not consistently available in our data set, have implications for survival.
In the multivariate analysis, patients with pretreatment LDH levels greater than the institutional upper limit of normal were found to have significantly worse PFS and OS. Elevated pretreatment LDH is associated with a more rapidly progressive disease and a higher burden of disease, which likely explains the worse survival outcomes. Disease status at the time of referral and the presence of stage III-IV disease were not associated with significant differences in PFS or OS, although the presence of bulky disease was associated with shorter OS. Consistent with prior analyses, pretreatment LDH likely serves as a surrogate for more aggressive disease.22,25 Further studies are needed to elucidate which patients are likely to have an optimal response to CAR-T cell therapy.
In our analysis, receipt of bridging therapy was associated with shorter PFS, although there was no significant difference in OS. Bridging therapy is often used as a temporizing measure in patients with a high disease burden or a rapidly progressive disease. These disease features have been shown to be associated with worse responses to CAR-T therapy in prior studies.26 The contrast in the association between PFS and OS may be explained by the choice or duration of bridging therapy or by the receipt of subsequent lines of therapy after CAR-T cells, including allogeneic stem cell transplant. These findings and optimal bridging therapy strategies warrant further evaluation in future studies.
An additional analysis demonstrated no difference in long-term cytopenia between patients who developed CRS and those who did not. Although the presence of CRS was associated with cytopenia at the 30 day time point, this association was not observed at later time points. This observation contrasts with findings from previous studies showing a possible association between CRS and prolonged hematologic toxicity.9 When evaluated by grade, severe CRS was also not significantly associated with cytopenia at any time point, although this analysis was limited by a small sample size and should be evaluated in larger studies.
Finally, there was no difference in ORR or CR rates between patients who developed CRS and those who did not, including when stratified by CRS grade. These findings are similar to previous findings in patients treated with axi-cel and lend further support to the idea that clinical CRS is not necessary for efficacy of CAR-T cell therapy, though further analysis is needed to fully evaluate this.27
This study is subject to the limitations experienced by many retrospective studies, including differences among study sites in patient selection, choice of therapy, location of administration, and management of toxicities. In addition, data regarding disease status before apheresis or administration of lymphodepleting chemotherapy, C-reactive protein levels, pretreatment ferritin levels, or cell dose were not consistently available. Despite these limitations, this study contributes to our understanding of the clinical implications of CRS and can inform patient counseling and management strategies in the future.
The development of clinically evident CRS, which includes presence of a fever greater than or equal to 38°C, has often been informally used as a surrogate marker for in vivo CAR-T cell activity and therefore as a possible predictor of outcomes. However, fever may also be a manifestation of the activity of other cells, including macrophages and monocytes, which may or may not directly track the T-cell mediated cytotoxicity against lymphoma. In this study, the CRS development was not associated with any differences in outcomes. Many patients have subtle symptoms before they develop clinical CRS, including low grade fever and chills. It is possible that these symptoms are evidence of some form of CAR-T cell activity, although the patient may not have met the criteria for CRS.
It is important for physicians not to conclude early in therapy that CAR-T cells were ineffective in patients who did not develop CRS and abandoned a reasonable period of observation in favor of alternative therapy that could negatively impact T cell function. This finding contributes to our understanding of the factors that influence the outcomes of patients undergoing CAR-T cell treatment. The finding that the use of steroids during CAR-T cell treatment did not affect outcomes confers stronger confidence in the use of steroids therapeutically or prophylactically in high-risk patients. Taken together, our findings will help understand the clinical consequences of CRS and shape future strategies for CRS management.
Acknowledgments
The authors thank the data managers at each Cell Therapy Consortium member institution who collected patient data and maintained the database.
This research was supported in part by a grant from Novartis Pharmaceuticals, Inc (D.L.P.), National Institutes of Health, National Cancer Institute grant P30 CA008748 (M.A.P.), and National Center for Advancing Translational Sciences of the National Institutes of Health award UL1-TR002494 (V.B.).
Authorship
Contribution: S.T.B. and O.O.O. were primarily involved in writing this manuscript; V.G.P. performed the statistical analysis and created the figures; and all other authors were involved in designing the research and editing the manuscript.
