Key Points
NMZL is most commonly an indolent disease with a probability of remaining untreated at five years of 25%.
Transformation of NMZL to large B-cell lymphoma is rare, with a cumulative incidence of 15% at 10 years.
Abstract
Nodal marginal zone lymphoma (NMZL) is a rare non-Hodgkin B-cell lymphoma that has historically been difficult to define, though is now formally recognized by the World Health Organization Classification. To better characterize the clinical outcomes of patients with NMZL, we reviewed a sequential cohort of 187 patients with NMZL to describe baseline characteristics, survival outcomes, and time-to-event data. Initial management strategies were classified into five categories: observation, radiation, anti-CD20 monoclonal antibody therapy, chemoimmunotherapy, or other. Baseline Follicular Lymphoma International Prognostic Index scores were calculated to evaluate prognosis. A total of 187 patients were analyzed. The five-year overall survival was 91% (95% confidence interval [CI], 87-95), with a median follow-up time of 71 months (range, 8-253) among survivors. A total of 139 patients received active treatment at any point, with a median follow-up time of 56 months (range, 13-253) among survivors who were never treated. The probability of remaining untreated at five years was 25% (95% CI, 19-33). For those initially observed, the median time to active treatment was 72 months (95% CI, 49-not reached). For those who received at least one active treatment, the cumulative incidence of receiving a second active treatment at 60 months was 37%. Transformation to large B-cell lymphoma was rare, with a cumulative incidence of 15% at 10 years. In summary, our series is a large cohort of uniformly diagnosed NMZL with detailed analyses of survival and time to event analyses. We showed that NMZL commonly presents as an indolent lymphoma for which initial observation is often a reasonable strategy.
Introduction
Nodal marginal zone lymphoma (NMZL) is a rare indolent lymphoma accounting for <2% of all non-Hodgkin lymphomas.1 Morphologically, NMZL is a primary nodal B-cell non-Hodgkin lymphoma that histologically resembles MZL of the spleen (splenic MZL) or extranodal sites (extranodal MZL). As one of the three recognized MZL subtypes, NMZL has only been formally recognized in the World Health Organization Classification in the last two decades, previously described by now-obsolete terms, such as monocytoid B-cell lymphoma and parafollicular B-cell lymphoma.2-5 In the recently updated fifth edition of the World Health Organization Classification, NMZL remains a distinct entity alongside the related but unique pediatric NMZL.1 Similarly, in the recently published International Consensus Classification of Mature Lymphoid Neoplasms, NMZL is recognized as a distinct disease entity but is noted to demonstrate heterogeneity.6
Given its overall rarity and historic difficulties in recognition and classification, detailed clinical characterization and description of outcomes in NMZL have been limited to relatively small case series.7-14 An early review of 124 non-mucosa–associated lymphoid tissue (MALT) MZLs between 1987 and 1998 noted four clinical subtypes of non-MALT MZL: (1) NMZL, (2) splenic MZL, (3) disseminated (splenic and nodal involvement), and (4) leukemic MZL (bone marrow and/or blood involvement without splenic/nodal involvement).10 Patients with NMZL were noted to have worse overall survival (OS) compared with the three other subtypes, but this study was limited in its use of MZL subtypes that are not recognized in current classification schemas. A similar early review of 21 cases between 1987 and 2001 noted a potentially more aggressive natural history in NMZL with proclivity for disseminated disease at presentation, though this description was also published before current classification schema and modern therapies.11 The largest published data set includes >4000 cases of NMZL from the Surveillance, Epidemiology, and End Results (SEER) database; while providing important epidemiological statistics, this dataset is limited by a lack of uniform pathology review, treatment details, and certain outcomes of interest, such as progression-free survival (PFS) and time-to-event outcomes.15 Although more recent reports have explored enhanced diagnostic methods and deeper understanding of molecular origins,16-18 there remains a distinct lack of understanding of the clinical behavior of NMZL using current classification schemes in the era of modern treatment.
Therefore, to further characterize clinical characteristics and outcomes of patients with NMZL, we reviewed a large cohort of patients with pathologically confirmed NMZL over the last two decades, with the goal of defining baseline characteristics, evaluating survival outcomes by treatment and prognostic schema, and providing time-to-event descriptions.
Methods
Patient cohort
Using a search of the Memorial Sloan Kettering (MSK) Lymphoma Outcomes Database, we identified patients with a diagnosis of NMZL between 1 January 2001 and 1 June 2021. Only patients with at least six months of follow-up were included. After conducting an initial query and excluding patients for incorrect diagnoses and inadequate follow-up, all potential cases were reviewed by three investigators (D.S., R.S., and D.Q.) to ensure appropriateness for inclusion. All patients were required to have undergone pathology review by an MSK hematopathologist for diagnostic confirmation for inclusion. Patients for whom MZL subtype was not clear based on initial medical record review were re-reviewed. Specifically, patients with nodal and extranodal involvement (disseminated MZL) that was indistinguishable between NMZL and non-nodal MZL were excluded. In addition, patients with potential splenic involvement based on imaging findings were included if their presentation was predominantly nodal based on clinical, radiographic, and pathology review. Data were collected by medical record review. Except for five patients with missing details of initial workup, all patients had baseline imaging (computed tomography or positron emission tomography/computed tomography) performed. Baseline Follicular Lymphoma International Prognostic Index (FLIPI) score was calculated to evaluate its impact on prognosis. Management strategies were classified into one of five categories: (1) observation, (2) radiation, (3) rituximab/anti-CD20 monotherapy (anti-CD20 mAb), (4) chemoimmunotherapy, or (5) other. Observation as a management strategy was determined via medical record review. Active treatment was considered as any treatment other than observation. The research protocol was approved by the MSK Institutional Review Board. This research was conducted in accordance with the Declaration of Helsinki.
