Abstract
Approximately 90% of patients with myelodysplastic syndromes (MDSs) have somatic mutations that are known or suspected to be oncogenic in the malignant cells. The genetic risk stratification of MDSs has evolved substantially with the introduction of the clinical molecular international prognostic scoring system, which establishes next-generation sequencing at diagnosis as a standard of care. Furthermore, the International Consensus Classification of myeloid neoplasms and acute leukemias has refined the MDS diagnostic criteria with the introduction of a new MDS/acute myeloid leukemia category. Monitoring measurable residual disease (MRD) has historically been used to define remission status, improve relapse prediction, and determine the efficacy of antileukemic drugs in patients with acute and chronic leukemias. However, in contrast to leukemias, assessment of MRD, including tracking of patient-specific mutations, has not yet been formally defined as a biomarker for MDS. This article summarizes current evidence and challenges and provides a conceptual framework for incorporating MRD into the treatment of MDS and future clinical trials.
Introduction
Measurable residual disease (MRD), the detection of residual malignant cells during complete hematologic remission, allows for disease monitoring and is the most important predictor of survival for acute leukemias.1-3 Although myelodysplastic syndromes (MDSs) are considered malignant preleukemic myeloid neoplasms and may share many features with subtypes of acute myeloid leukemia (AML), MRD has not yet been effectively applied as a biomarker in MDS.4-8
MDSs are a heterogeneous group of biologically and clinically distinct subentities characterized by ineffective and dysplastic hematopoiesis; therefore, standardizing clinical response criteria has been difficult.8 The most recently proposed International Working Group (IWG) 2023 response criteria for higher-risk MDS is the first to consider MRD status as an exploratory end point and recommends its reporting as a response category.9 However, the IWG 2023 criteria do not provide details about the application of MRD testing or a formal definition of MRD response.
The available evidence shows that MRD assessment in MDS is likely to be context-dependent and influenced by biological and clinical prognostic factors, such as genetic subtype, disease stage, and treatment strategy. Furthermore, the analytical performance and applicability (subgroup vs general testing) of diagnostic tests as well as time points and sample sources, are important and must be considered in the assessment of MRD in MDS. Widespread implementation of MRD diagnostics in MDS is currently limited because of the cost and unproven clinical utility.
We propose that MRD can be an important biomarker in MDS, which would allow for pharmacodynamic assessment, prediction of survival, disease monitoring, and treatment decision–making. In this manuscript, firstly, we review methodologic considerations, such as multiparametric flow cytometry (MFC) vs next-generation sequencing (NGS) as well as source material considerations of bone marrow (BM) vs peripheral blood (PB). Next, we consider MRD in the context of clonal hematopoiesis (CH) and for the different clinical settings of nonintensive treatment of older or frail patients with MDS and allogeneic hematopoietic cell transplantation (HCT). Finally, we consider open questions and prospects for the future, including emerging technologies and efforts toward standardization of MRD evaluation.
Methodologic considerations
MFC or NGS
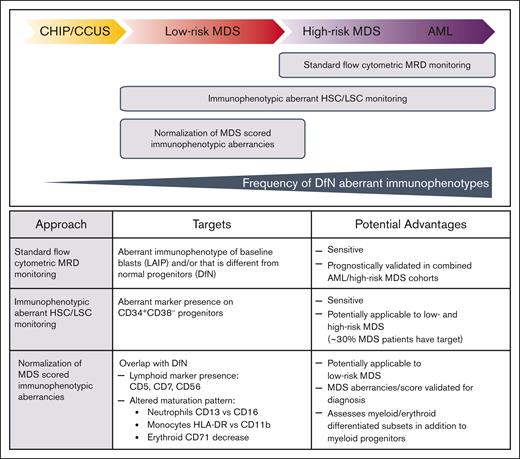
MFC-MRD, which is considered technically difficult to standardize but has a short turnaround time, quantifies MRD as progenitor cells that express leukemia-associated or different from normal aberrant immunophenotypes; these are identified in ∼90% of AMLs but are probably less frequent in MDS.3,10 MFC-MRD has a limit of detection (LOD) from 0.1% to 0.01% (10–3-10−4), although higher sensitivities (10−5-10−6) are reported for leukemic stem cell (LSC) detection using immunophenotypic aberrant hematopoietic stem cell (HSC) populations.11 MFC assessment of different from normal dysplastic maturation12-14 could supplement MFC-MRD quantitation of aberrant blast or stem cells (Figure 1).15 However, interpretation of MFC-MRD in MDS may be limited by residual CH-related changes of hematopoietic cells as well as the challenge of discriminating between lower-risk dysplastic clones and leukemic blasts.16,17
MFC has been used as an MRD test for MDS in a few studies that included patients with high-risk MDS in older AML cohorts.16,18 Evidence from nonintensive treatment trials in patients with AML ineligible for HCT shows a significantly higher relapse risk for patients with MFC-MRD–positive (MFC-MRD+) results than for those with MFC-MRD–negative results.19-22 In the peritransplant setting, tracking of leukemic blasts could be accomplished using MFC-MRD,23,24,25 but only a few studies have examined this approach outside of AML treatment.26-29
The genetic landscape of MDS has been studied and reviewed in detail by several authors.7,30-33 Approximately 90% of the patients with MDS will have at least 1 oncogenic lesion, but no single mutation is pathognomonic for MDS.30,31,33 Because recurrent hotspot mutations and gene fusions that are detectable via real-time quantitative polymerase chain reaction are less frequent in MDS compared with that in AML, an alternative approach, such as targeted error-corrected NGS, is possibly the most useful method for MRD assessment in MDS.6,33-37 However, no single MRD method has perfect sensitivity and specificity in MDS.
