TO THE EDITOR:
Mature T-cell neoplasms (MTCNs) are a clinically and biologically heterogeneous family of lymphoid malignancies that together represent between 10% and 15% of all non-Hodgkin lymphoma. MTCNs are primarily classified by histological and immune-phenotypical features. In addition, MTCNs are defined by their dominant clinical presentation as nodal, extranodal, leukemic, and primary-cutaneous.1,2 The primary treatment approach for MTCNs varies considerably based on subtype.3 Advancements in the first-line treatment setting include the addition of brentuximab vedotin to chemotherapy in certain subtypes of CD30-expressing peripheral T-cell lymphomas.4 In the relapsed setting, there is considerable overlap in treatment options including allogeneic stem cell transplant, which can provide a cure in up to half of eligible patients, albeit with a significant risk of nonrelapse mortality.5-8 However, other immunotherapies have had significantly less impact in MTCNs compared with mature B-cell neoplasms.9,10 One of the main reasons for this is because of the absence of a specific target. The lack of a uniformly expressed tumor target across all the subtypes of MTCNs has a significant impact on the development of targeted therapies.
CD38 is a multifunctional ectoenzyme and transmembrane protein that is frequently expressed in multiple hematologic malignancies and, at lower frequency and intensity, in subsets of normal myeloid and lymphoid cells.11 Targeting CD38 with therapeutic monoclonal antibodies, such as daratumumab and isatuximab, has transformed the treatment of untreated and relapsed/refractory multiple myeloma (MM), a malignancy that exhibits high CD38 expression.12 Case reports and preclinical studies targeting CD38 have also shown some efficacy in T-cell acute lymphoblastic leukemia, and a phase 2 clinical trial demonstrated moderate results in extranodal NK/T-cell lymphoma.13-16 However, the frequency and density of CD38 expression across the spectrum of MTCNs has not been examined. In this letter, we report the expression and intensity of CD38 across various subtypes of MTCN. We also assess the potential of CD38 as a treatment target in 2 preclinical mouse models of MTCN treated with daratumumab.
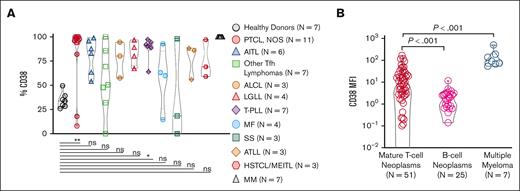
We retrospectively searched all MTCNs diagnosed at Thomas Jefferson University Hospital (TJUH; Philadelphia, PA) from January 2013 to April 2020. Cases in which immune-phenotypical characterization of the tumor biopsy detected an abnormal T-cell population were included. A retrospective investigation of flow cytometry data acquired at time of fresh sample collection was conducted on CD38 expression in neoplastic cells across various MTCN subtypes (n = 51) including: peripheral T-cell lymphoma not otherwise specified (PTCL-NOS; n = 11), angioimmunoblastic T-cell lymphoma (n = 6), other PTCL with a follicular helper T-cell phenotype (n = 7), anaplastic large cell lymphoma (n = 3), large granular lymphocytic leukemia (n = 4), T-cell prolymphocytic leukemia (T-PLL; n = 7), mycosis fungoides (n = 4), Sézary syndrome (n = 3), adult T-cell leukemia/lymphoma (n = 3), hepatosplenic T-cell lymphoma (n = 2), and monomorphic epitheliotropic intestinal T-cell lymphoma (n = 1). Flow cytometry (FC) was performed on either BDCalibur or BD FACSCanto cytometers (BD Biosciences; Franklin Lakes, NJ) and data analyzed using Kaluza software (Beckman Coulter Life Sciences; Sharon Hill, PA). Using a standard T-cell panel, CD38 expression was assessed on phenotypically aberrant T-cell populations representing the neoplastic cells in various subtypes of MTCN (supplemental Table). The abnormal population was selected from the CD45 gate and defined as abnormal if displaying loss of ≥1 of the mature T-cell markers CD2, CD3, CD5, and/or CD7, and/or a significantly skewed CD4:CD8 ratio. CD38 expression on the phenotypically abnormal malignant population was defined as positive if displaying any fluorescence signaling. The intensity of CD38 expression was determined using the mean fluorescence intensity (MFI) of the phenotypically abnormal malignant population. With this strategy, we analyzed 51 unique patients with MTCN with at least 1 biopsy that met the FC criteria identifying the predominant malignant population, which could be assessed for CD38 expression. The percentages of CD38-expressing cells within the phenotypically abnormal malignant T-cell populations, within T cells from healthy donors, and within MM samples for comparison are represented in Figure 1A. Across the MTCN spectrum, almost all subtypes trended toward higher CD38 expression than CD3+ T cells from healthy donor peripheral blood mononuclear cells, with PTCL-NOS (P = .0026) and T-PLL (P = .0202) showing significantly increased expression. We did not observe any statistical differences in the percent of CD38+ cells when grouped by compartment (data not shown). In addition, the MFI of CD38-expressing malignant cells are compared with healthy donor CD3+ T cells and MM malignant cells in Figure 1B. MTCNs have significantly increased CD38 MFI compared with mature B-cell neoplasms (P < .0001). Together, these data demonstrate intrapatient heterogeneity of the CD38 receptor density with an increased CD38 expression in most subtypes of MTCN, with highest expression in PTCL-NOS and T-PLL. The study was performed under approved institutional review board and institutional animal care and use committee protocols and was performed in accordance with the Declaration of Helsinki.