Primary analyses
Descriptive statistics were used for baseline characteristics. Differences in baseline characteristics by initial management strategy were evaluated for significance using the Fisher exact test and Kruskal-Wallis rank sum test. The primary objective was to determine median OS of the entire cohort. OS was defined as the time from diagnosis to the time of death or last follow-up. PFS was also assessed and defined as the time from initiation of management (including observation) to the time of documented progression, death, or last follow-up. PFS and OS probabilities were estimated by the Kaplan-Meier method and compared for various features using the log-rank test, with a P value ≤.05 considered significant. In particular, survival estimates by stage, FLIPI score (individual score and risk category), and initial management strategy were compared using the log-rank test for OS.
In addition, associations of progression of disease at 24 months (POD24) and event-free survival at 12 months (EFS12) with OS were evaluated. OS analysis according to POD24 was stratified among those who received initial active management vs those who were observed, and then calculated for those who either experienced a progression event within 24 months of diagnosis (“early progressors”) or had at least 24 months of follow-up in the absence of a progression event (“non-early progressors”). Residual OS was calculated from the progression event for “early progressors” and from the 24-month timepoint for “non-early progressors.” EFS12 was defined as experiencing an event (disease progression, relapse, transformation, unplanned retreatment of NMZL after initial management, or death) within 12 months of diagnosis. OS analysis according to EFS12 was stratified among those who received initial active management vs those who were observed, and then calculated for those who either experienced an event within 12 months of diagnosis (“early event experiencers”) or had at least 12 months of follow-up in the absence of an event (“non-early event experiencers”). Patients who died of non-lymphoma causes within 12 months and patients who transformed at initial diagnosis were excluded. Residual OS was calculated from the event for “early event experiencers” and from the 12-month timepoint for “non-early event experiencers.”
Given the heterogeneity of treatments and comparatively small number of patients who received “other” treatment as an initial management strategy, such patients were excluded from analyses that were stratified by initial management strategy.
Time-to-event analyses
Time-to-event analyses were performed for time to first and second active treatment and time to transformation to large cell lymphoma. Time to first active treatment was defined as the time from diagnosis to the time of any treatment other than observation. Time to second active treatment was defined as the time from first active treatment to the time of the next active treatment. Time to treatment outcomes were evaluated by cumulative incidence function with death as a competing risk. Patients who underwent observation or treatment other than radiation, anti-CD20 mAb, or chemoimmunotherapy as an initial management were excluded from time to second active treatment analyses. Cumulative incidence of death did not have an impact on time to first active treatment but was a factor on cumulative incidence of second active treatment at later time points. Therefore, Kaplan-Meier estimates were calculated for time to first active treatment, whereas cumulative incidence function estimates were calculated for time to second active treatment. Time to transformation started from the date of diagnosis and was evaluated by cumulative incidence function with death as a competing risk. Three patients with transformed disease at initial presentation (with underlying NMZL) were excluded from time-to-transformation analyses. As above, patients who received “other” treatment as an initial management strategy were excluded from time-to-event analyses given the heterogeneity and small numbers of patients.
Results
Patient characteristics and initial management
A total of 187 patients were identified. Baseline characteristics and initial management strategy are shown in Table 1. The median age at diagnosis was 62 years (interquartile range, 50-69). Clinical stage was broadly distributed, with 71 patients (41%) having stage 1 to 2 disease (limited stage) at presentation and 104 patients (59%) having stage 3 to 4 disease (advanced stage). Thirty-eight patients with stage 1 to 2 disease had documented negative baseline bone marrow examination. Fifty-three patients had baseline bone marrow involvement. Among those evaluable for baseline FLIPI (n = 139), 61 (44%), 35 (25%), and 43 (31%) had scores of between 0 and 1 (low), 2 (intermediate), and ≥3 (high), respectively.
Baseline characteristics
| Characteristic . | N = 187 (%) . |
|---|---|
| Sex | |
| Female | 99 (53) |
| Male | 88 (47) |
| Age, y (range) | 62 (50-69) |
| Elevated LDH | 42 (28) |
| Unknown | 35 |
| Hemoglobin <12 g/dL | 34 (21) |
| Unknown | 26 |
| Clinical stage | |
| 1-2∗ | 71 (41) |
| 3-4 | 104 (59) |
| Unknown | 12 |
| >4 nodal sites | 55 (30) |
| Unknown | 3 |
| Disease bulk | |
| <5 cm | 122 (74) |
| 5-10 cm | 35 (21) |
| ≥10 cm | 8 (4.9) |
| Unknown | 23 |
| FLIPI score | |
| 0-1 | 61 (44) |
| 2 | 35 (25) |
| 3+ | 43 (31) |
| Unknown | 48 |
| Bone marrow involvement (morphology) | 53 (46) |
| Unknown | 71 |
| Viral status | |
| Hepatitis B status | |
| Previous infection | 6 (3.2) |
| Susceptible | 123 (66) |
| Unknown | 58 |
| Hepatitis C antibody | |
| Positive | 4 (2.1) |
| Negative | 120 (64) |
| Unknown | 63 |
| Initial management | |
| Observation | 89 (48) |
| Radiation | 18 (9.6) |
| Anti-CD20 mAb | 26 (14) |
| Chemoimmunotherapy | 44 (24) |
| Other | 10 (5.3) |
| Characteristic . | N = 187 (%) . |
|---|---|
| Sex | |
| Female | 99 (53) |
| Male | 88 (47) |
| Age, y (range) | 62 (50-69) |
| Elevated LDH | 42 (28) |
| Unknown | 35 |
| Hemoglobin <12 g/dL | 34 (21) |
| Unknown | 26 |
| Clinical stage | |
| 1-2∗ | 71 (41) |
| 3-4 | 104 (59) |
| Unknown | 12 |
| >4 nodal sites | 55 (30) |
| Unknown | 3 |
| Disease bulk | |
| <5 cm | 122 (74) |
| 5-10 cm | 35 (21) |
| ≥10 cm | 8 (4.9) |
| Unknown | 23 |
| FLIPI score | |
| 0-1 | 61 (44) |
| 2 | 35 (25) |
| 3+ | 43 (31) |
| Unknown | 48 |
| Bone marrow involvement (morphology) | 53 (46) |
| Unknown | 71 |
| Viral status | |
| Hepatitis B status | |
| Previous infection | 6 (3.2) |
| Susceptible | 123 (66) |
| Unknown | 58 |
| Hepatitis C antibody | |
| Positive | 4 (2.1) |
| Negative | 120 (64) |
| Unknown | 63 |
| Initial management | |
| Observation | 89 (48) |
| Radiation | 18 (9.6) |
| Anti-CD20 mAb | 26 (14) |
| Chemoimmunotherapy | 44 (24) |
| Other | 10 (5.3) |
LDH, lactate dehydrogenase.