NGS-MRD also has several known limitations that have to be addressed before broader application in MDS.38 From a technological perspective, the 2 most important limitations are the standardization of the bioinformatics analysis platform and the intrinsic error rate due to rare events in a given sample interfering with the clear discrimination of the target from noise.3,37 Because of its intrinsic error rate, conventional NGS, now commonly used for the diagnosis of MDS/AML, has an LOD between 2% and 5% variant allele frequency (VAF).36,37 Although a positive MRD test result above this LOD during complete remission (CR) could be useful prognostically, a lower LOD is needed to give a meaningful discrimination of relapse risk between positive and negative tests in most instances. Technical advancements such as molecular tagging (unique molecular identifiers) and duplex sequencing allow for error correction, leading to LODs far below 0.1%, although achieving such sensitivity is mainly determined by the amount of input DNA or number of cells and costs.36,37
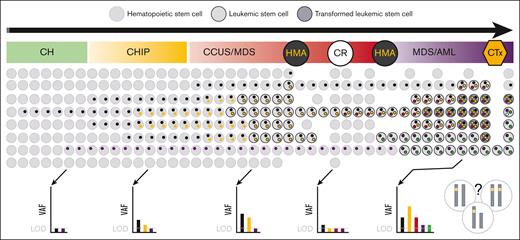
From a biological point of view, the results of NGS-MRD do not provide the full picture of MDS/AML, defined by clonal diversity and evolution with sometimes indetermined potential due to CH (Figure 2).39,41,42 Consequently, distinguishing between residual HSCs carrying clonal mutations of no pathogenic significance and LSCs via NGS-MRD is challenging. Furthermore, the ability to track MRD using NGS is also limited in MDS with germ line predisposition when no additional genetic markers are present because germ line mutations are noninformative for MRD (supplemental Table 1).3-5 Fundamentally, 2 approaches to target selection in NGS-MRD can be distinguished: sequencing of predefined target panels vs patient-specific mutation monitoring. However, to date, it is unknown which approach is superior. This is a moving target, and the decision will depend on a fine balance between the costs vs additionally acquired information and evidence related to outcome benefit.
Clonality and NGS-MRD. Schematic depiction of the polyclonal evolution and trackable somatic mutations from CH, through CHIP and CCUS to MDS/AML. Each colored dot within the cell represents a distinct mutation, with 2 different transformed clones (dark circles) developing over time and one outcompeting the other (so called clone sweeping39). NGS of the bulk population can only detect genetic alterations with a frequency above the LOD, which depends on the error-corrected sequencing methodology used. The VAF represents the variant frequency within the bulk population without information on the co-occurrence of variants within a single subclone of that population that is under constant intrinsic competition and extrinsic pressure (treatment). Depending on the bulk composition, the same VAF can represent different mutational states on a single-cell level (demonstrated by chromosomes in the right lower corner of the figure), such as biallelic vs monoallelic mutation, homozygous vs hemizygous or heterozygous mutations. Importantly, such allelic imbalances, eg, biallelic TP53 mutation, are not limited to MDS/AML but can also be found in CH, CHIP, and CCUS. CTx, chemotherapy. The figure was adapted and modified from the study by Stauber et al.40
Clonality and NGS-MRD. Schematic depiction of the polyclonal evolution and trackable somatic mutations from CH, through CHIP and CCUS to MDS/AML. Each colored dot within the cell represents a distinct mutation, with 2 different transformed clones (dark circles) developing over time and one outcompeting the other (so called clone sweeping39). NGS of the bulk population can only detect genetic alterations with a frequency above the LOD, which depends on the error-corrected sequencing methodology used. The VAF represents the variant frequency within the bulk population without information on the co-occurrence of variants within a single subclone of that population that is under constant intrinsic competition and extrinsic pressure (treatment). Depending on the bulk composition, the same VAF can represent different mutational states on a single-cell level (demonstrated by chromosomes in the right lower corner of the figure), such as biallelic vs monoallelic mutation, homozygous vs hemizygous or heterozygous mutations. Importantly, such allelic imbalances, eg, biallelic TP53 mutation, are not limited to MDS/AML but can also be found in CH, CHIP, and CCUS. CTx, chemotherapy. The figure was adapted and modified from the study by Stauber et al.40
Source material: BM or PB
Studies in patients with AML demonstrated a comparable clinical impact of MRD testing between PB and BM aspirate specimens.43-47 PB as a source for MRD testing may be more suitable than BM in MDS because PB is generally more informative about CH, is not affected by dilution or fibrosis, and is more easily accessible, thereby, showing greater consistency in addition to a lower cost for serial examinations. However, sensitivity is considered to be ∼1 log lower with PB than with BM, and there are concerns about the accurate quantification of the myeloid clonal burden during phases of neutropenia and concurrent relative lymphocytosis.3,48 MRD testing is most useful in patients who reach CR and show no morphological signs of the underlying disease. However, in cases in which hematopoiesis is not restored because of drug toxicity or limited stem cell reserve, a skewed lymphoid-to-myeloid cell ratio may be a relevant problem.48 In a research context, precise calculations of the VAF of somatic mutations could be crucial for monitoring the pharmacodynamics of new drugs in patients who are refractory and fail to achieve CR. Until prospective studies confirm that PB can effectively replace BM in MRD testing for MDS and that both molecular and flow cytometric testing yield comparable results in PB, BM should continue to be regarded as the current gold standard. Circulating cell-free tumor DNA could provide an alternative MRD target in the PB during the phase of neutropenia.49,50
The European LeukemiaNet (ELN) MRD Working Party actively pursues the goal of standardization and has published a detailed consensus document in 2021, updating the recommendations on MRD in AML.3,51 The currently recommended MRD threshold that has been established by prospective trials for AML in the first CR is 1 in 1000 cells (0.1%; 10−3). We propose that the ELN MRD recommendations of optimized technical requirements, a minimal detection limit, and standardized reporting should also be implemented in the MRD assessment of MDS (supplemental Table 1).
