TO THE EDITOR:
Sourisseau et al1 recently published a study in this journal on a risk-based strategy on letermovir use for prevention of cytomegalovirus (CMV) infection in patients receiving an allogeneic hematopoietic cell transplant (allo-HCT). In the study, 2 time periods were analyzed. In the first (2015-2017), patients underwent transplantation before the implementation of letermovir use, whereas in the second (2018-2021), letermovir was administered to all patients who were at high risk, and those who were at low risk received corticosteroid treatment before week 14 from transplantation. The authors observed that patients who were at high risk who had received letermovir presented a lower incidence of CMV infection compared with those in the same risk group not treated with letermovir (P < .001). The same trend was observed in the low-risk groups.
Estimating the individual risk at baseline for developing CMV infection after allo-HCT is important,2,3 especially in the current era in which the efficacy of letermovir for CMV infection and disease prevention has been proven.4 Nonetheless, although well tolerated, letermovir may be an unfeasible option in some contexts owing to its high cost and uncertainty on the optimal length of treatment and development of resistance to prevent CMV reactivation. Moreover, risk scores consider and weigh different factors, changing from one population to another. For instance, the use of cord blood (UCB) has been designated as criteria for allocating patients in a CMV high-risk group as published by Marty et al.4 However, we consider that classifying all UCB transplants (UCBTs) as high risk is still up for debate. Thus, based on this premise, using a large cohort of UCBT recipients, we developed a pretransplant prognostic score predicting CMV infection.
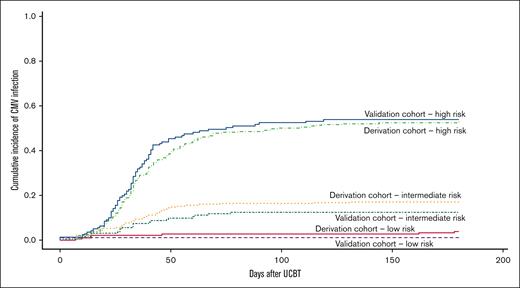
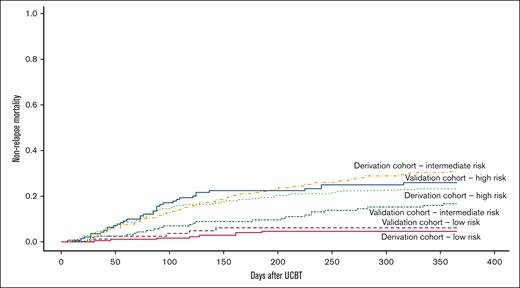
Demographic and clinical data of 1406 UCBT recipients (2010-2019) were retrospectively obtained from the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC) and Eurocord registries. Approval for this study was granted following all due considerations of ethical matters on behalf of Eurocord and SFGM-TC. The study was conducted in accordance with the Declaration of Helsinki. The outcome variables for CMV infection were CMV viremia leading to preemptive treatment or proven organ infection (CMV disease) from days 0 to 180 after UCBT. Data on potential use of letermovir were not available, but we assumed that most patients did not receive the treatment considering the inclusion period of our study and the availability of letermovir. HLA mismatches were considered as any mismatch in the HLA class I (antigenic level) or II (allele level) typing between UCB and recipient. All-cause mortality between days 0 and 180 was considered a competing risk for CMV infection. In contrast with other graft types, UCB is always CMV seronegative. Our study population was divided into a derivation cohort (n = 984, 70%) and a validation cohort (n = 422, 30%). Our risk score was created testing known variables associated with CMV infection after allo-HCT2,3 in bivariate analyses using Fine-Gray model for competing risks in the derivation cohort. Variables with a subdistribution hazard ratio (SHR) >1 and a P value of <.05 were then tested in a Fine-Gray multivariable regression model. As reported by other groups,2,3,5 beta coefficients obtained from that model were used to assign a scoring system according to the sum of their scores and categorized into low (0), intermediate (1-26), and high (27-29). All statistical analyses were performed using SPSS v.23 and R Studio v.1.3.1093. In the derivation cohort, the median age was 23.5 years (range, 0.3-73.2 years). The most frequent diagnoses were acute myeloid leukemia (n = 317, 32.2%) and acute lymphoblastic leukemia (n = 235, 23.9%). Most recipients were CMV seropositive (n = 516, 52.4%). In the validation cohort, the median age was 21.8 years (range, 0.2-69.1 years). The 2 most frequent diagnoses were also acute myeloid leukemia and acute lymphoblastic leukemia, with 36.5% (n = 154) and 22.5% (n = 95), respectively. CMV seropositivity was observed in 53.3% (n = 225). CMV infections were observed in 26.4% (n = 260) and 25.6% (n = 108) of the derivation cohort and the validation cohort, respectively. The median time from UCBT to the development of CMV infection was 33 days for both cohorts. Bivariate analyses showed the following significant risk factors in the pre-UCBT context: age ≥ 36 years (P = .002), patient CMV seropositivity (<0.0001), and ≥2 HLA mismatches (P = .007). Based on these findings and the beta coefficients of the multivariable analysis, 1 scoring point was assigned if age ≥ 36 years at UCBT, 2 points if HLA mismatches ≥ 2, and 26 points to patient CMV seropositivity. Thus, for each cohort, 3 risk groups were created according to their scores. Table 1 shows the number of CMV infections according to the risk (scoring) group in both cohorts. In the derivation cohort, according to the created risk groups, most recipients fell into the intermediate-risk category (n = 393, 44.0%). As expected, CMV infection rates were the highest in recipients falling into the high-risk group (52.8% in the derivation cohort and 53.9% in the validation cohort). In contrast, the rates of CMV infection within the low-risk group in both derivation and validation cohorts were low (3.8% and 1.2%, respectively). As observed, in both the derivation and validation cohorts, patients in the high-risk group were more prone to develop CMV infection compared with those in the low-risk group (Figure 1). One-year nonrelapse mortality by risk group and cohort is depicted in Figure 2.