Not all patients with stage 1 to 2 disease underwent baseline bone marrow examination (refer to the text).
Initial management strategies varied. The most common approaches were observation (n = 89, 48%) and chemoimmunotherapy (n = 44, 24%), followed by anti-CD20 mAb (n = 26, 14%), radiation (n = 18, 9.6%), and other (n = 10, 5.3%). Specifically for chemoimmunotherapy, regimens consisted of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) (n = 17), bendamustine plus rituximab (n = 16), rituximab plus fludarabine (n = 4), R-CVP (rituximab, cyclophosphamide, vincristine, and prednisone) (n = 4), R-CHEP (rituximab, cyclophosphamide, doxorubicin, etoposide, and prednisone) (n = 1), R-EPOCH (rituximab, etoposide, vincristine, cyclophosphamide, and doxorubicin) (n = 1), and rituximab, cyclophosphamide, and prednisone (n=1). Specifically for therapies in the “other” category, regimens consisted of fludarabine (n = 3), CHOP (n = 1), CVP (n = 1), clarithromycin on a clinical trial (NCT00461084) (n = 1), cyclophosphamide in a patient with concomitant rheumatological disease (n = 1), ledipasvir plus sofosbuvir in a patient with previously untreated hepatitis C (n = 1), an investigational idiotype vaccination at an outside institution (n = 1), and resection only of multiple involved lymph nodes in one patient.
Forty-eight patients (28%) never received therapy, with a median follow-up time of 56 (range, 13-253) months among those who were never treated. Differences in baseline characteristics by initial management strategy are shown in supplemental Table 1. Management differed significantly by stage (P < .001). Patients with limited stage disease (n = 68) were more commonly managed with observation (60%) or radiation (25%) as compared with anti-CD20 mAb (3%) and chemoimmunotherapy (12%). In contrast, in patients with advanced-stage disease (n = 97), initial management was more commonly with anti-CD20 mAb (24%) or chemoimmunotherapy (36%), though observation was still common (39%). Management also differed significantly with FLIPI score (P = .008). Patients with low and intermediate scores were more likely to be observed or radiated. In contrast, patients with high FLIPI scores were more commonly treated with chemoimmunotherapy or anti-CD20 mAb. As shown in supplemental Table 1, the initial management differed statistically in the presence of >4 nodal sites and bone marrow involvement, but did not differ by sex, age, or lactate dehydrogenase level.
In patients who received initial therapy with R-CHOP, long-term cardiovascular complications and secondary malignancies were rare. One patient suffered a myocardial infarction one year after R-CHOP administration, one patient developed bladder adenocarcinoma six years after R-CHOP administration, and one patient developed clonal cytopenias of undetermined significance 13 years after R-CHOP administration.
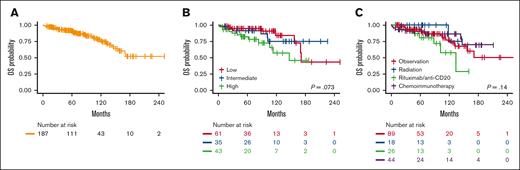
Survival estimates
Survival estimates are shown in Table 2 and Figure 1. Among survivors, the median follow-up time was 71 months (range, 8-253). For the entire cohort, the median OS was not reached (95% confidence interval [CI], 157 months-not reached). Overall, 5-year OS was 91% (95% CI, 87-95), and 10-year OS was 76% (95% CI, 67-85). Five-year OS decreased as FLIPI risk group increased, but differences did not reach statistical significance (P = .073). Similarly, 5-year OS did not differ by first management strategy (P = .13). PFS estimates and Kaplan-Meier curves are shown in supplemental Table 2 and supplemental Figure 1.