CH of indeterminate potential and clonal cytopenia of undetermined significance
The prevalence of CH is generally age-related, and its detection is assay-dependent.52-54 When the sensitivity of sequencing reaches ∼1% VAF, 85% of persons with an age of 80 years or older will have age-related CH.55 CH of indeterminate potential (CHIP), defined by somatic mutations with a VAF of 2% or higher, and clonal cytopenia of undetermined significance (CCUS), defined by CHIP with persistent cytopenia, are potentially preneoplastic states and inherent features in the pathogenesis of MDS.4,5,55-57 However, the occurrence of somatic mutations in CH, CHIP, and CCUS are stochastic events, and the kinetics of clone growth leading to progression to MDS/AML are unpredictable in most cases (Figure 2).39-42,58,59 Complicating matters, copy number alterations, independently or co-occurring with single nucleotide variants, have also been shown to play an important role in leukemogenesis.60,61
The number, combinations, and VAFs of somatic mutations show a strong association with progression from CCUS to myeloid neoplasm.41,52,62-64 CH is a risk factor for therapy-related myeloid neoplasms in patients who received cytotoxic treatment for primary malignancies.65-68 CH involving somatic mutations in TP53 and PPM1D is common in patients developing therapy-related MDS.66,68-70 Recent evidence suggests that thalidomide analogs such as lenalidomide also provide a growth advantage to TP53 mutated HSCs.68 Longitudinal measurements of mutant driver genes and clone size may allow for the early identification of progression into MDS. However, there is insufficient evidence to suggest that monitoring of CH or CCUS could be beneficial for populations at high risk, such as individuals with somatic TP53 mutations who received cytotoxic therapy. Reduction in cost and further improvements in sequencing and data analysis could lead to clone-specific targeted interventions as part of a secondary prevention.
Studies in patients with AML and MDS suggest that the persistence of CH, especially somatic mutations in 1 or more of the DTA (DNMT3A, TET2, and ASXL1) genes, during CR after chemotherapy or before HCT is not associated with an increased risk of relapse.3,71-74 It is important to bear in mind that only AML entities that are characterized by certain driver mutations (NPM1, bZIP in-frame mutated CEBPA) or gene fusions (CBFB::MYH11 and RUNX1::RUNX1T1) are typically cured without allogeneic HCT, presumably because their LSCs are chemotherapy-sensitive.51 Patients with AML with adverse-risk genetic abnormalities, including mutated TP53 and myelodysplasia-related gene mutations or cytogenetic abnormalities, whether primary or secondary, should receive HCT as part of their therapy.51 The recipient’s CH should disappear after HCT, which can be tracked via MRD testing, but the time point at which residual DTA mutations should be undetectable after transplantation is not established.44,49,75,76 In addition, donor-derived CH must be carefully excluded, especially if untargeted NGS is used for MRD monitoring.77,78 A retrospective NGS-MRD study of 131 patients with AML who underwent HCT showed that residual DTA mutations had no prognostic significance on days 90 and 180 after HCT.72 This study indicated that kinetics (an increase in VAF of DTA mutations between 2 time points) may be a better prognosticator of relapse.79 In the future, serial single-cell sequencing analyses will likely provide an answer to which mutations or combinations of mutations of residual CH have an impact on clinically relevant end points.80,81
Clinical considerations
Adapting the MRD assessment approach based on treatment goals together with considerations of cost and inconvenience is reasonable. Because effective treatment options are currently unavailable for most patients with MDS who are not eligible for HCT, MRD testing may not be justified for most real-world patients receiving palliative treatment outside of clinical trials. The subsequent sections will explore various clinical scenarios that may have different implications for MRD results.
Nonintensive treatment of MDS
Cytogenetic response, a complete or partial disappearance of chromosomal abnormalities, was introduced as a response criterion for MDS by the IWG in 2000 to enable prospective evaluation and comparability between clinical trials, although no data were available at that time to support a relationship between cytogenetic response and clinical outcome.82 Since then, most clinical trials that have included cytogenetic response criteria as an end point have demonstrated this association.8 We argue that defining MRD criteria for MDS is necessary for the same reasons that cytogenetic response criteria were established: to ensure successful clinical research and clear comparisons between trials.
Regular MRD assessment of patients with MDS who are not eligible for transplant should currently be focused in clinical research. Except for hypoplastic MDS or MDS with <5% BM blasts and isolated 5q deletion (MDS-del[5q]) treated with immunosuppressive agents or lenalidomide, respectively, most patients with low-risk MDS will initially receive supportive care when they need treatment because of cytopenia.7
We advocate that reporting MRD responses is important for understanding the efficacy of investigational new drugs. One example is the phase 2 portion of the MDS3001 study, which evaluated the efficacy of imetelstat, a competitive inhibitor of telomerase activity, in 57 patients who were red blood cell (RBC) transfusion-dependent with lower-risk MDS.83 Treatment with imetelstat resulted in a clinically meaningful 37% reduction in the 8-week RBC transfusion dependence rate. Pertinent to the utility of MRD, the reduction in the VAF of somatic SF3B1 mutations correlated with transfusion independence, suggesting that the SF3B1 VAF could be a surrogate molecular marker that predicted response (prolonged transfusion independence).
Residual mutations of CH further complicate MRD analysis after nonintensive therapies because they represent the remaining founder clones with residual hematopoietic potential that cannot be eradicated without the use of HCT thus far.42,84 An improvement in the treatment efficacy of targeting culprit subclones would make MRD testing more attractive as a surrogate marker for progression-free survival (PFS). Because it is biologically implausible that increasing VAF of mutations paralleling the progression of subclones would not influence critical outcomes,85 incorporating MRD analysis in the response criteria and definitions of progressive disease seems to be a reasonable goal.