Increased CD38 expression in MTCN. (A) These results represent a retrospective analysis of data acquired from fresh specimens from multiple biopsy sites (bone marrow, lymph nodes, spinal fluid, peripheral blood, pleural fluid, tonsils, soft tissue, parotid, and small bowel) from 51 patients, 7 healthy controls, and 7 MM controls, which were examined by flow cytometry. The percentage of CD38 from within the phenotypically abnormal malignant population across the MTCN spectrum was also compared with CD38 staining on CD3+ cells in peripheral blood of healthy donors and samples from patients with MM. PTCL-NOS (P = .0026) and T-PLL (P = .0202) were statistically significantly increased as analyzed by the Kruskal-Wallis test. (B) The CD38 MFI of the gated neoplastic T-cell population from patients with MTCN compared with that of samples from patients with B-cell neoplasms (P < .0001) and patients with MM (P < .0001) by Mann-Whitney analysis. Each data point represents a unique patient with MTCN.
Increased CD38 expression in MTCN. (A) These results represent a retrospective analysis of data acquired from fresh specimens from multiple biopsy sites (bone marrow, lymph nodes, spinal fluid, peripheral blood, pleural fluid, tonsils, soft tissue, parotid, and small bowel) from 51 patients, 7 healthy controls, and 7 MM controls, which were examined by flow cytometry. The percentage of CD38 from within the phenotypically abnormal malignant population across the MTCN spectrum was also compared with CD38 staining on CD3+ cells in peripheral blood of healthy donors and samples from patients with MM. PTCL-NOS (P = .0026) and T-PLL (P = .0202) were statistically significantly increased as analyzed by the Kruskal-Wallis test. (B) The CD38 MFI of the gated neoplastic T-cell population from patients with MTCN compared with that of samples from patients with B-cell neoplasms (P < .0001) and patients with MM (P < .0001) by Mann-Whitney analysis. Each data point represents a unique patient with MTCN.
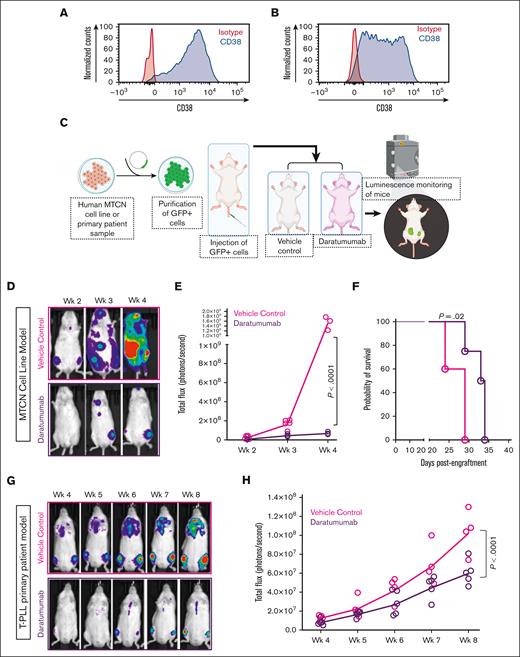
Next, we studied the in vivo efficacy of the anti-CD38 antibody, daratumumab, in CD38-expressing MTCN-bearing mice in 2 xenograft models (Figure 2). Using the firefly luciferase–green fluorescent protein (GFP) lentivirus (Cellomics Technology; Halethorpe, MD), we generated a MTCN cell line (HuT-78) and a sample from a patient with T-PLL (phenotype: CD2+, CD3+, CD4+, CD5+, CD7+, CD8−, CD38+, CD56−, CD57−) that express luciferase for efficient in vivo tracking and detection of tumor progression. CD38 surface expression was examined by FC. Both HuT-78 luciferase and the T-PLL luciferase cells expressed CD38, as shown in Figure 2A and 2B, respectively. Then, neoplastic cells were injected intravenously into immunodeficient Rag−/−γc−/− mice. After confirmation of cell engraftment and expansion using bioluminescence imaging, mice were randomized to 2 groups: vehicle treated and daratumumab treated. Starting at 1 week after cell injection in the HuT-78 luciferase model (3 weeks after cell injection in the T-PLL model), mice received 8 mg/kg subcutaneous treatment with daratumumab (Darzalex Faspro; kindly donated by the TJUH pharmacy) or vehicle control, once weekly. Disease burden was monitored by in vivo imaging weekly, starting 1 week after treatment initiation using the IVIS imager (Perkin-Elmer; Waltham, MA), and luminescence pixel intensity was quantified