OS estimates
| Characteristic . | N . | Event N . | Median survival . | 60 mo OS (95% CI) . | 120 mo OS (95% CI) . | P value∗ . |
|---|---|---|---|---|---|---|
| Overall | 187 | 36 | —† (157, —) | 91% (87%-95%) | 76% (67%-85%) | |
| Stage | 175 | 34 | .073 | |||
| 1-2 | — (170, —) | 94% (88%-100%) | 82% (70%-97%) | |||
| 3-4 | — (134, —) | 88% (81%-95%) | 67% (56%-82%) | |||
| FLIPI score | 139 | 26 | .033 | |||
| 0 | — (—, —) | 100% (100%-100%) | 100% (100%-100%) | |||
| 1 | 171 (157, —) | 94% (87%-100%) | 82% (67%-100%) | |||
| 2 | — (—, —) | 91% (82%-100%) | 75% (58%-96%) | |||
| 3 | — (113, —) | 85% (70%-100%) | 68% (42%-100%) | |||
| 4+ | 106 (84, —) | 78% (60%-100%) | 45% (22%-92%) | |||
| FLIPI risk group | 139 | 26 | .073 | |||
| 0-1 (low) | 171 (170, —) | 95% (89%-100%) | 84% (70%-100%) | |||
| 2 (intermediate) | — (—, —) | 91% (82%-100%) | 75% (58%-96%) | |||
| 3+ (high) | 147 (106, —) | 82% (70%-95%) | 57% (38%-85%) | |||
| Initial management | 177 | 31 | .14 | |||
| Observation | — (157, —) | 94% (88%-99%) | 77% (65%-91%) | |||
| Radiation | — (118, —) | 100% (100%-100%) | 75% (43%-100%) | |||
| Anti-CD20 mAb | 134 (102, —) | 81% (65%-100%) | 57% (33%-99%) | |||
| Chemoimmunotherapy | — (—, —) | 90% (82%-100%) | 86% (75%-99%) |
| Characteristic . | N . | Event N . | Median survival . | 60 mo OS (95% CI) . | 120 mo OS (95% CI) . | P value∗ . |
|---|---|---|---|---|---|---|
| Overall | 187 | 36 | —† (157, —) | 91% (87%-95%) | 76% (67%-85%) | |
| Stage | 175 | 34 | .073 | |||
| 1-2 | — (170, —) | 94% (88%-100%) | 82% (70%-97%) | |||
| 3-4 | — (134, —) | 88% (81%-95%) | 67% (56%-82%) | |||
| FLIPI score | 139 | 26 | .033 | |||
| 0 | — (—, —) | 100% (100%-100%) | 100% (100%-100%) | |||
| 1 | 171 (157, —) | 94% (87%-100%) | 82% (67%-100%) | |||
| 2 | — (—, —) | 91% (82%-100%) | 75% (58%-96%) | |||
| 3 | — (113, —) | 85% (70%-100%) | 68% (42%-100%) | |||
| 4+ | 106 (84, —) | 78% (60%-100%) | 45% (22%-92%) | |||
| FLIPI risk group | 139 | 26 | .073 | |||
| 0-1 (low) | 171 (170, —) | 95% (89%-100%) | 84% (70%-100%) | |||
| 2 (intermediate) | — (—, —) | 91% (82%-100%) | 75% (58%-96%) | |||
| 3+ (high) | 147 (106, —) | 82% (70%-95%) | 57% (38%-85%) | |||
| Initial management | 177 | 31 | .14 | |||
| Observation | — (157, —) | 94% (88%-99%) | 77% (65%-91%) | |||
| Radiation | — (118, —) | 100% (100%-100%) | 75% (43%-100%) | |||
| Anti-CD20 mAb | 134 (102, —) | 81% (65%-100%) | 57% (33%-99%) | |||
| Chemoimmunotherapy | — (—, —) | 90% (82%-100%) | 86% (75%-99%) |
The bold values are statistically significant (p values less than 0.05).
Log-rank test.
Em dash refers to not reached.
Overall Survival Estimates. Kaplan-Meier estimates for OS in the entire cohort (A), by baseline FLIPI risk group (B), and first management strategy (C).
Overall Survival Estimates. Kaplan-Meier estimates for OS in the entire cohort (A), by baseline FLIPI risk group (B), and first management strategy (C).
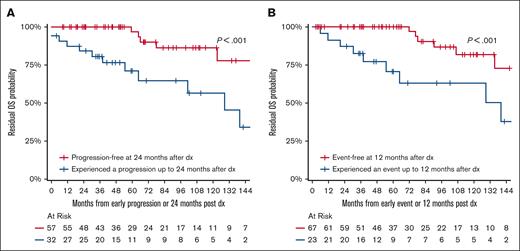
Survival analyses according to POD24 and EFS12 are shown in Tables 3 and 4 and Figure 2. Among the 89 patients who received immediate active therapy and were eligible for POD24 analysis, 32 patients experienced progression or death within 24 months (POD24), and 57 did not experience progression or death within 24 months (no POD24). The 10-year OS in the POD24 group vs no POD24 group was 56% (95% CI, 36-85) vs 86% (95% CI, 74-100) (P < .001) (Figure 2A). Among the 93 patients who received immediate active therapy and were eligible for EFS12 analysis, 26 experienced an event within 12 months (EFS12), and 67 did not experience an event within 12 months (no EFS12). The 10-year OS in the EFS12 group vs no EFS12 group was 55% (95% CI, 37-82) vs 82% (95% CI, 68-98) (P < .001) (Figure 2B). Additional metrics for POD24 and EFS12 are shown in Tables 3 and 4.