This premise would also apply to future drugs with a mechanism of action that causes differentiation of neoplastic cells into normal blood cells instead of eradicating them, thereby improving suboptimal hematopoiesis but not leading to a potential reduction in the clonal burden. Only after studying such associations can we learn about the role of MRD and clinical benefit. Consequently, MRD assessment should be incorporated into the design of clinical trials investigating new agents for the treatment of MDS while implementing the recommendations of the US Food and Drug Administration on regulatory considerations for the use of MRD as a surrogate efficacy end point.86
HSCs with del(5q) are selectively resistant to lenalidomide. Tehranchi et al showed that similar to a molecular MRD measurement, the 5q deletion remained detectable in all patients with MDS-del(5q) using fluorescence in situ hybridization of sorted CD34+, CD38–/low, and CD90+ HSCs at the time of CR during lenalidomide treatment, even in patients with complete cytogenetic response (CCyR).87 A retrospective analysis of the phase 2 MDS-003 and the phase 3 MDS-004 studies showed that 103 of 181 (57%) patients achieved a cytogenetic response with lenalidomide, of whom 84 of 103 (81.6%) also achieved RBC transfusion independence at ≥26 weeks.88 The case of lenalidomide and MDS-del(5q) is a good example demonstrating that MRD testing, on one hand, shows the efficacy of specific treatment at the genetic level and, on the other hand, provides evidence that a cure, in a strict sense, is not possible because the malignant stem cell is not eradicated.
Patients with low-risk MDS-del(5q) who are treated with lenalidomide have a median AML-free survival of ∼3.5 years.88 Patients who are eligible for transplant may benefit from the early detection of subclonal TP53 mutations at diagnosis and regular monitoring during lenalidomide treatment.68,89-92 In a prospective multicenter study of the German MDS study group involving 67 MDS-del(5q) patients, median overall survival (OS) was significantly different between patients with (n = 59) and those without (n = 8) a TP53 mutation at diagnosis (3.55 years vs not reached; P = .002).90 Because the expansion of a TP53 subclone is associated with treatment failure and progression during treatment with lenalidomide, TP53 MRD testing would allow better stratification of patients for early HCT or clinical trials.91
High-risk MDS is treated with hypomethylating agents (HMAs), and response is associated with the number and type of somatic mutations.45,85,93-96 The decrease in VAF of certain high-risk or clearly transforming mutations indicating partial or complete elimination of subclones was associated with better PFS after treatment with HMAs such as azacitidine or decitabine, alone or in combination with other drugs, in several cohort studies (Table 1; supplemental Table 2). There seems to be a strong concordance between molecular and clinical responses, but the exact threshold of mutation clearance indicating the highest outcome difference during treatment with HMAs is not known. VAF thresholds of 1% and 5% have been described as meaningful in this setting and have to be put in context of baseline risk groups such as TP53.84,85,97
Summary of important studies with patients with MDS and reported MRD results including allogeneic HCT
| Study . | Population . | Study design . | Intervention . | MRD methodology . | Results . |
|---|---|---|---|---|---|
| Mixed populations, including palliative therapy | |||||
| Welch et al98 | MDS (n = 26), AML (de novo, n = 54; relapsed, n = 36) | Prospective, uncontrolled trial (n = 84) and extension cohort (n = 32) | 10-day or 5-day decitabine | WES and NGS gene panel (LOD not specified) | Rate of any mutation clearance associated with morphological response |
| Hunter et al95 | MDS (n = 210), MDS/MPN (n = 16), AML (N = 102), and t-MN (n = 60) | Retrospective | HMA therapy (7% additional agents) | NGS gene panel (VAF ≥5%) | TP53 mutation clearance associated with longer median survival (15.6 [negative] vs 7.7 [positive] months; P = .001) |
| Sallman et al97 | MDS (n = 40), AML (n = 11), and MDS/MPN (n = 4) | Phase 1b/2 | Eprenetapopt plus azacitidine | NGS (PB; LOD 0.1%) | TP53 mutation clearance associated with CR |
| Steensma et al83 | ESA relapsed/ refractory lower-risk MDS (N = 57) | Phase 2 | Imetelstat | NGS (BM and PB) | SF3B1 VAF reduction correlated with duration of transfusion independence |
| Yun et al84 | MDS (n = 95), secondary AML (n = 52), and MDS/MPN (n = 10) | Retrospective | HMA (74%), intensive chemotherapy (45%), and HCT (24%) | NGS gene panel (BM and PB; MRD VAF ≥5%) | MRD negativity (median OS not reached vs 18.5 months; P = .002) and TP53 mutation clearance <5% were associated with better OS |
| Sallman et al100 | MDS (N = 95) | Phase 1b | Magrolimab plus azacitidine | MFC (LOD 0.02%) | Small, heterogeneous high-risk cohort with 26% TP53 mutant MDS CR rate 33%, MRD negativity rate 23% Trend for improved OS in patients who became MRD– |
| Nannya et al 85 | MDS (n = 384) | Retrospective | Azacitidine | NGS gene panel (≥1%; LOD not specified) | Except for DDX41, posttreatment (≥ 4 cycles) clone size correlated with response observations |
| Before transplant | |||||
| Festuccia et al27 | MDS (n = 285; 23% had advanced to AML before HCT) CMML (n = 4) | Retrospective | HCT | MFC-MRD (LOD 0.001%-0.1%) plus cytogenetics/FISH | MRD status associated with CIR |
| Dillon et al71 | MDS (N = 48) | Subgroup analysis of a prospective phase 3 trial | RIC (n = 23) vs MAC (n = 25) | NGS 10-gene panel (PB) | MRD status associated with OS (55% vs 79%; P = .045) and CIR (40% vs 11%; P = .022) at 3 years Higher relapse rate in MRD+ patients randomly assigned to RIC vs MAC: 60% vs 8% (P = .010) |
