TO THE EDITOR:
We read with great interest and appreciate the article by Carreño-Tarragona et al published in a recent issue of Blood Advances on titled “CNL and aCML should be considered as a single entity based on molecular profiles and outcomes.”1 We commend the authors on their detailed clinical and mutational analysis of a relatively large cohort of patients with these rare diseases. Based on similar baseline clinical characteristics, molecular profile, and survival, the authors conclude that these diseases should be classified as a single entity. Although provocative, there are natural concerns that this analysis raises. Firstly, despite shared mutational features, morphologic differences exist between these 2 entities, namely bone marrow dysplasia in the case of atypical chronic myeloid leukemia (aCML),2 as the authors acknowledge. These distinguishing features may be irrelevant unless related to differences in clinical outcomes. Despite the efforts of the authors, the sample size of 61 patients is insufficient to compare survival outcomes across these 2 diseases. A significantly larger cohort is required to fully understand the difference in survival between patients with chronic neutrophilic leukemia (CNL) and aCML.
In order to further investigate survival differences between the patients with aCML and CNL, we used the National Cancer Database (NCDB), which was sourced from hospital registry oncology data from >1500 facilities that capture >70% of newly diagnosed cancer cases in the United States, with >34 million historical records. Certified tumor registrars use standard data item and coding definitions for data gathering, which undergoes monitoring for integrity and quality assurance.3 Patients were identified using ICD-O-3 codes for aCML (9876) and CNL (9963). The Kaplan-Meier method was used to estimate overall survival (OS), and comparisons among groups were made using the log-rank test. Because this analysis included deidentified data from the NCDB, it did not require institutional review board review. It was conducted in accordance with the Declaration of Helsinki.
We identified a total of 702 patients with aCML and 294 patients with CNL (Table 1). Similar age, sex, and comorbidity burden were observed between the 2 diseases. Data from NCDB do not include baseline hematologic parameters, physical exam findings, or cytogenetic information. Mutational data are also not widely available in the NCDB, although JAK2 mutational results were available in 222 aCML and 104 CNL cases and detected in 18.8% and 15.3% of patients with aCML and CNL, respectively. No other mutational information was available. Detailed treatment information is similarly not reported in this data set.
Baseline characteristics of aCML and CNL patients identified in the NCDB
| Characteristic . | aCML, N = 702 . | CNL, N = 294 . | P value . |
|---|---|---|---|
| Age, y median (IQR) | 72 (62, 80) | 71 (64, 79) | .5 |
| Sex, N (%) | .5 | ||
| Female | 267 (38.0%) | 118 (40.1%) | |
| Male | 435 (62.0%) | 176 (59.9%) | |
| Race, N (%) | .4 | ||
| Black | 63 (9.0%) | 21 (7.1%) | |
| White | 599 (85.3%) | 254 (86.4%) | |
| Other | 40 (5.7%) | 19 (6.5%) | |
| JAK2 mutated, N (%)∗ | 29 (13.8%) | 16 (15.3%) | .10 |
| CCI, N (%) | .4 | ||
| 0 | 509 (72.5%) | 220 (74.8%) | |
| 1 | 107 (15.2%) | 44 (15.0%) | |
| 2 | 47 (6.7%) | 21 (7.1%) | |
| ≥3 | 39 (5.6%) | 9 (3.1%) | |
| Unknown | 7 (1.0%) | 1 (0.3%) | |
| Year of diagnosis, N (%) | .8 | ||
| 2004-2008 | 106 (15.1%) | 47 (16.0%) | |
| 2009-2013 | 182 (25.9%) | 78 (26.5%) | |
| 2014-2018 | 286 (40.7%) | 110 (37.4%) | |
| 2019-2020 | 128 (18.2%) | 59 (20.1%) |
| Characteristic . | aCML, N = 702 . | CNL, N = 294 . | P value . |
|---|---|---|---|
| Age, y median (IQR) | 72 (62, 80) | 71 (64, 79) | .5 |
| Sex, N (%) | .5 | ||
| Female | 267 (38.0%) | 118 (40.1%) | |
| Male | 435 (62.0%) | 176 (59.9%) | |
| Race, N (%) | .4 | ||
| Black | 63 (9.0%) | 21 (7.1%) | |
| White | 599 (85.3%) | 254 (86.4%) | |
| Other | 40 (5.7%) | 19 (6.5%) | |
| JAK2 mutated, N (%)∗ | 29 (13.8%) | 16 (15.3%) | .10 |
| CCI, N (%) | .4 | ||
| 0 | 509 (72.5%) | 220 (74.8%) | |
| 1 | 107 (15.2%) | 44 (15.0%) | |
| 2 | 47 (6.7%) | 21 (7.1%) | |
| ≥3 | 39 (5.6%) | 9 (3.1%) | |
| Unknown | 7 (1.0%) | 1 (0.3%) | |
| Year of diagnosis, N (%) | .8 | ||
| 2004-2008 | 106 (15.1%) | 47 (16.0%) | |
| 2009-2013 | 182 (25.9%) | 78 (26.5%) | |
| 2014-2018 | 286 (40.7%) | 110 (37.4%) | |
| 2019-2020 | 128 (18.2%) | 59 (20.1%) |
CCI, Charlson Comorbidity Index; IQR, interquartile range.