Residual OS by POD24
| Initial management/POD24 group . | N (%) . | Event N . | 36 mo OS (95% CI) . | 60 mo OS (95% CI) . | 120 mo OS (95% CI) . | P value∗ . |
|---|---|---|---|---|---|---|
| Active treatment | 18 | <.001 | ||||
| Progression-free at 24 mo | 57 (64%) | 100% (100%-100%) | 97% (90%-100%) | 86% (74%-100%) | ||
| Progression within 24 mo | 32 (36%) | 80% (68%-96%) | 71% (55%-91%) | 56% (37%-85%) | ||
| Observation | 15 | .046 | ||||
| Progression-free at 24 mo | 53 (65%) | 98% (94%-100%) | 93% (85%-100%) | 76% (59%-97%) | ||
| Progression within 24 mo | 28 (35%) | 88% (75%-100%) | 82% (67%-100%) | 59% (38%-92%) |
| Initial management/POD24 group . | N (%) . | Event N . | 36 mo OS (95% CI) . | 60 mo OS (95% CI) . | 120 mo OS (95% CI) . | P value∗ . |
|---|---|---|---|---|---|---|
| Active treatment | 18 | <.001 | ||||
| Progression-free at 24 mo | 57 (64%) | 100% (100%-100%) | 97% (90%-100%) | 86% (74%-100%) | ||
| Progression within 24 mo | 32 (36%) | 80% (68%-96%) | 71% (55%-91%) | 56% (37%-85%) | ||
| Observation | 15 | .046 | ||||
| Progression-free at 24 mo | 53 (65%) | 98% (94%-100%) | 93% (85%-100%) | 76% (59%-97%) | ||
| Progression within 24 mo | 28 (35%) | 88% (75%-100%) | 82% (67%-100%) | 59% (38%-92%) |
The bold values are statistically significant (p values less than 0.05).
Log-rank test.
Residual OS by EFS12
| Initial management/EFS group . | N (%) . | Event N . | 36 mo OS (95% CI) . | 60 mo OS (95% CI) . | 120 mo OS (95% CI) . | P value∗ . |
|---|---|---|---|---|---|---|
| Active treatment | 19 | <.001 | ||||
| Event-free at 12 mo | 67 (72%) | 100% (100%-100%) | 100% (100%-100%) | 82% (68%-98%) | ||
| Event within 12 mo | 26 (28%) | 73% (58%-92%) | 62% (45%-86%) | 55% (37%-82%) | ||
| Observation | 16 | .9 | ||||
| Event-free at 12 mo | 74 (83%) | 94% (88%-100%) | 88% (79%-97%) | 67% (52%-87%) | ||
| Event within 12 mo | 15 (17%) | 92% (77%-100%) | 92% (77%-100%) | 79% (56%-100%) |
| Initial management/EFS group . | N (%) . | Event N . | 36 mo OS (95% CI) . | 60 mo OS (95% CI) . | 120 mo OS (95% CI) . | P value∗ . |
|---|---|---|---|---|---|---|
| Active treatment | 19 | <.001 | ||||
| Event-free at 12 mo | 67 (72%) | 100% (100%-100%) | 100% (100%-100%) | 82% (68%-98%) | ||
| Event within 12 mo | 26 (28%) | 73% (58%-92%) | 62% (45%-86%) | 55% (37%-82%) | ||
| Observation | 16 | .9 | ||||
| Event-free at 12 mo | 74 (83%) | 94% (88%-100%) | 88% (79%-97%) | 67% (52%-87%) | ||
| Event within 12 mo | 15 (17%) | 92% (77%-100%) | 92% (77%-100%) | 79% (56%-100%) |
The bold values are statistically significant (p values less than 0.05).
Log-rank test.
POD24 and EFS12 Analyses. OS analyses according to POD24 (A) and EFS12 (B).
Of the 37 deaths, five (14%) were as a result of lymphoma progression. Three of these five patients had experienced transformation to an aggressive, large-cell lymphoma. Eleven deaths were as a result of other causes. Six patients died of infection-related causes; only one of these patients was on treatment for transformed lymphoma. Two infection-related deaths were secondary to COVID-19 infection in patients who were not undergoing therapy. As for the other three infection-related deaths, one occurred in a patient with a primary immunodeficiency syndrome, one occurred in a patient being treated for a nonspecific connective tissue disease, and one occurred in a patient without clear risk factors. There were four deaths owing to non-lymphoma malignancies (acute myeloid leukemia, esophageal cancer, colorectal cancer, and non–small cell lung cancer). One patient with lymphoma who was on treatment with R-mini-CHOP died of a suspected cerebrovascular event in the middle of treatment, and one patient died after a mechanical fall. Twenty patients died of unknown causes.
Time-to-event analyses
In total, 139 patients received any treatment at some point, with a median follow-up time of 56 (interquartile range, 27-103) months among those who were never treated. The estimated 5-year untreated rate was 25% (95% CI, 19-33). For those observed initially, the median time to first active treatment was 72 months (95% CI, 49-not reached). For those who received at least one active treatment, the cumulative incidence of second active treatment (in the presence of death as a competing risk) at 60 months was 37%. Time to second active treatment differed significantly from first active treatment. For example, in those treated with frontline radiation, the cumulative incidence of second active treatment at 24 months was 6% vs those initially treated with anti-CD20 mAb or chemoimmunotherapy, in whom the cumulative incidence of second active treatment at 24 months was 44% or 34%, respectively (P = .02) (Table 5).