| Craddock et al 23,112 | AML (n = 164) and MDS (n= 80) | Phase 2 randomized trial | Standard RIC (n = 108) vs intensified FLAMSA-Bu RIC (n = 108)` | MFC (BM; LOD 0.02%-0.05%) | Pretransplant MRD positivity associated with 2-year CIR in MDS: 50.0% vs 21.1% (P = .020) |
| Ma et al 119 | MDS-EB (n = 103) | Retrospective | HCT | MFC (BM; LOD <0.01%-0.05%) | MRD status associated with DFS and OS |
| After transplant | |||||
| Bernal et al26 | AML (n = 49) and MDS (n = 38) | Retrospective | MAC (16%) and RIC (84%) | MFC (BM; >0.01%) | Positive pretransplant MRD associated with positive MRD on day +100 Positive MRD on day +100 associated with relapse (OR, 6.55) |
| Duncavage et al114 | MDS (N = 90) | Retrospective | RIC (42%) and MAC (58%) | NGS (BM; VAF ≥0.5%) | 37% of patients tested as MRD+ on day +30 and 31% on day +100 MRD positivity on days +30 and +100 was associated with higher risk of disease progression or death |
| Nakamura et al 49 | AML (n= 37) and MDS (n = 14) | Retrospective | HCT (MAC 100%; 92% cord blood) | Personalized droplet digital PCR assay (circulating tumor DNA from serum or DNA from matched BM; median LOD 0.04%) | MRD positivity (either BM or serum) at 1 and 3 months associated with higher 3-year CIR and risk of death Greater or equal to 1.5-fold increase in ctDNA between 1 and 3 months after HCT was associated with highest risk of relapse (HR = 28.5; P = .0001) and death (HR, 17.4; P = .0009) |
| Study . | Population . | Study design . | Intervention . | MRD methodology . | Results . |
|---|---|---|---|---|---|
| Mixed populations, including palliative therapy | |||||
| Welch et al98 | MDS (n = 26), AML (de novo, n = 54; relapsed, n = 36) | Prospective, uncontrolled trial (n = 84) and extension cohort (n = 32) | 10-day or 5-day decitabine | WES and NGS gene panel (LOD not specified) | Rate of any mutation clearance associated with morphological response |
| Hunter et al95 | MDS (n = 210), MDS/MPN (n = 16), AML (N = 102), and t-MN (n = 60) | Retrospective | HMA therapy (7% additional agents) | NGS gene panel (VAF ≥5%) | TP53 mutation clearance associated with longer median survival (15.6 [negative] vs 7.7 [positive] months; P = .001) |
| Sallman et al97 | MDS (n = 40), AML (n = 11), and MDS/MPN (n = 4) | Phase 1b/2 | Eprenetapopt plus azacitidine | NGS (PB; LOD 0.1%) | TP53 mutation clearance associated with CR |
| Steensma et al83 | ESA relapsed/ refractory lower-risk MDS (N = 57) | Phase 2 | Imetelstat | NGS (BM and PB) | SF3B1 VAF reduction correlated with duration of transfusion independence |
| Yun et al84 | MDS (n = 95), secondary AML (n = 52), and MDS/MPN (n = 10) | Retrospective | HMA (74%), intensive chemotherapy (45%), and HCT (24%) | NGS gene panel (BM and PB; MRD VAF ≥5%) | MRD negativity (median OS not reached vs 18.5 months; P = .002) and TP53 mutation clearance <5% were associated with better OS |
| Sallman et al100 | MDS (N = 95) | Phase 1b | Magrolimab plus azacitidine | MFC (LOD 0.02%) | Small, heterogeneous high-risk cohort with 26% TP53 mutant MDS CR rate 33%, MRD negativity rate 23% Trend for improved OS in patients who became MRD– |
| Nannya et al 85 | MDS (n = 384) | Retrospective | Azacitidine | NGS gene panel (≥1%; LOD not specified) | Except for DDX41, posttreatment (≥ 4 cycles) clone size correlated with response observations |
| Before transplant | |||||
| Festuccia et al27 | MDS (n = 285; 23% had advanced to AML before HCT) CMML (n = 4) | Retrospective | HCT | MFC-MRD (LOD 0.001%-0.1%) plus cytogenetics/FISH | MRD status associated with CIR |
| Dillon et al71 | MDS (N = 48) | Subgroup analysis of a prospective phase 3 trial | RIC (n = 23) vs MAC (n = 25) | NGS 10-gene panel (PB) | MRD status associated with OS (55% vs 79%; P = .045) and CIR (40% vs 11%; P = .022) at 3 years Higher relapse rate in MRD+ patients randomly assigned to RIC vs MAC: 60% vs 8% (P = .010) |
| Craddock et al 23,112 | AML (n = 164) and MDS (n= 80) | Phase 2 randomized trial | Standard RIC (n = 108) vs intensified FLAMSA-Bu RIC (n = 108)` | MFC (BM; LOD 0.02%-0.05%) | Pretransplant MRD positivity associated with 2-year CIR in MDS: 50.0% vs 21.1% (P = .020) |
| Ma et al 119 | MDS-EB (n = 103) | Retrospective | HCT | MFC (BM; LOD <0.01%-0.05%) | MRD status associated with DFS and OS |
| After transplant | |||||
| Bernal et al26 | AML (n = 49) and MDS (n = 38) | Retrospective | MAC (16%) and RIC (84%) | MFC (BM; >0.01%) | Positive pretransplant MRD associated with positive MRD on day +100 Positive MRD on day +100 associated with relapse (OR, 6.55) |
| Duncavage et al114 | MDS (N = 90) | Retrospective | RIC (42%) and MAC (58%) | NGS (BM; VAF ≥0.5%) | 37% of patients tested as MRD+ on day +30 and 31% on day +100 MRD positivity on days +30 and +100 was associated with higher risk of disease progression or death |
| Nakamura et al 49 | AML (n= 37) and MDS (n = 14) | Retrospective | HCT (MAC 100%; 92% cord blood) | Personalized droplet digital PCR assay (circulating tumor DNA from serum or DNA from matched BM; median LOD 0.04%) | MRD positivity (either BM or serum) at 1 and 3 months associated with higher 3-year CIR and risk of death Greater or equal to 1.5-fold increase in ctDNA between 1 and 3 months after HCT was associated with highest risk of relapse (HR = 28.5; P = .0001) and death (HR, 17.4; P = .0009) |
A more extensive version of this table can be found in supplemental Table 2.
CIR, cumulative incidence of relapse; CMML, chronic myelomonocytic leukemia; DFS, disease-free survival; DTA, DNMT3A, TET2, and ASXL1; ESA, erythropoiesis-stimulating agent; FISH, fluorescence in situ hybridization; FLAMSA-Bu, fludarabine, cytarabine, amsacrine, and busulfan; HR, hazard ratio; MAC, myeloablative conditioning; MDS-EB, MDS with excess blasts; MPN, myeloproliferative neoplasm; OR, odds ratio; PCR, polymerase chain reaction; RIC, reduced intensity conditioning; t-MN, therapy-related myeloid neoplasm; WES, whole-exome sequencing.