CMV infection after UCBT by risk (scoring) group in both cohorts (N = 1278)
| Derivation cohort (n = 893) . | Validation cohort (n = 385) . | ||||||
|---|---|---|---|---|---|---|---|
| Risk group . | n (%) . | % CMV infection . | P value . | Risk group . | n (%) . | % CMV infection . | P value . |
| Low (0) | 182 (20.4) | 3.8 | <.0001 | Low (0) | 83 (21.6) | 1.2 | <.0001 |
| Intermediate (1-26) | 393 (44.0) | 17.0 | Intermediate (1-26) | 161 (41.8) | 12.4 | ||
| High (27-29) | 318 (35.6) | 52.8 | High (27-29) | 141 (36.6) | 53.9 | ||
| Derivation cohort (n = 893) . | Validation cohort (n = 385) . | ||||||
|---|---|---|---|---|---|---|---|
| Risk group . | n (%) . | % CMV infection . | P value . | Risk group . | n (%) . | % CMV infection . | P value . |
| Low (0) | 182 (20.4) | 3.8 | <.0001 | Low (0) | 83 (21.6) | 1.2 | <.0001 |
| Intermediate (1-26) | 393 (44.0) | 17.0 | Intermediate (1-26) | 161 (41.8) | 12.4 | ||
| High (27-29) | 318 (35.6) | 52.8 | High (27-29) | 141 (36.6) | 53.9 | ||
Data were missing from 128 recipients.
One-year nonrelapse mortality by risk group and cohort: 4.6% derivation cohort–low risk, 6.1% validation cohort–low risk, 16.7% validation cohort–intermediate risk, 23.3% derivation cohort–high risk, and 25.9% validation cohort–high risk, and 30.9% derivation cohort–intermediate risk; P < .0001.
One-year nonrelapse mortality by risk group and cohort: 4.6% derivation cohort–low risk, 6.1% validation cohort–low risk, 16.7% validation cohort–intermediate risk, 23.3% derivation cohort–high risk, and 25.9% validation cohort–high risk, and 30.9% derivation cohort–intermediate risk; P < .0001.
Previous CMV risk score studies have been performed mainly considering adult graft sources.2,3 Our study used a large population of UCBT recipients from different transplant centers to evaluate the feasibility of establishing a clinical score to stratify the risk of CMV infection in patients undergoing UCBT.
Recipient CMV serostatus is considered the dominant risk factor for CMV infection after an allo-HCT.6,7 In our study, recipient CMV seropositivity was the main predictive variable for CMV infection after UCBT. When performing the multivariable analysis in the derivation cohort, those with CMV seropositivity had a 13.4-times higher likelihood of developing CMV infection after UCBT than CMV-seronegative recipients (P < .0001). We further defined other 2 predictive variables for CMV: ≥ 36 years at UCBT and HLA mismatch UCB. These 2 risk factors have been previously identified as contributors to the potential development of CMV after HCT.2,3,6-8 Importantly, because of the high impact of CMV seropositivity in our risk score, we performed a subanalysis exclusively including CMV-seropositive patients, which corroborated that age ≥ 36 years at UCBT and HLA mismatch UCB continue to play an important role.
Overall, our study gives an evaluation of pretransplant factors predicting the risk of CMV infection in UCBT recipients. Sourisseau et al1 concluded that a risk-based strategy for letermovir maintained the high efficacy of this medication in patients who are at high risk, allowing to exclude this treatment in some patients who are at low risk. Therefore, the use of scoring systems remains important to detect the risk for CMV infection. In addition, despite obtaining outstanding results using letermovir, it is important to consider the subclinical CMV reactivation9 and, more importantly, that its discontinuation may lead to delayed-onset CMV infection,10 thus, extended treatment duration remains a debate.11
In contrast to the status quo, we demonstrated that not all UCBT recipients should be automatically assigned to a high-risk group because our results showed that CMV infection varied within UCBT recipients with minimal risk in those categorized as low risk. In the setting of UCBT, our score allows to stratify CMV risk, which could contribute to CMV prevention and treatment.
Acknowledgments: The authors thank all the participating centers for their dedication and involvement in patient care.
Contribution: E.G., H.R., and M.R.F. designed the study; M.R.F. analyzed results; F.V. and M.R.F. wrote the first draft of the manuscript; B.C., C.K., G.M.S., and H.R. reviewed the data; and all authors contributed to the writing and approval of the last version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Monica Rivera Franco, Eurocord, Hôpital Saint Louis APHP, Institut de Recherche de Saint-Louis (IRSL) EA3518, Université de Paris Cité, Paris, France 75010; e-mail: monica.riverafranco-ext@aphp.fr.