Cumulative incidence of second active treatment or death by frontline active treatment∗
| . | 12-mo Cum. Inc. (95% CI) . | 24-mo Cum. Inc. (95% CI) . | 36-mo Cum. Inc. (95% CI) . | 60-mo Cum. Inc. (95% CI) . | P value . |
|---|---|---|---|---|---|
| First active treatment | 0.22 (0.15-0.33) | 0.31 (0.22-0.43) | 0.37 (0.27-0.49) | 0.37 (0.27-0.49) | .02 |
| Radiation | 0 | 0.06 (0.01-0.39) | 0.11 (0.03-0.44) | 0.11 (0.03-0.44) | |
| Anti-CD20 mAb | 0.31 (0.17-0.57) | 0.44 (0.28-0.7) | 0.53 (0.36-0.78) | 0.53 (0.36-0.78) | |
| Chemoimmunotherapy | 0.26 (0.16-0.44) | 0.34 (0.22-0.53) | 0.37 (0.25-0.56) | 0.37 (0.25-0.56) | |
| Death | 0.03 (0.01-0.11) | 0.03 (0.01-0.11) | 0.03 (0.01-0.11) | 0.05 (0.02-0.14) | .07 |
| Radiation | 0 | 0 | 0 | 0 | |
| Anti-CD20 mAb | 0.04 (0.01-0.29) | 0.04 (0.01-0.29) | 0.04 (0.01-0.29) | 0.09 (0.02-0.38) | |
| Chemoimmunotherapy | 0.05 (0.01-0.18) | 0.05 (0.01-0.18) | 0.05 (0.01-0.18) | 0.05 (0.01-0.18) |
| . | 12-mo Cum. Inc. (95% CI) . | 24-mo Cum. Inc. (95% CI) . | 36-mo Cum. Inc. (95% CI) . | 60-mo Cum. Inc. (95% CI) . | P value . |
|---|---|---|---|---|---|
| First active treatment | 0.22 (0.15-0.33) | 0.31 (0.22-0.43) | 0.37 (0.27-0.49) | 0.37 (0.27-0.49) | .02 |
| Radiation | 0 | 0.06 (0.01-0.39) | 0.11 (0.03-0.44) | 0.11 (0.03-0.44) | |
| Anti-CD20 mAb | 0.31 (0.17-0.57) | 0.44 (0.28-0.7) | 0.53 (0.36-0.78) | 0.53 (0.36-0.78) | |
| Chemoimmunotherapy | 0.26 (0.16-0.44) | 0.34 (0.22-0.53) | 0.37 (0.25-0.56) | 0.37 (0.25-0.56) | |
| Death | 0.03 (0.01-0.11) | 0.03 (0.01-0.11) | 0.03 (0.01-0.11) | 0.05 (0.02-0.14) | .07 |
| Radiation | 0 | 0 | 0 | 0 | |
| Anti-CD20 mAb | 0.04 (0.01-0.29) | 0.04 (0.01-0.29) | 0.04 (0.01-0.29) | 0.09 (0.02-0.38) | |
| Chemoimmunotherapy | 0.05 (0.01-0.18) | 0.05 (0.01-0.18) | 0.05 (0.01-0.18) | 0.05 (0.01-0.18) |
Cum. Inc., cumulative incidence.
The bold values are statistically significant (p values less than 0.05).
Patients with observation or “other” as initial management strategy were excluded from this analysis.
Overall, 25 patients experienced transformation to aggressive lymphoma. Three patients presented with transformed disease at original diagnosis (with underlying NMZL). Of the remaining 22 patients, 11 transformed while undergoing observation, and 11 transformed after at least one active therapy. Transformed disease was diffuse large B-cell lymphoma (DLBCL) in 24 patients and plasmablastic lymphoma in one patient. Of the DLBCL transformations, 18 were nongerminal center B-cell (non-GCB) immunophenotype, four were GCB immunophenotype, and two did not have sufficient data to be classified. Two patients with GCB lymphoma had clonal typing; in one patient, clonal relation to NMZL was confirmed, and in the other the DLBCL was clonally unrelated. Excluding 3 patients who presented with transformed disease, the cumulative incidence of transformation with death as a competing risk at 24, 60, and 120 months was 5%, 12%, and 15%, respectively (supplemental Table 3; supplemental Figure 2). With a median follow-up of 30 months, eight patients died after transformation. Median OS after transformation was 64 months (95% CI, 43-not reached).
Discussion
To our knowledge, this is one of the largest dedicated evaluations of baseline characteristics, survival, and time-to-event analyses in patients with NMZL. The existing literature on NMZL is limited in its use of prior classification schemas and generally does not reflect outcomes in the current therapeutic era. Our series describes the characteristics and outcomes of NMZL in the modern era and aids in the prognostication and management of patients.
A major goal of our study was to understand management strategies in NMZL. For initial management of NMZL, the current paradigm as outlined in the National Comprehensive Cancer Network guidelines mirrors that of other indolent B-cell lymphomas, in particular follicular lymphoma. In our series, although most patients had advanced-stage disease at initial presentation, observation was chosen in nearly half (48%) of the patients, reflecting a commonly indolent presentation. In addition, the estimated 5-year untreated rate was 25% (95% CI, 19-33), highlighting a cohort of patients with prolonged lack of clinically actionable progression. As shown in supplemental Table 1, initial management varied significantly by stage, number of nodal sites, and FLIPI score, likely reflecting decision-making based on perceived treatment indications. For example, among patients who received chemoimmunotherapy as initial management, most (48%) had FLIPI ≥3 as compared with FLIPI from 0 to 1 (26%) or 2 (26%); similarly, most patients treated with initial chemoimmunotherapy had advanced-stage disease (81%). However, even for those observed as initial management, 48% had advanced-stage disease and 23% had FLIPI ≥3, indicating that stage and other prognostic factors comprising FLIPI score did not necessarily determine initial management decisions.
OS was long. With >5 years of median follow-up, median OS was not reached. Ten-year OS was 76%. OS did not differ significantly by stage, FLIPI risk group, or first management strategy. Although we evaluated PFS (supplemental Table 2), progression alone is challenging to properly capture in a retrospective series in which patients were managed with non-uniform treatment and surveillance strategies. However, it is clear that progression (and variables that reflect progression and additional events, such as POD24 and EFS12) is a clinically relevant end point in indolent lymphomas, including MZL, as demonstrated in a prospective, international cohort of >1300 patients with MZL led by the Fondazione Italiana Linformi.19 In this report, among 321 patients who required immediate therapy, POD24 was confirmed in 59 (18%) patients, and such patients experienced a significantly worse OS than those without POD24 (3-year OS: 53% vs 95%; hazard ratio, 19.5; 95% CI, 8.4-45).19 An equally important longitudinal, prospective effort capturing >600 patients with indolent lymphoma from the epidemiological databases of the University of Iowa/Mayo Clinic showed that patients who achieve EFS12 have a subsequent OS that is not statistically different from the general population.20 Our report reinforces such findings for NMZL, noting a statistically significant difference in OS based on POD24, and observing excellent long-term survival in those who are event-free within 12 months of diagnosis.