Treatment response is usually short-lived with currently available agents, which may explain why MRD assessment has not been useful in the palliative setting of high-risk MDS in routine care. However, this does not mean that MRD assessment has no merit but may instead indicate that the current therapeutic options for MDS are limited. What would it mean if HMA therapy did not lead to a temporary suppression of TP53-mutated clones?85,93,95,97-100 The answer is that such a therapy would be less effective and bridge fewer patients with MDS/AML to HCT, which is the only option for cure.85,95,101-103
Pretransplant setting: prognostication and treatment decision–making
Evidence has emerged indicating that MDS with ∼10% to 19% BM blasts shares important biological and clinical similarities with AML when patients are stratified based on genetics.5,6 Many studies that investigated the role of MRD in AML included a subgroup of MDS/AML, which allowed basic principles of MRD analysis to be applied to the results of studies that enrolled patients with AML as the majority (Table 1; supplemental Table 2).16,18,23,71,104 The creation of the new entity MDS/AML in the recently published International Consensus Classification has introduced facts that affect the care of many patients with MDS outside of clinical trials.5 It is a reality that many academically affiliated transplant centers will use available MRD technologies, including less sensitive conventional techniques, in individual cases with the intent to improve the survival of their patients with MDS who are eligible for transplant. Ideally, MRD measurements should be performed in special reference laboratories.
When nonintensive or intensive treatments are used as a bridge to HCT, pretransplant MRD assessment can provide valuable prognostic information to influence the conditioning regimen and the posttransplantation plan.27 Many retrospective studies have evaluated the prognostic impact of somatic mutations at the time of HCT on the outcome of patients with MDS and, without implementing MRD assessment, proposed different genes associated with an unfavorable prognosis.105-111 Factoring in all consistent results and giving most weight to the largest study (Lindsley et al109), which analyzed the PB of 1514 patients with MDS using NGS (reporting a VAF threshold of 2%) before performing allogenic HCT, we can draw the following conclusions. Firstly, mutations in TP53 are consistently associated with the highest risk of relapse and decreased OS,29,105-108,110,111 which is not influenced by the conditioning intensity.109 Secondly, mutations in RAS pathway genes are associated with shorter OS because of an increased risk of relapse,108,110 specifically among patients aged 40 years or older who might not have received myeloablative conditioning.109
Post hoc analyses of prospective studies in MDS/AML that incorporated MRD assessment after intensive treatment and/or before HCT consistently show a higher risk of relapse for patients with MRD positivity than that for patients with MRD negativity.16,18,23,71,112 By performing 10-gene NGS-MRD in 48 CR samples from a randomized trial of younger patients up to age 65 years who were eligible for transplant, Dillon et al demonstrated that myeloablative conditioning mitigated the relapse risk associated with MRD positivity of non-DTA mutations in MDS.71 Because most patients with MDS are older than 70 years or have other adverse factors beyond genetics, myeloablative conditioning is frequently not an option, and other strategies to reduce relapse risk and improve OS must be explored. In a trial comparing reduced intensity regimens that included patients with MDS (33%; 80/244), Craddock et al showed that achieving a complete donor T-cell chimerism at 3 months, a potential surrogate marker for the graft-vs-leukemia effect, but not the intensification of the conditioning regimen reversed the negative impact of pretransplant MFC-MRD positivity on relapse incidence and OS.23 Pretransplant MRD positivity is also not a contraindication to HCT because clinical trials such as the VidazaAllo Study have demonstrated a better OS after HCT than that by continuing HMA treatment.102 In summary, these data suggest that patients with MDS without MRD may avoid myeloablative conditioning and that MRD positivity is useful to steer patients at high risk into clinical trials.95,102,103
Posttransplant setting: avoiding relapse
Because relapse of MDS after HCT is associated with a very poor prognosis, there is a great need for early detection and prevention through targeted intervention.113 MFC, NGS, polymerase chain reaction, and CD34+-sorted donor chimerism analyses have been successfully used to detect MRD in the posttransplant setting (Table 1; supplemental Table 2). Duncavage et al performed NGS-MRD in BM samples from 86 consecutive adult patients with MDS and secondary AML 30 and 100 days after HCT to assess mutation clearance and related risk of relapse.114 Before HCT, 86 of 90 (96%) analyzed patients had at least 1 detectable somatic mutation via whole-exome sequencing, and 68 of 86 (79%) with the use of a generic myeloid NGS panel of 40 recurrently mutated genes. At day 30 after transplant, 26 of 86 (30%) patients tested as MRD+ (only 1 patient had a sole DTA variant), defined by a VAF of ≥0.5% in the myeloid NGS panel. After adjustment for conditioning regimen, MRD positivity ≥0.5% was associated with a lower 1-year PFS compared with no detectable mutations at this threshold at 30 days after transplant (30.8% vs 57.1%; hazard ratio for progression or death, 2.09; 95% confidence interval [CI], 1.18-3.70; P = .02). Importantly, patients with mutations detectable at VAF ≥0.1% on day 30 had a statistically higher risk of progression (P < .003 using Gray test) and a shorter PFS (P = .021 using proportional hazards and χ2 test) than those without mutations detectable at a VAF <0.1%. However, only the results of a more elaborate NGS, which also detects patient-specific nonmyeloid-related somatic mutations, were reported for this threshold. Furthermore, MRD positivity on day 100 after transplant, which was detected in 18 of 58 (31%) patients by incorporating patient-specific nonmyeloid-related somatic mutations, was also associated with a lower 1-year PFS (27.8% vs 77.5%; hazard ratio for progression or death, 2.51; 95% CI, 1.26-5.01; P = .01). In a multivariable analysis, age >60 years, secondary AML, TP53 mutation, and MRD positivity ≥0.5% on days 30 and 100 were independently associated with disease progression or death.