Conflict-of-interest disclosure: D.L.P. reports honorarium for consulting or advisory board participation from Novartis, Kite/Gilead, Incyte, Gerson Lehrman Group, Janssen (Johnson & Johnson), Jazz, Adepcet Bio, DeCART, Bristol Myers Squibb (BMS), bluebird bio, Kadmon, Angiocrine, and Mirror Biologics; research support from Novartis; patents and royalties related to CAR T-cell therapy for malignancies licensed to Novartis and Tmunity; and stock/equity in Genentech/Roche (spouse former employment). S.J.S. reports membership on the advisory board for BeiGene, Celgene/BMS/Juno, Genentech, Roche, Genmab, Incyte, Janssen, MorphoSys, Mustang Biotech, and Novartis; research grants from Genentech/Roche, Merck, and Novartis; is a member on the steering committee for Fate Therapeutics, Legend Biotech, and Nordic Nanovector; received honoraria from Incyte and Novartis; consults for Novartis; and reports a patent on “combination therapies of CAR and PD-1 inhibitors” with royalties paid to Novartis. L.J.N. reports honoraria from ADC Therapeutics, BMS, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, Janssen, MorphoSys, Novartis, and Takeda, and research support from BMS, Caribou Biosciences, Epizyme, Genentech, Genmab, Gilead/Kite, Janssen, IGM Biosciences, Novartis, and Takeda. M.-A.P. reports honoraria from AbbVie, AlloVir, Astellas, BMS, Celgene, Equilium, Exevir, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Novartis, Nektar Therapeutics, Omeros, OrcaBio, Takeda, VectivBio AG, and Vor Biopharma; serves on data and safety monitoring boards (DSMBs) for Cidara Therapeutics, Medigene, Sellas Life Sciences, and Servier, serves on the scientific advisory board of NexImmune; has ownership interests in NexImmune and Omeros; and received institutional research support for clinical trials from Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. M.R.B. reports membership on an advisory board or consultancy for Kite/Gilead, Novartis, CRISPR Therapeutics, Autolus Therapeutics, BMS, Incyte, Sana Biotechnology, Iovance Biotherapeutics, and served on a speakers bureau for BMS, Sanofi, Agios, and Incyte. J.P.M. reports honoraria for consulting or advisory board participation for Kite, BMS, Novartis, Caribou, AlloVir, NEKTAR, Janssen. R.T.M. is an advisor or consultant for AlloVir, CRISPR Therapeutics, Incyte, and Novartis; reports honoraria from Incyte; research support from AlloVir, and Novartis; participation in a data and safety monitoring board for Athersys, Vor Pharma, and Novartis; a patent with Athersys, Inc; and serves on the scientific advisory board of Artiva and Lyrik Therapeutics. A.I.C. reports institutional research funding from Novartis and Fate Therapeutics and is an advisor for Kite. V.B. reports research funding from Incyte, BMS, Gamide Cell, Incyte, Citius Pharmaceuticals, Fate Therapeutics; consultancy with Incyte, Karyopharma, ADC, BMS, Gamida Cell, Fate Therapeutics and DSMB; and membership at Miltenyi Biotec. J.E.M. receives institutional research funding from Gilead, CRISPR, Precision BioSciences, Scripps, Fate Therapeutics, ADC. P.A.R. reports research support/funding from BMS, Kite Pharma, Inc/Gilead, MorphoSys, Calibr, Tessa Therapeutics, Fate Therapeutics, Xencor, and Novartis Pharmaceuticals Corporation; speaker’s bureau from Kite Pharma, Inc/Gilead; consultancy on advisory boards of AbbVie, Novartis Pharmaceuticals Corporation, BMS, Janssen, BeiGene, Karyopharm Therapeutics Inc, Takeda Pharmaceutical Company, Kite Pharma, Inc/Gilead, Sana Biotechnology, Nektar Therapeutics, Intellia Therapeutics, and Bayer; and received honoraria from Novartis Pharmaceuticals Corporation. O.O.O. reports consultancy with and serves on the advisory boards for Pfizer, Kite, Gilead, AbbVie, Janssen, TGR Therapeutics, ADC, Novartis, Curio Science, Nektar; receives institution funding from Kite, Pfizer, Daichi Sankyo, Allogene; and receives honoraria from Pfizer and Gilead. The remaining authors declare no competing financial interests.
Correspondence: Olalekan O. Oluwole, Division of Hematology and Oncology, Vanderbilt University Medical Center, 777 Preston Research Building, 2220 Pierce Ave, Nashville, TN 37232; e-mail: olalekan.oluwole@vumc.org.
References
Author notes
Data are available upon request from the corresponding author, Olalekan O. Oluwole (olalekan.oluwole@vumc.org).