Still, given the limitations in assessing progression in retrospective analyses, we used time-to-event models, in particular time to next treatment and cumulative incidence of treatment initiation, which capture objective time points and indicate a perceived need for treatment. The 5-year probability of remaining treatment-free was 25% in this cohort; although exact treatment indications (such as those outlined in the Group d’Etude des Lymphomes Folliculaires criteria21) were not assessed in this retrospective study, these patients may represent a cohort of patients with NMZL who may never need treatment owing to indolence. Among those who were observed as initial management, 41 of 89 (46%) eventually required treatment, with a median time to first active treatment of 72 months. These time to treatment analyses show that most patients with NMZL have long periods of clinical indolence.
Another important finding pertains to those treated with upfront radiation, which was primarily in limited stage disease. In these patients, the cumulative incidence of second active treatment was only 11% at five years and no such patients have died; this shows a potential for long-term disease control with localized radiation in limited-stage disease. Finally, in those treated with anti-CD20 mAb or chemoimmunotherapy as initial management, cumulative incidence of second active treatment was greater than those treated with upfront radiation (37% at five years), reflecting more advanced and aggressive disease features at the time of treatment. Nevertheless, survival outcomes were still encouraging in this group.
Our findings on survival are comparable with limited prior reports. Five-year OS in NMZL has variably been reported between 55% and ∼90%.7-14 Drawing conclusions from prior reports and comparing with our data is hindered by heterogeneity of prior pathology classifications and treatment practices over time, as well as some prior reports providing data only on MZL in aggregate as opposed to distinguishing among subtypes. In the largest evaluation ever undertaken of 4724 patients with NMZL identified from the SEER database, 5- and 10-year OS was 64.2% and 44.3%, respectively.15 We report greater survival than that reported in the SEER analysis, with 5- and 10-year OS of 91% and 76%, respectively. Interestingly, lymphoma-specific OS from the SEER database nearly matches these findings, with 5- and 10-year OS of 86.3% and 78.7%, respectively. Notably, the SEER publication reported on patients diagnosed between 1995 and 2009, whereas ours reported on those diagnosed between 2001 and 2021. The improvement in survival in our cohort is most likely secondary to the incorporation of anti-CD20 therapy into first-line management strategies and the plethora of novel therapies for relapsed and refractory indolent lymphomas.22 In particular, ibrutinib and zanubrutinib, two inhibitors of Bruton tyrosine kinase, were approved by the US Food and Drug Administration for the treatment of relapsed or refractory MZL, including NMZL, over the course of our observational report (though ibrutinib approval has recently been withdrawn for MZL).23-25
Among all deaths, only five were because of lymphoma. A large, prospective series recently reported causes of death in low-grade B-cell lymphomas in the rituximab era.26 Our findings are generally similar with this report in finding a low incidence of lymphoma-related death compared with non-lymphoma-related deaths. Aggregation of this data reflects most patients with NMZL die of non-lymphoma etiologies.
A second major goal of our study was to evaluate the ability of baseline FLIPI scores to accurately prognosticate patients at diagnosis.27,28 In our study, FLIPI correlated with PFS but not with OS. A limitation in our report is missing data that disallowed FLIPI calculation in 48 (25%) patients. Prior reports have attempted similar evaluations of FLIPI in patients with NMZL.9,29,30 An early report of 47 patients with NMZL between 1994 and 2004 found prognostic utility of FLIPI on OS in multivariate analyses.9 Only three patients in this study received upfront therapy with a rituximab-containing regimen. In a more recent publication of 56 patients between 1996 and 2014, high-risk FLIPI was associated with inferior PFS and OS when compared to a joint group of patients who are at low or intermediate risk on univariate analysis but was not significant on multivariate analysis.30 Finally, in a larger cohort of 144 patients with MZL between 2003 and 2010, FLIPI had a strong prognostic influence on PFS and OS in the overall cohort, but notably was unable to differentiate groups in the subcohort of patients with NMZL.29 Our results show statistically significantly differences in PFS by FLIPI risk group without significant difference in OS. Prospective confirmation and validation would be needed before wider adoption, and it is likely that a prognostic schema more reflective of underlying biology would be useful.
Finally, transformation events were similar to those reported in other indolent lymphomas, such as follicular lymphoma, with only 25 patients experiencing transformation. Studies on transformation in MZL, let alone NMZL, are limited, but our findings are generally consistent with previous publications, which have shown that transformation is a rare event in NMZL.31-33 Acknowledging a small number of patients, we found a relatively long survival after transformation of 64 months (95% CI, 43-not reached).
The strength of this analysis lies in the large, dedicated cohort of patients with NMZL, and emphasis on time-to-event outcomes. However, it is limited by its single-center and retrospective nature. Prospective studies comprising multiple, unaffiliated institutions are more likely to reflect current management strategies and outcomes, and real-world analyses of management outcomes from clinical practice outside of academic centers would be useful. In particular, indications for treatment and progression events are distinctly challenging to accurately capture in retrospective series, and we advise cautious interpretation of the progression data reported in this study. Prospective, epidemiological databases, such as the Lymphoma Epidemiology of Outcomes Study (NCT04996706) are critical to accurately characterize and deepen our understanding of rare lymphomas, such as NMZL.