Unfortunately, there are few prospective data on the treatment of MRD of MDS after HCT, almost exclusively from AML studies that included a few patients with high-risk MDS.104,115,116 In the RELAZA2 study, Platzbecker et al used quantitative polymerase chain reaction of leukemia-specific fusion genes or mutant NPM1 as well as donor chimerism analysis of sorted CD34+ cells from PB (threshold mixed chimerism <80%) to detect MRD and initiate treatment with azacitidine. One-year relapse-free survival was 46% (95% CI, 32-59) in the 53 patients who tested MRD+, 5 of whom had MDS, who received the preemptive treatment.104 Although the efficacy of this preemptive approach is also supported by a retrospective study,117 randomized controlled trials between patients tested as MRD+ and MRD-negative would be needed to give a definitive answer. Here, an NGS panel–based MRD assay might be more informative than MFC, for the detection of posttransplant emerging subclones with therapeutic targets.116
Proposition for future MRD analysis in MDS
Tailor MRD to goals of therapy
MRD assessment, ideally a combination of NGS-MRD and MFC-MRD, should be incorporated in all clinical trials in MDS. Although CR is the ultimate goal of any MDS treatment because of the association with improved OS, we acknowledge that hematologic improvement (HI) is also an important and meaningful clinical end point associated with improved quality of life that should be explored in clinical trials.9 Genetic and morphologic responses do not perfectly correlate, as demonstrated by CCyR, which is associated with improved survival but does not always lead to CR in patients with high-risk MDS receiving HMA treatment.118 For this reason, in contrast to AML, we propose that the complete MRD response category should always include CCyR and be distinct from morphological responses such as CR or HI. Furthermore, variants in DTA genes should be documented (DTA+/−) but generally not considered as MRD+.
The 2 clinical scenarios (1) treatment with palliative intent and (2) treatment with curative intent should be distinguished when applying MRD response criteria. In the former scenario, the application of MRD measurement is currently reserved only for clinical trials; in the latter, MRD assessment may already be offered in individual cases. This would have 2 advantages. In the palliative setting, in which the focus is on PFS and HI, the interaction of morphology and residual subclones would be easier to describe and investigate (eg, HI with MRD+ DTA+). In the curative setting, in which the main goal is to predict and prevent relapse, the morphological response might be of lesser importance after induction treatment because of HCT (eg, marrow CR with complete MRD response DTA+). The proposed provisional MRD criteria (Table 2) serve as a basis for discussion and will certainly need to be adjusted by suggestions from the stakeholders’ community9 and with the results of further studies.
Proposition for MRD response criteria in MDS
| Category . | Defining criteria . |
|---|---|
| MRDCR | CCyR∗ or normal karyotype, and complete MRD response: negative results (lower LOD at least 0.1%) in all MRD tests (NGS, MFC, and PCR) that were used |
| MRDLL | CCyR∗ or normal karyotype, and any MRD above the LOD of the assay but below the level of 0.1% |
| MRD+ | CCyR∗ or normal karyotype, and any MRD tests positive ≥0.1% |
| –DTA+/− | Used as an additional MRD test qualifier: eg, MRDCR DTA+ and MRD+ DTA− |
| MFC-MRD− | MFC is used as a standalone test without other genetic or molecular tests MFC-MRD–: no detection of any leukemic clones using MFC (lower LOD, 0.1%) |
| MFC-MRD+ | MFC is used as a standalone test without other genetic or molecular tests MFC-MRD+: detection of leukemic clones using MFC with a frequency ≥ 0.1% |
| MRD relapse | Previous documentation of MRDCR, MRDLL, or MFC-MRD− after treatment MRD relapse confirmed in a second consecutive samples Newly detected MRD+ Newly detected MFC-MRD+ Greater or equal to 1 log10 increase of VAF of previously detected DTA variants after day +100 of allogeneic HCT† |
| Category . | Defining criteria . |
|---|---|
| MRDCR | CCyR∗ or normal karyotype, and complete MRD response: negative results (lower LOD at least 0.1%) in all MRD tests (NGS, MFC, and PCR) that were used |
| MRDLL | CCyR∗ or normal karyotype, and any MRD above the LOD of the assay but below the level of 0.1% |
| MRD+ | CCyR∗ or normal karyotype, and any MRD tests positive ≥0.1% |
| –DTA+/− | Used as an additional MRD test qualifier: eg, MRDCR DTA+ and MRD+ DTA− |
| MFC-MRD− | MFC is used as a standalone test without other genetic or molecular tests MFC-MRD–: no detection of any leukemic clones using MFC (lower LOD, 0.1%) |
| MFC-MRD+ | MFC is used as a standalone test without other genetic or molecular tests MFC-MRD+: detection of leukemic clones using MFC with a frequency ≥ 0.1% |
| MRD relapse | Previous documentation of MRDCR, MRDLL, or MFC-MRD− after treatment MRD relapse confirmed in a second consecutive samples Newly detected MRD+ Newly detected MFC-MRD+ Greater or equal to 1 log10 increase of VAF of previously detected DTA variants after day +100 of allogeneic HCT† |
DTA, DNMT3A, TET2, and ASXL1; LL, low level of detection (<0.1%); MRDCR, complete MRD response.
International Working Group 2023 response criteria (unchanged from IWG 2006).9
Corroboration via sorted donor chimerism analyses is recommended.
An optimal gene panel for NGS-MRD has not yet been defined for MDS. The calculation in the molecular international prognostic scoring system requires the analysis of 31 genes for risk stratification at diagnosis.33 This panel can be used as a starting point for further refinements of NGS-MRD diagnostics in MDS (supplemental Table 3). As a minimum, we consider the 10-gene panel, which has been described as prognostic in patients with MDS and AML, before conditioning for HCT (supplemental Table 4).71 All detected mutations should be considered as potential MRD markers (supplemental Table 1).