JAK2 results were available for 222 aCML and 104 CNL cases.
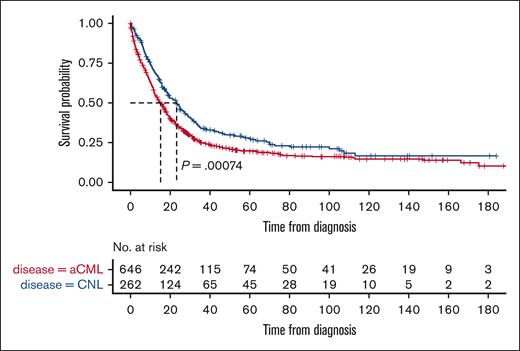
A total of 503 aCML and 183 CNL patients died during a median follow-up of 71.7 months (95% confidence interval [CI], 63.2-86.4 months). The median OS of patients with aCML was 15.2 months (95% CI, 13.2-17.3) compared with 23.1 months (95% CI, 18.8-28.2) for those with CNL: a difference that was statistically significant (P = .00074; Figure 1). To eliminate the possibility of potential confounding diagnoses, we performed a sensitivity analysis including only patients who had negative JAK2 mutational results. This analysis of 183 and 88 patients with aCML and CNL having wild-type JAK2, respectively, demonstrated similar results as the primary analysis with a significantly shorter median OS in patients with aCML than in those with CNL (17.0 months [95% CI, 13.6-19.5] vs 27.1 months [95% CI, 22.8-41.9]; P = .0063).
Kaplan-Meier curve of the OS of patients with aCML and CNL. Patients with aCML (red line) had a significantly shorter OS than patients with CNL (blue line).
Kaplan-Meier curve of the OS of patients with aCML and CNL. Patients with aCML (red line) had a significantly shorter OS than patients with CNL (blue line).
There are limitations of NCDB data, the most important 1 being that central histomorphology review by an expert hematopathologist was not performed to confirm these rare diagnoses. Although limited in baseline characteristics, extended mutational information, and treatment details, our analysis of a comparatively larger cohort of patients with aCML and CNL suggests a significant survival difference between these 2 entities when defined morphologically. The NCDB median OS of aCML cases (15.2 months) is similar to what is presented in the article by Carreño-Tarragona et al (17.7 months)1 and similar to what has been reported from another series of aCML cases (12.4 months).4 Similarly, we found a median OS of 23.1 months for patients with CNL, which is longer than estimated in this article (15.2 months),1 although similar to another series of patients with CNL (23.5 months).5 Therefore, despite inherent limitations in NCDB studies, survival in this analysis is generally consistent with previous investigations.
Although survival differences are not sufficient to distinguish diagnoses, the disparate survival outcomes of patients with aCML and CNL reported in this study support meaningful differences in these entities despite similar baseline clinical and mutational characteristics. Our data advocate that although mutational information is paramount for determining prognosis, incorporation of histomorphologic information is also of prognostic relevance. Notably, both the International Consensus Classification and World Health Organization kept these diagnoses distinct in their updated classification, although aCML was renamed myelodysplastic/myeloproliferative neoplasms with neutrophilia in the latter framework.2,6 Ultimately, additional evaluations of CNL, aCML, and other myelodysplastic/myeloproliferative neoplasm–overlapping syndromes will incorporate both clinical and morphologic evaluations in order to enhance our understanding of the natural history and outcomes of these rare myeloid neoplasms.
Contribution: D.T., D.S., and J.M. wrote the paper, and D.T. performed the statistical analysis.
Conflict-of-interest disclosure: D.T. receives contracted research funding to his institution from CTI Biopharma, Astellas Pharma, and Gilead, and consulting fees from CTI Biopharma, Novartis, AbbVie, Sierra Oncology, GlaxoSmithKline (GSK), and Cogent Biosciences. J.M. receives contracted research funding to his institution from AbbVie, CTI BioPharma Corp, Celgene/Bristol Myers Squibb, Geron Corporation, Incyte Corporation, Kartos Therapeutics, Novartis, PharmaEssentia, and Roche; is on the data and safety monitoring board at Karyopharm Therapeutics; and has received consulting fees from Galecto, Sierra Oncology, GSK, Imago BioSciences, and Constellation Pharmaceuticals/MorphoSys.
Correspondence: Douglas Tremblay, Division of Hematology/Oncology, The Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, 1 Gustave L Levy Pl, Box 1079, New York, NY 10029; e-mail: douglas.tremblay@mssm.edu.
References
Author notes
Data are available on request from the corresponding author, Douglas Tremblay (douglas.tremblay@mssm.edu).