In conclusion, we present a large series of NMZL in the modern therapeutic era. Despite commonly disseminated disease at presentation, our work shows that NMZL can be an indolent lymphoma for which observation is a reasonable initial management strategy. Transformation events are uncommon. The FLIPI score may predict progression but requires further study. Prospective studies are necessary to confirm and validate the above results to refine prognostication and treatment paradigms in NMZL.
Acknowledgment
This research was funded in part through the National Institutes of Health/National Cancer Institute Cancer Center support grant P30 CA008748.
Authorship
Contribution: R.S., E.D., D.Q., and D.S. designed the study; E.D. analyzed results and created the figures; R.S. and D.J.D. wrote the manuscript; and all authors were involved in taking care of the patients involved and provided critical feedback on study design, data collection, data interpretation, and manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests related to the presented work. Outside the scope of this work, R.S., E.D., M.O., Z.D.E.-P., P.A.H., D.J.S., A.M.I., and C.N.O. have no disclosures. A.D. served as a consultant for Incyte, EUSA Pharma, Loxo and receives research support from Roche and Takeda. B.S.I. has received honorarium from GT Medical Technologies, Inc. C.L.B. is currently employed by Genentech. P.C.C. has stock or stock options in Bristol Myers Squibb, Johnson and Johnson, Pfizer, AstraZeneca, GSK, Novartis. L.F. receives research funding and consultancy fees from Genmab, AbbVie and Roche/Genentech and has received honoraria/participated on advisory boards for ADCT, SeaGen and AstraZeneca. S.M.H. research support from ADC Therapeutics, Affimed, Aileron, Celgene, Crispr Therapeutics, Daiichi Sankyo, Forty Seven, Inc, Kyowa Hakko Kirin, Millennium/Takeda, Seattle Genetics, Trillium Therapeutics, and Verastem/SecuraBio, consultancy fees from Acrotech Biopharma, ADC Therapeutics, Astex, Auxilus Pharma, Merck, C4 Therapeutics, Celgene, Cimieo Therapeutics, Daiichi Sankyo, Janssen, Kura Oncology, Kyowa Hakko Kirin, Myeloid Therapeutics, ONO Pharmaceuticals, Seattle Genetics, SecuraBio, Shoreline Biosciences, Inc, Takeda, Trillium Therapeutics, Tubulis, Verastem/SecuraBio, Vividion Therapeutics and Yingli Pharma Limited. N.K. receives research funding form SeaGen. O.B.L. served on advisory board for Morphosys, Inc. J.K.L. serves as a consult for TG therapeutics and Epizyme. M.J.M has received honoraria from Genentech, Roche, GlaxoSmithKline, Bayer, Pharmacyclics, Janssen, SeaGen, Immunovaccine Technologies, Takeda, and Epizyme, has participated in a consulting or advisory role for Genentech, Bayer, Merck, Juno Therapeutics, Roche, Teva, Rocket Medical, SeaGen, Daiichi Sankyo, Takeda, and Epizyme, and has received research funding from Genentech, Roche, GlaxoSmithKline, IGM Biosciences, Bayer, Pharmacyclis, Janssen, Rocket Medical, SeaGen, Immunovaccine Technologies. A.J.M. has research support from ADC Therapeutics, Beigene, Miragen, SeaGen, Merck, Bristol Myers Squibb, Incyte, and SecuraBio, honorarium from Affimed, Imbrium Therapeutics L.P./Purdue, Janpix Ltd, Merck, Seattle Genetics, and Takeda. A.N. has grant/research support from Pharmacyclics/AbbVie; Kite/Gilead, Cornerstone, consultancy role from Janssen, Morphosys, Cornerstone, Epizyme, EUSA, TG therapeutics, ADC Therapeutics, and AstraZeneca. M.L.P. has advisory/consultancy roles with Novartis, Synthekine, BeiGene, Kite and MustangBio. S.V. has advisory/consultancy roles with Novartis, Synthekine, BeiGene, Kite MustangBio. A.D.Z. has received financial compensation for consulting from Genentech/Roche, Gilead, Celgene, Janssen, Amgen, Novartis, Adaptive Biotechnology, Morphosys, AbbVie, AstraZeneca, MEI Pharma, research funding from MEI Pharmaceuticals, Genentech/Roche, BeiGene, NIH SPORE -PI (receives salary support and funds awarded to MSK) and has served on the data monitoring committee for BeiGene (Chair) and BMS/Celgene/Juno. G.S. has received financial compensations for participating on advisory boards or consulting from AbbVie, Bayer, BeiGene, BMS/Celgene, Epizyme, Genentech/Roche, Genmab, Incyte, Ipsen, Janssen, Kite/Gilead, Loxo, Miltenyi, Molecular Partners, Morphosys, Nordic Nanovector, Novartis, Rapt, Regeneron and Takeda and is a shareholder in Owkin. A.K. has received research funding from AbbVie Pharmaceuticals, Adaptive Biotechnologies, Celgene, Pharmacyclics, Seattle Genetics, AstraZeneca, and Loxo Oncology/Lilly and has received financial compensation for participating on advisory board for Celgene, Genentech, Kite Pharmaceuticals, Loxo Oncology/Lilly, AstraZeneca.
The current affiliation for C.L.B. is Genentech, New York, NY.
Correspondence: Robert Stuver, Department of Medicine, Lymphoma Service, Memorial Sloan Kettering Cancer Center, 530 East 74th St, New York, NY; e-mail: stuverr@mskcc.org.
References
Author notes
Data are available on request from the corresponding author, Robert Stuver (stuverr@mskcc.org).
The full-text version of this article contains a data supplement.