Time points of MRD assessment
The optimal MRD measurement time points are not known and will always reflect the design of published clinical trials that demonstrate outcome differences between patients with MRD+ and MRD-negative results. No evidence-based recommendation can be given for the setting of palliative treatment. Outside of clinical trials, a pragmatic suggestion would be to perform MRD testing in patients who have a long-lasting remission with HMAs and wish to reduce therapy or who have indeterminate cytopenia despite achieving CCyR. For patients treated with the intention of cure, we pragmatically suggest performing MRD testing in BM for remission assessment before HCT as well as on days +30 and +100 after HCT. These time points would allow for conditioning regimens (myeloablative vs reduced intensity) and immunosuppression (faster vs normal tapering of immunosuppressive agents) to be adjusted as well as an optional donor lymphocyte infusion to be planned. If a molecular marker is present, further NGS-MRD assessments could be performed every 4 to 8 weeks using PB samples. Any MRD+ results should be confirmed by further testing to estimate clone kinetics.
Potential role of new methodologies
A major drawback of NGS-MRD is that the reported VAF represents the average frequency within a bulk cell population, making it impossible to provide information on the co-occurrence of multiple variants within a single subclone of that cell population.80 Especially in MDS, in which CH is an integral part of its pathogenesis, the inability to distinguish residual CH from LSCs is still an obstacle to clearly establishing the presence of MRD in some cases. Single-cell analysis has great potential to revolutionize MRD assessment in this regard because it is able to resolve clonal architecture. For example, sequencing single cells from enriched LSCs at diagnosis and during remission could explain which combinations of mutations are found in the same cell and steer more sensitive NGS-MRD detection. Recently, Dillon et al have shown in a proof-of-principle study in 3 patients with AML that a tailored single-cell analysis integrating patient-specific mutations and structural variants from whole-genome sequencing as well as cell surface markers is able to determine the exact genetic alterations that are present in a single cell.81 Single-cell MRD analysis is in the early stages of development. Further studies, ideally in the context of prospective clinical trials, are necessary to demonstrate feasibility on a large scale.
Another promising approach to detecting MRD is to perform NGS in CD34+ (or alternatively, CD117+) selected cells from PB after magnetic cell separation or flow cytometric sorting.76 In an analysis of 40 patients with MDS/AML in CR after HCT, Stasik et al demonstrated an impressively high sensitivity of 100% and specificity of 91% for detecting molecular relapse.76 The lower limit of MRD detection was 10−6, ∼10-fold more sensitive than the measurement of donor chimerism as performed in the RELAZA2 study, and PB was superior to BM as a source of CD34+ cells.
Regarding minimally invasive MRD assessment, serial analysis of circulating cell-free tumor DNA for leukemia-specific mutations in serum may be the optimal approach for cytopenic patients with MDS/AML. Previous studies in the post-HCT setting in patients with MDS/AML have demonstrated the principal feasibility of this methodology, which must be standardized and prospectively investigated in different clinical scenarios.49,50
Standardization efforts
The standardization of MRD methods is the key to accomplish reproducibility and comparability. The MRD working group of the ELN has published a blueprint on how to successfully carry out such an endeavor in AML. Reproducibility must be demonstrated in clinical trials using a published standardized methodology. This means that in addition to technological advancement, considerable standardization efforts will be necessary in MDS in the future. A first step should be the definition of uniform MRD criteria.
Open questions
Because the extent of discordance between MRD measured via MFC and NGS is currently unknown in MDS, we recommend that both methods be prospectively studied in parallel to determine clinically meaningful detection thresholds. In addition, when NGS-MRD testing is used at specific time points in clinical trials, the comparison of BM and PB source materials is recommended. The potential role of LSC-based detection of MRD is unknown for MDS and should be explored. If patients are randomly assigned between intensive and nonintensive therapy, MRD assessment should be used to answer the question of whether MRD negativity has the same value after both treatment types and what specific mutations are affected by either strategy. Copy number abnormalities and allelic imbalances, including copy-neutral loss of heterozygosity, are important in the pathogenesis of MDS but have rarely been discussed in the context of MRD. Furthermore, the significance of uncommon mutations from agnostic NGS approaches should be explored in more granularity to answer the question whether all non-DTA mutations or combinations thereof are predictive for relapse or progression. Single-cell sequencing is providing increasing insight into the role of subclones in treatment resistance and relapse. This technology could be used to determine the stage (diagnosis or relapse) at which escape clones emerge and, thus, possibly predict their occurrence.
Summary
A negative MRD test result indicates that there is no evidence of disease present above a predefined test threshold. However, although MRD measurements give an important prognostic estimate, this estimate is not absolute because relapse is also observed in patients with MRD-negative results, and MRD assessment is potentially hampered by source material processing, technique used, benign CH, and the time point of investigation. The landscape of MRD in MDS continues to evolve with the introduction of new methods such as single-cell sequencing; however, a formal working MRD definition is needed. We propose MRD response criteria built on currently available evidence. Because there remains no curative therapy for most patients with MDS, implementation of MRD testing is an important part of clinical trial design and should be a secondary end point to achieve intertrial comparability and efficacy quantification and to improve our understanding of the relationship between residual CH and relapse. Clinically useful evidence to establish MRD as a biomarker will require both high-quality randomized controlled trials and large collaborations.
Acknowledgments
This work was supported by funding from the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
The views expressed in this work do not represent the official views of the National Institutes of Health or the United States Government.
Authorship
Contribution: E.S. and S.Z.P. conceptualized the study; E.S., S.F., P.D.A., and S.Z.P. interpreted data and drafted the manuscript; E.S. wrote the first draft; and all authors have seen and approved the manuscript being submitted.
Conflict-of-interest disclosure: E.S. received honoraria from Amgen. The remaining authors declare no competing financial interests.
Correspondence: Steven Z. Pavletic, National Cancer Institute, Center for Cancer Research, National Institutes of Health, 10 Center Drive, Room CRC 4-3130, Bethesda, MD 20892-1907; e-mail: pavletis@mail.nih.gov.
References
Author notes
The full-text version of this article contains a data supplement.