using Living Image software (Perkin-Elmer version 4.7.3) (Figure 2C). Representative whole-body images of HuT-78 luciferase mice with patient-derived xenografts (PDXs) treated with vehicle (n = 5; red) or daratumumab (n = 4; black), showed notably reduced disease burden and slowed progression when compared with control animal treated with vehicle (Figure 2D). Tumor burden was quantified in Living Image as total flux (photons/s) determined for each group of animals at each imaging time point. In the HuT-78 PDX model, mice treated with daratumumab had significantly decreased total flux compared with animals receiving vehicle after 4 weeks of treatment (Figure 2E; P < .0001). Mice treated with daratumumab also had significantly improved survival outcomes vs mice treated with vehicle (Figure 2F; P = .02). Similarly, in the primary T-PLL model, mice with PDXs in the daratumumab treatment group (n = 5) had significantly reduced tumor burden and progression compared with mice treated with vehicle (n = 4; Figure 2G) as well as significant reductions in total flux (Figure 2H; P < .0001). To our knowledge, this is the first proof-of-concept demonstration that immunotherapeutic targeting of CD38 leads to reduced disease burden and consequent reduced disease progression in a preclinical model of MTCN.
Preclinical efficacy of daratumumab in CD38-expressing MTCN-bearing mice in 2 different xenograft models. (A) CD38 expression in HuT-78 depicted as histogram of CD38 compared with isotype control-stained cells. (B) CD38 expression in luciferase-GFP–transduced T-PLL cells from patients depicted as histogram of CD38 compared with isotype control-stained cells. (C) Schematic diagram showing experimental design: HuT-78 or primary T-PLL cells from patients were transduced with the lentivirus expressing firefly luciferase-GFP, sorted to >99% purity, and transplanted into Rag−/−γc−/− mice with 2 or 4 million cells, respectively. Mice with PDXs were evaluated for leukemia cell engraftment on an IVIS imager (Perkins-Elmer). After confirming the engraftment of leukemia cells in vivo (1 week for HuT-78 and 3 weeks for primary T-PLL), cohorts of mice were randomly assigned to treatment with vehicle or 8 mg/kg daratumumab (Darzalex Faspro formulation with subcutaneous administration) once a week. Mice were imaged weekly using an IVIS imager, starting 1 week after treatment initiation. Figure made with biorender.com. (D) Representative bioluminescence images of HuT-78 mice with PDXs demonstrating leukemia burden in vehicle-treated (red) and daratumumab-treated (black) cohorts at weeks 2, 3, and 4 after treatment initiation. (E) Luciferase bioluminescence intensity was quantified by measuring total flux (photons/s) in mice using the IVIS-100 and Living Image software at each time point (vehicle n = 5, but 2 vehicle mice died before the week-4 imaging time point; daratumumab n = 4; P < .0001). (F) Survival curve of HuT-78 mice with PDXs treated with vehicle (pink) compared with daratumumab (purple; P = .02). (G) Representative bioluminescence images of mice with xenografts derived from patients with T-PLL demonstrating leukemia burden in vehicle and daratumumab-treated cohorts over time. (H) Luciferase bioluminescence presented as total flux (photons/second) over time of mice with xenografts derived from patients with T-PLL treated with daratumumab (n = 5, purple) vs vehicle (n = 4, pink; P < .0001).
Preclinical efficacy of daratumumab in CD38-expressing MTCN-bearing mice in 2 different xenograft models. (A) CD38 expression in HuT-78 depicted as histogram of CD38 compared with isotype control-stained cells. (B) CD38 expression in luciferase-GFP–transduced T-PLL cells from patients depicted as histogram of CD38 compared with isotype control-stained cells. (C) Schematic diagram showing experimental design: HuT-78 or primary T-PLL cells from patients were transduced with the lentivirus expressing firefly luciferase-GFP, sorted to >99% purity, and transplanted into Rag−/−γc−/− mice with 2 or 4 million cells, respectively. Mice with PDXs were evaluated for leukemia cell engraftment on an IVIS imager (Perkins-Elmer). After confirming the engraftment of leukemia cells in vivo (1 week for HuT-78 and 3 weeks for primary T-PLL), cohorts of mice were randomly assigned to treatment with vehicle or 8 mg/kg daratumumab (Darzalex Faspro formulation with subcutaneous administration) once a week. Mice were imaged weekly using an IVIS imager, starting 1 week after treatment initiation. Figure made with biorender.com. (D) Representative bioluminescence images of HuT-78 mice with PDXs demonstrating leukemia burden in vehicle-treated (red) and daratumumab-treated (black) cohorts at weeks 2, 3, and 4 after treatment initiation. (E) Luciferase bioluminescence intensity was quantified by measuring total flux (photons/s) in mice using the IVIS-100 and Living Image software at each time point (vehicle n = 5, but 2 vehicle mice died before the week-4 imaging time point; daratumumab n = 4; P < .0001). (F) Survival curve of HuT-78 mice with PDXs treated with vehicle (pink) compared with daratumumab (purple; P = .02). (G) Representative bioluminescence images of mice with xenografts derived from patients with T-PLL demonstrating leukemia burden in vehicle and daratumumab-treated cohorts over time. (H) Luciferase bioluminescence presented as total flux (photons/second) over time of mice with xenografts derived from patients with T-PLL treated with daratumumab (n = 5, purple) vs vehicle (n = 4, pink; P < .0001).
The finding of CD38 expression on the malignant T cells across a broad spectrum of MTCN subtypes in this study is noteworthy because it opens novel avenues for immunotherapeutic targeting in MTCNs that currently have limited treatment options. To our knowledge, this is the largest and most diverse characterization of CD38 expression using FC across a broad spectrum of MTCN. There have been few reports on CD38 expression in MTCN. By FC, the frequency of CD38 expression was previously reported to be low in a large cohort of patients with Sézary syndrome.17 CD38 expression determined by immunohistochemistry has previously been described in strictly nodal PTCL and the highest levels of CD38 were detected in PTCL-NOS, in accordance with our FC data.18 NK/T-cell lymphoma is often CD38+ by IHC with frequently high expression,14 but we were not able to assess that in our data set with FC. Further studies into the clinical efficacy of anti-CD38 treatment in different subtypes of MTCN are needed, as well as an investigation into possible roles for CD38 in the pathogenesis of these diseases. These findings provide a strong rationale for developing anti-CD38–targeted agents in patients with MTCN.
Acknowledgment: The authors thank the TJUH pharmacy for their kind donation of daratumumab for use in these studies.
Contributions: C.I. and W.T.J. wrote the manuscript, designed and performed research, analyzed and interpreted data, and performed statistical analysis; K.M. and C.I. performed in vivo experiments, imaging, and data collection; A.V., L.N., G.U., and J.G. collected data; J.E.B. edited the manuscript; A.B. and R.K. collected data and edited the manuscript; P.S. provided conceptual design input, performed statistical analysis, and edited the manuscript; N.N. provided input on conceptual design and edited the manuscript; B.C. provided reagents, experimental design feedback, and edited the manuscript; P.P. kindly provided the luciferase virus and training critical to developing the luciferase PDX mouse models used in this study, and edited the manuscript; N.C. and P.P. provided samples and contributed to conceptual design and edited the manuscript; and A.M. designed the research, analyzed and interpreted data, performed statistical analysis, developed figures, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anjali Mishra, Sidney Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA; e-mail: Anjali.mishra@jefferson.edu; and Pierluigi Porcu, Sidney Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA; e-mail: Pierluigi.Porcu@jefferson.edu.
References
Author notes
∗C.I. and W.T.J. contributed equally to this work.
For data sharing, contact the corresponding author, Anjali Mishra (Anjali.mishra@jefferson.edu).
The full-text version of this article contains a data supplement.